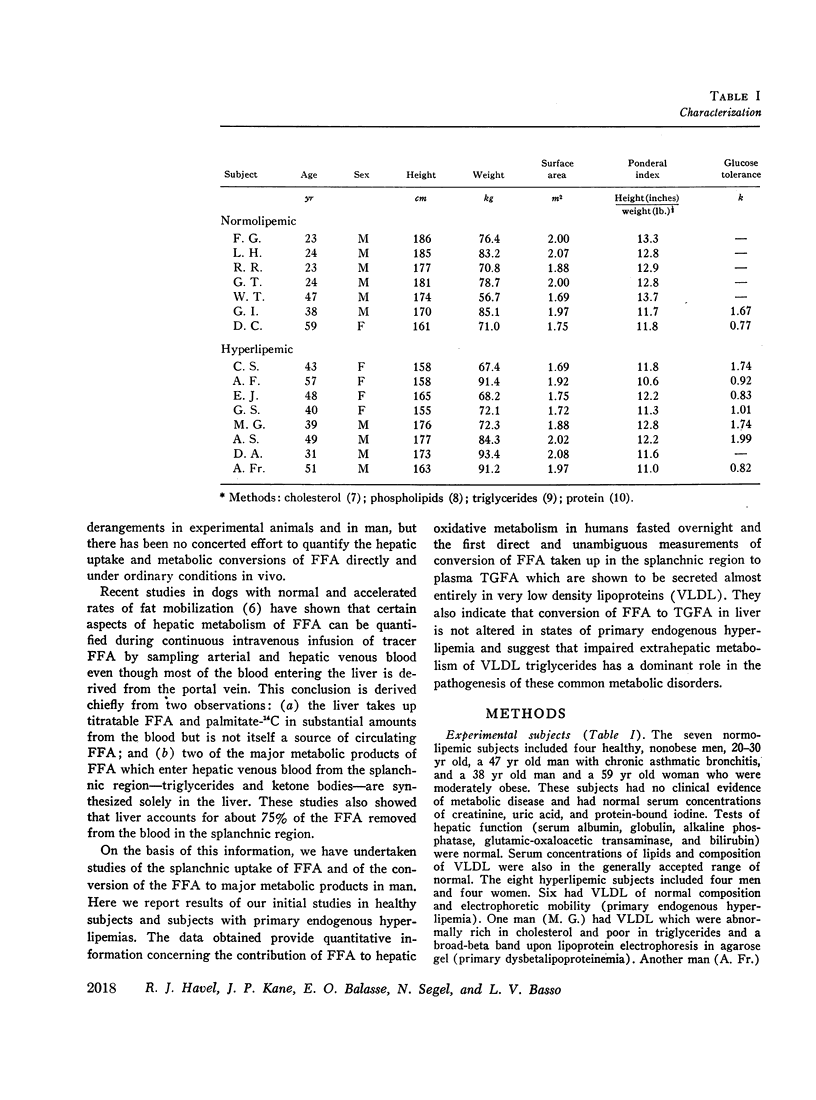
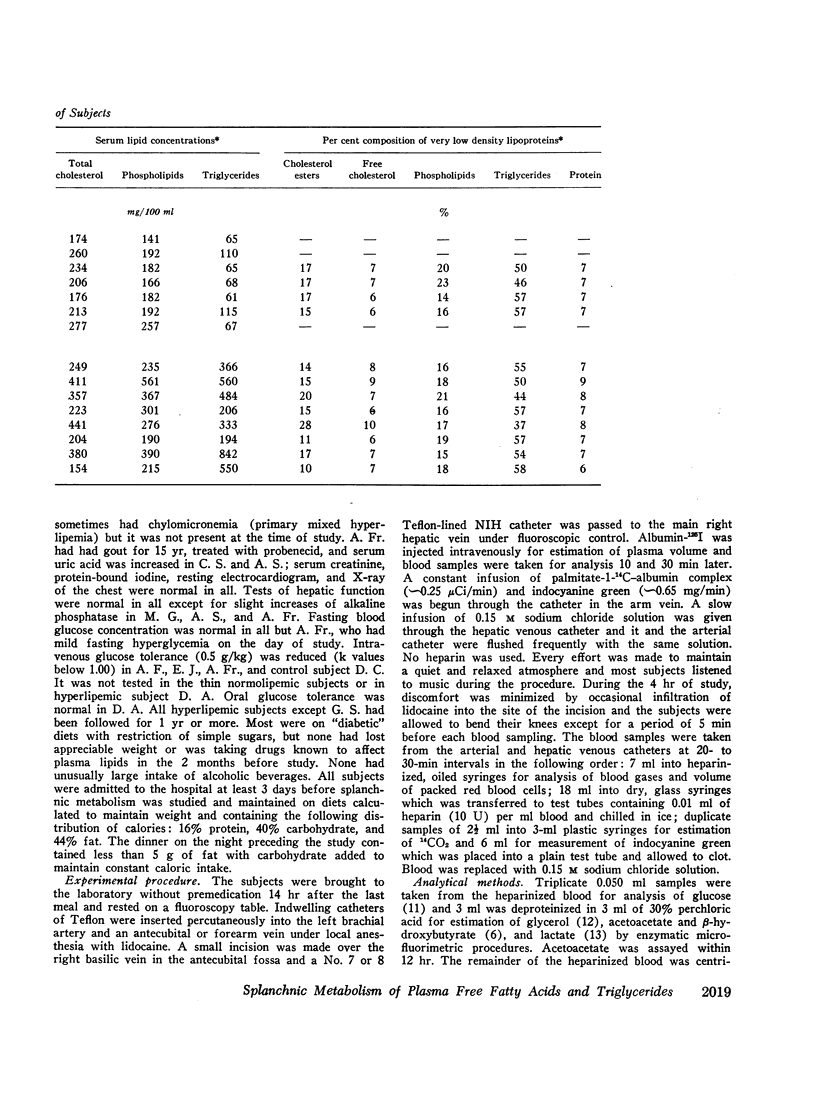
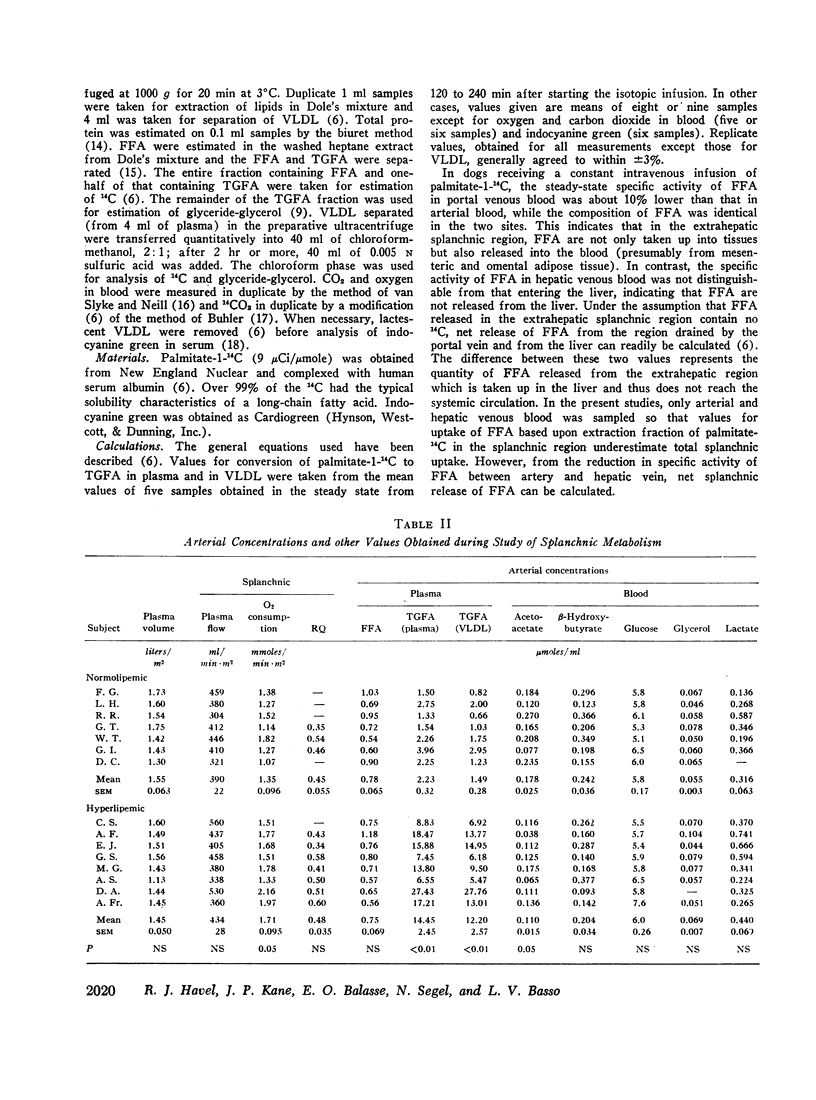
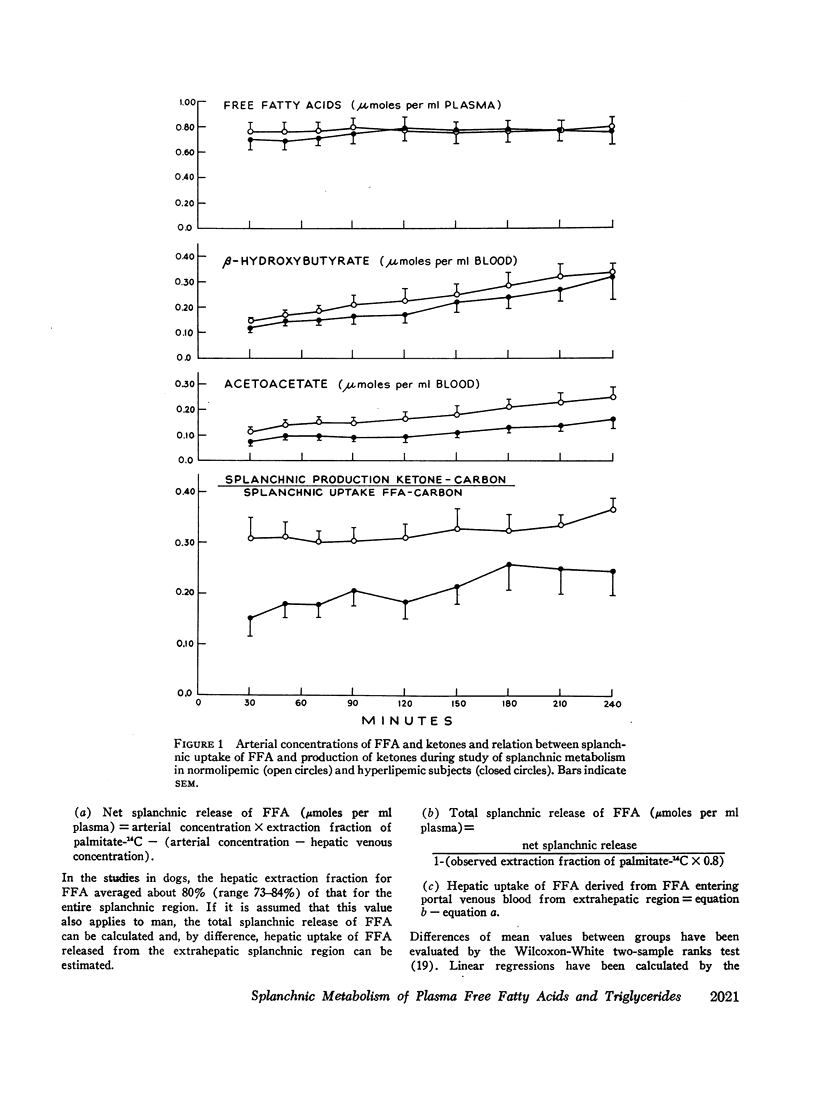
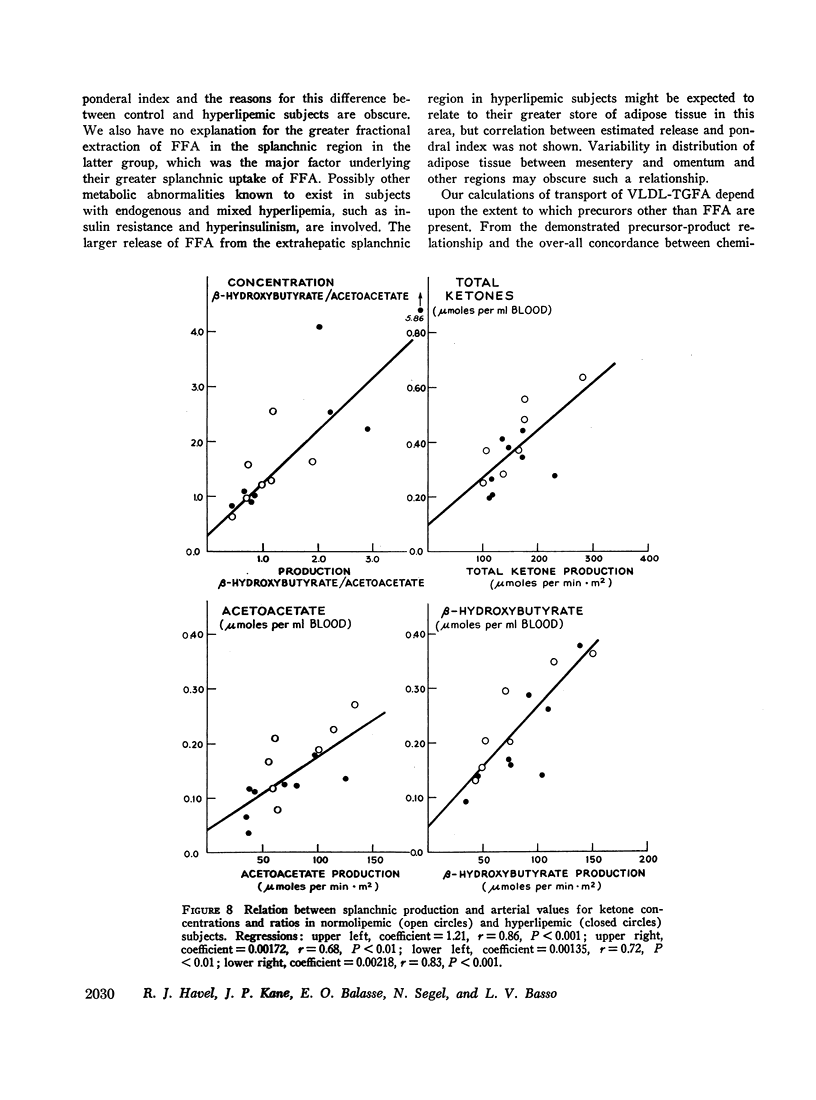
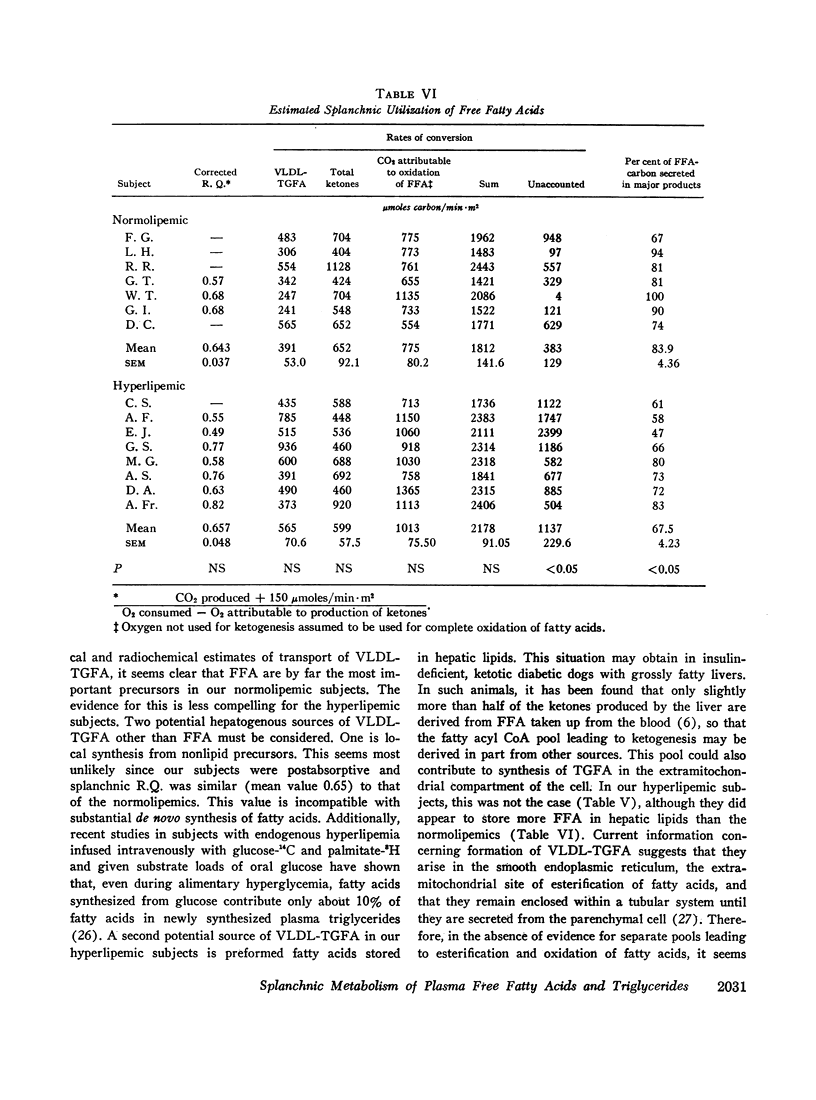
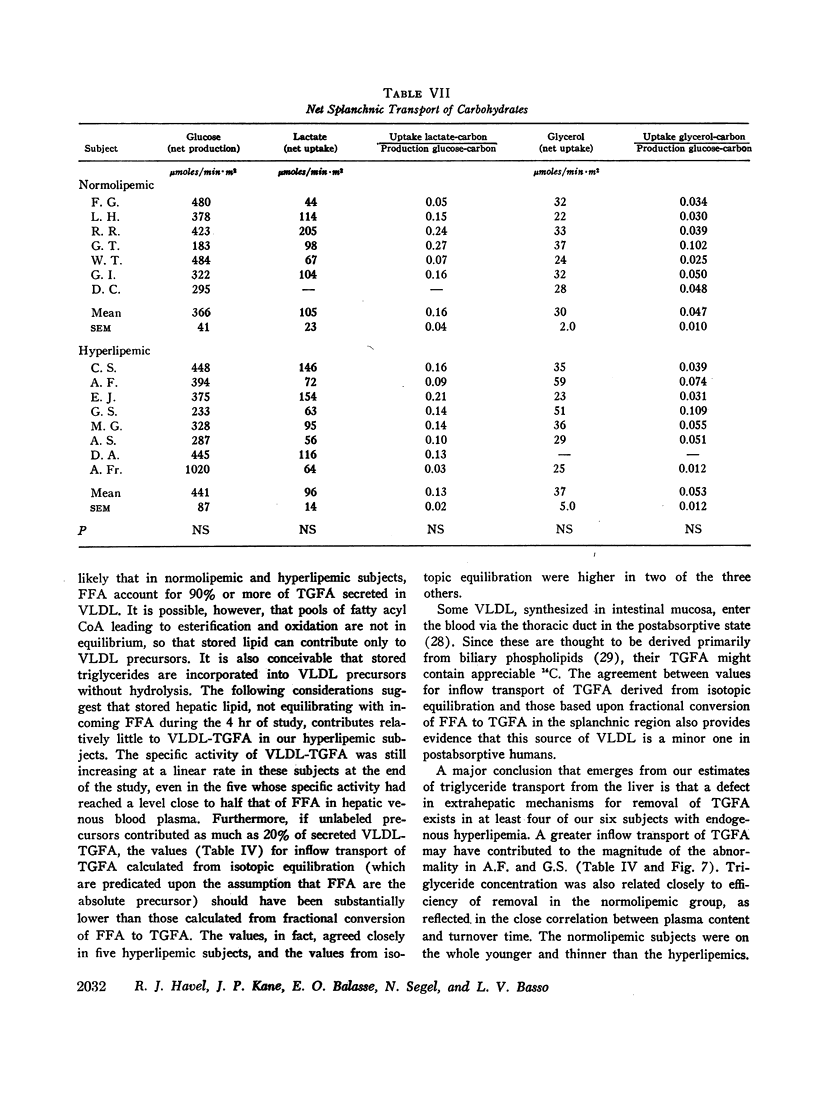
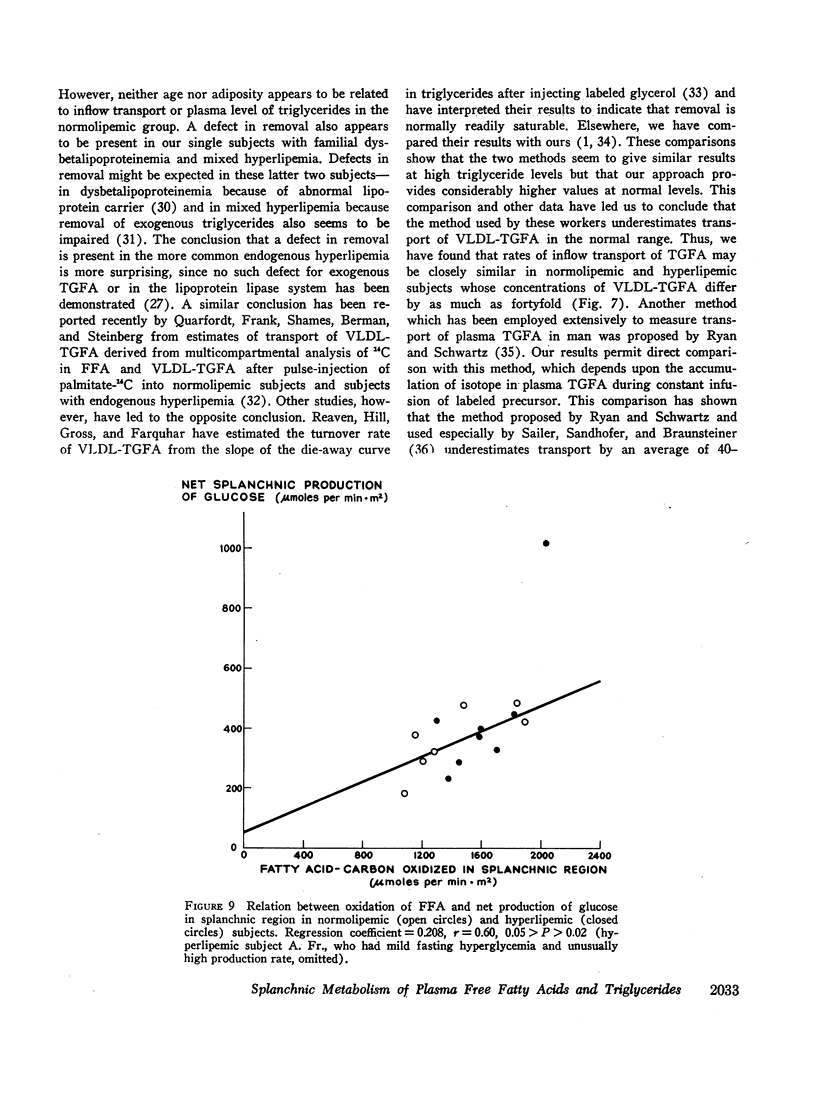
Abstract
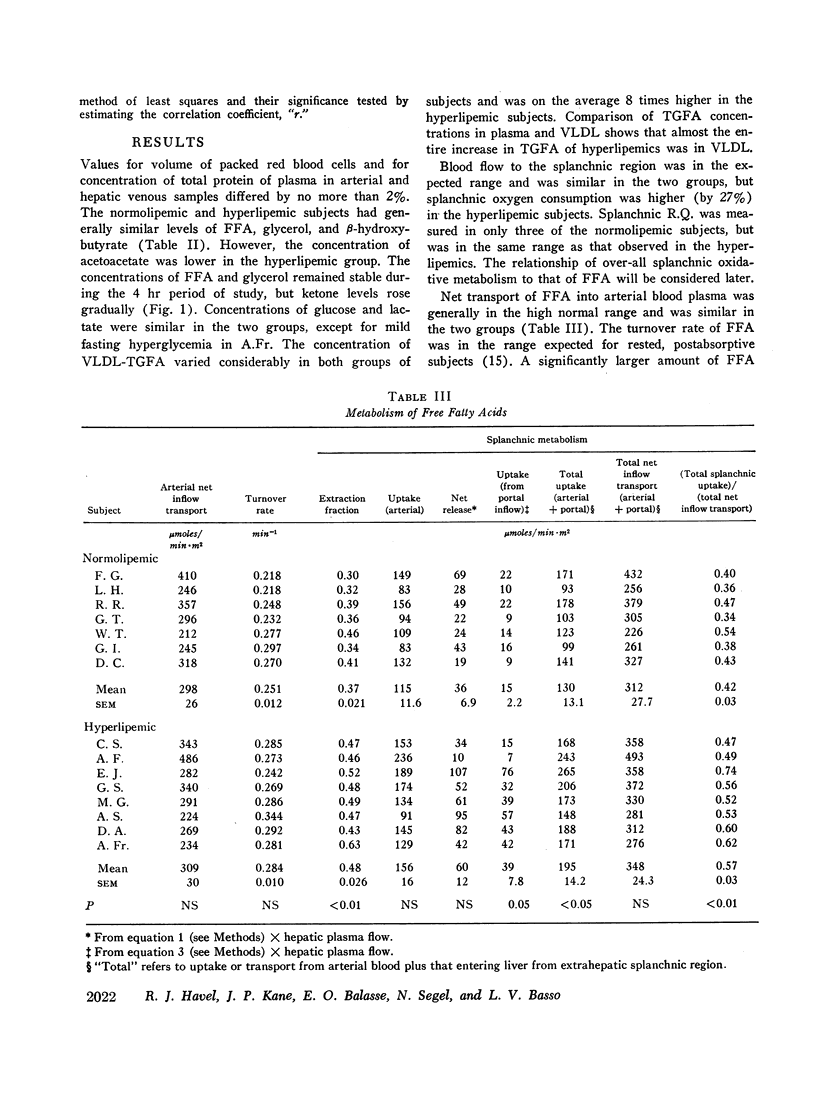
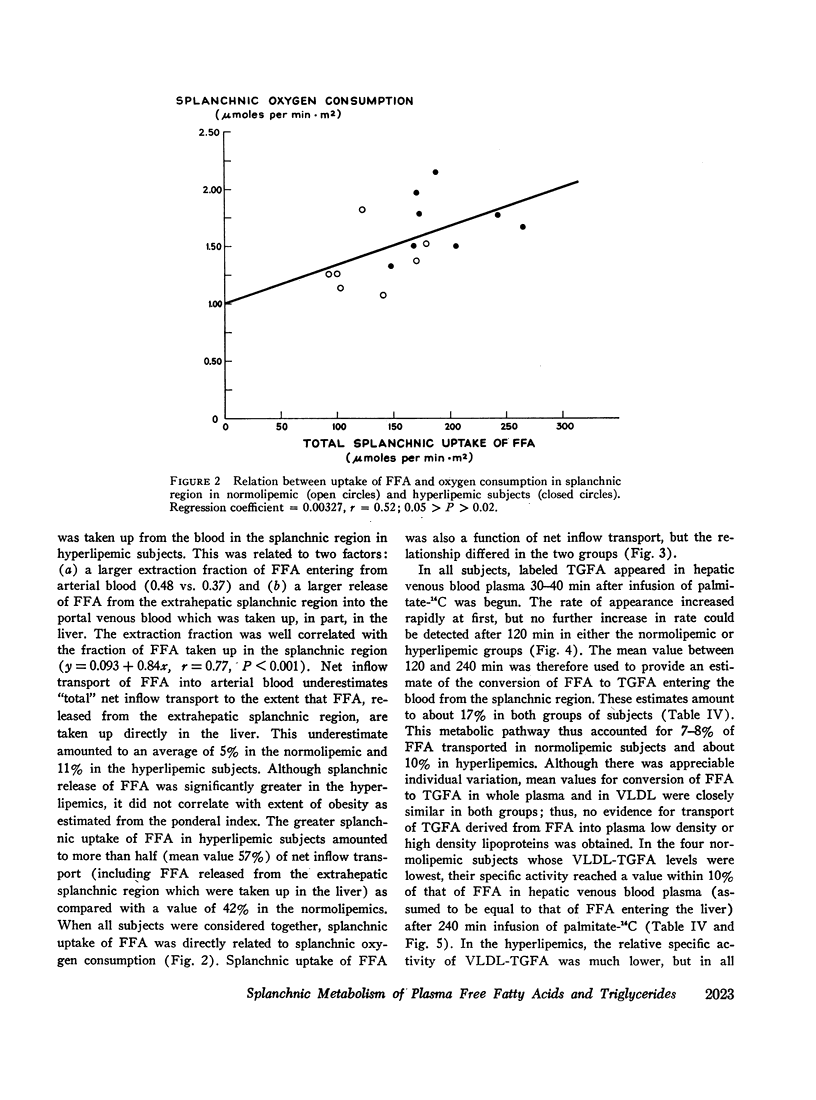
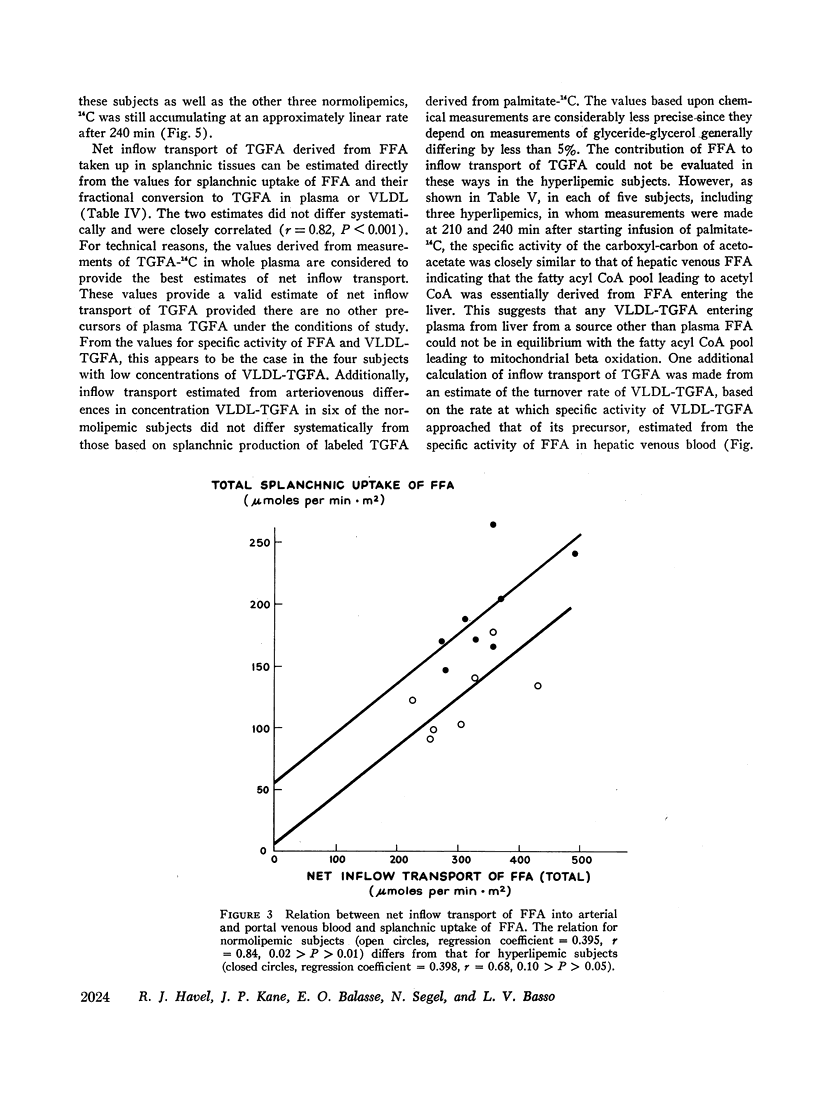
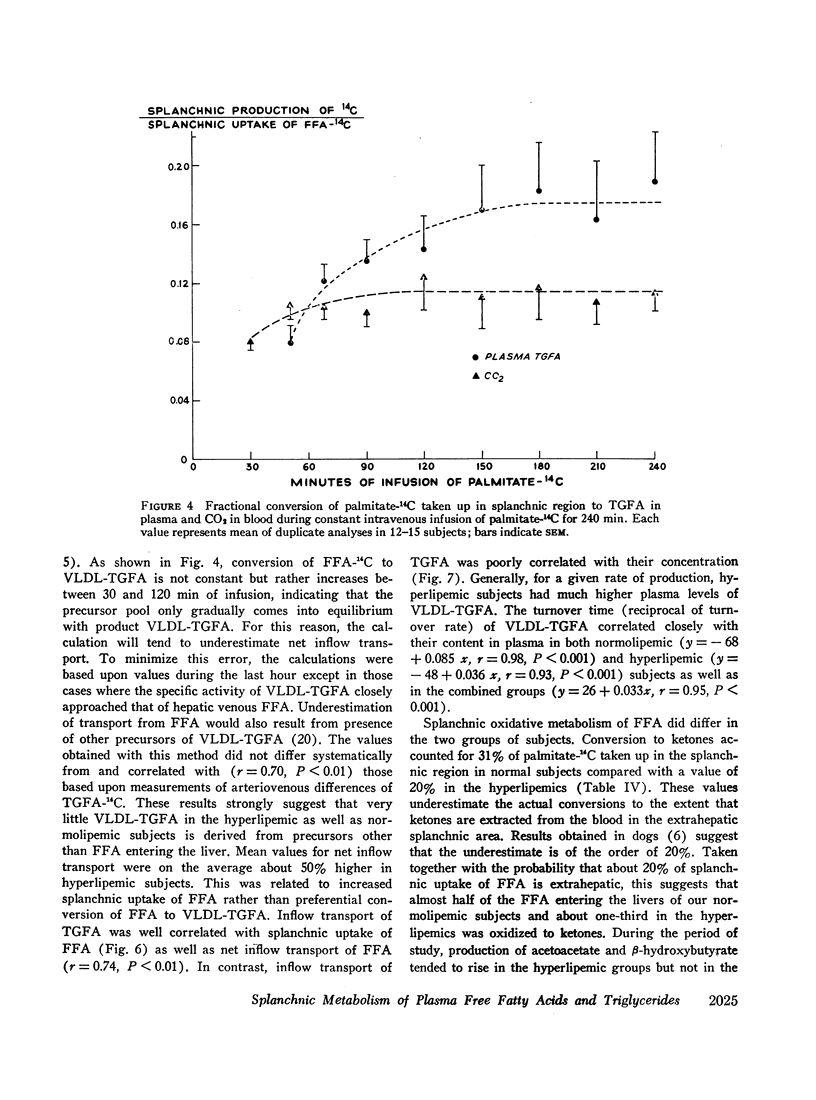
Transport of free fatty acids from the blood into the splanchnic region and their conversion to triglycerides of very low density lipoproteins, together with estimates of splanchnic oxidation of free fatty acids to ketones and to carbon dioxide and water, have been made in the postabsorptive state in seven normolipemic subjects, six with primary endogenous hyperlipemia and one each with primary dysbetalipoproteinemia and mixed hyperlipemia. Net systemic transport of free fatty acids into the blood was the same in normolipemic and hyperlipemic groups, but a greater fraction was taken up in the splanchnic region in the latter. Transport into the blood in very low density lipoproteins of triglyceride fatty acids derived from free fatty acids was proportional and bore the same relationship to splanchnic uptake of free fatty acids in the two groups. In normolipemic subjects, near equilibration of specific activities after 4 hr infusion of palmitate-1-14C showed that almost all triglyceride fatty acids of very low density lipoproteins and acetoacetate were derived from free fatty acids taken up in the splanchnic region. In the hyperlipemic subjects, equilibration of free fatty acidcarbon with acetoacetate was almost complete, but not with triglyceride fatty acids, owing at least in part to increased pool size. Comparison of the rate of equilibration of triglyceride fatty acids-14C with rate of inflow transport from the splanchnic region, together with other data, indicated that most of the circulating triglyceride fatty acids of very low density lipoproteins in hyperlipemic subjects were also derived from free fatty acids. Although mean inflow transport of triglyceride fatty acids was greater in the hyperlipemic subjects, it correlated poorly with their concentration and it appeared that efficiency of mechanisms for extrahepatic removal must be a major determinant of the concentration of triglycerides in blood plasma of the normolipemic as well as the hyperlipemic subjects. Estimates of splanchnic respiratory quotient supported the concept that oxidation of free fatty acids accounts for almost all of splanchnic oxygen consumption in the postabsorptive state. Splanchnic oxygen consumption was greater in the hyperlipemics, but fractional oxidation of free fatty acids to ketones was higher in normolipemic subjects. Calculations of splanchnic balance indicate that a larger fraction of free fatty acids was stored in lipids of splanchnic tissues in the hyperlipemics. No differences were found between the two groups in net splanchnic transport of glucose, lactate, or glycerol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORCHGREVINK C. F., HAVEL R. J. TRANSPORT OF GLYCEROL IN HUMAN BLOOD. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:946–949. doi: 10.3181/00379727-113-28539. [DOI] [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Basso L. V., Havel R. J. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970 Mar;49(3):537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. Origin and characteristics of endogenous lipid in thoracic duct lymph in rat. J Lipid Res. 1966 Jan;7(1):158–166. [PubMed] [Google Scholar]
- CARLSON L. A. DETERMINATION OF SERUM TRIGLYCERIDES. J Atheroscler Res. 1963 Jul-Aug;3:334–336. doi: 10.1016/s0368-1319(63)80012-5. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A., EKELUND L. G. Splanchnic production and uptake of endogenous triglycerides in the fasting state in man. J Clin Invest. 1963 May;42:714–720. doi: 10.1172/JCI104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDBERG S. J., KLEIN R. F., TROUT D. L., BOGDONOFF M. D., ESTES E. H., Jr The incorporation of plasma free fatty acids into plasma triglycerides in man. J Clin Invest. 1961 Oct;40:1846–1855. doi: 10.1172/JCI104409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Jan 5;276(1):34–contd. doi: 10.1056/NEJM196701052760107. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J. Conversion of plasma free fatty acids into triglycerides of plasma lipoprotein fractions in man. Metabolism. 1961 Dec;10:1031–1034. [PubMed] [Google Scholar]
- HAVEL R. J., NAIMARK A., BORCHGREVINK C. F. Turnover rate and oxidation of free fatty acids of blood plasma in man during exercise: studies during continuous infusion of palmitate-1-C14. J Clin Invest. 1963 Jul;42:1054–1063. doi: 10.1172/JCI104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Balasse E. O., Williams H. E., Kane J. P., Segel N. Splanchnic metabolism in von Gierke's disease (glycogenosis type I). Trans Assoc Am Physicians. 1969;82:305–323. [PubMed] [Google Scholar]
- Havel R. J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med. 1969;15:117–154. [PubMed] [Google Scholar]
- KETTERER S. G., WIEGAND B. D., RAPAPORT E. Hepatic uptake and biliary excretion of indocyanine green and its use in estimation of hepatic blood flow in dogs. Am J Physiol. 1960 Sep;199:481–484. doi: 10.1152/ajplegacy.1960.199.3.481. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Bovine ketosis. Vet Rec. 1966 Feb 5;78(6):187–192. doi: 10.1136/vr.78.6.187. [DOI] [PubMed] [Google Scholar]
- LOOMIS M. E. An enzymatic fluorometric method for the determination of lactic acid in serum. J Lab Clin Med. 1961 Jun;57:966–969. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYERS J. D. Net splanchnic glucose production in normal man and in various disease states. J Clin Invest. 1950 Nov;29(11):1421–1429. doi: 10.1172/JCI102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. D. THE HEPATIC BLOOD FLOW AND SPLANCHNIC OXYGEN CONSUMPTION OF MAN-THEIR ESTIMATION FROM UREA PRODUCTION OR BROMSULPHALEIN EXCRETION DURING CATHETERIZATION OF THE HEPATIC VEINS. J Clin Invest. 1947 Nov;26(6):1130–1137. doi: 10.1172/JCI101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDOLPH W., MAAS D., RICHTER J., HASINGER F., HOFMANN H., DOHRN P. UBER DIE BEDEUTUNG VON ACETACETAT UND BETA-HYDROXYBUTYRAT IM STOFFWECHSEL DES MENSCHLICHEN HERZENS. Klin Wochenschr. 1965 Apr 15;43:445–451. doi: 10.1007/BF01483852. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Hill D. B., Gross R. C., Farquhar J. W. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 1965 Nov;44(11):1826–1833. doi: 10.1172/JCI105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. G., Schwartz T. B. Dynamics of plasma triglyceride turnover in man. Metabolism. 1965 Dec;14(12):1243–1254. doi: 10.1016/s0026-0495(65)80004-x. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Sailer S., Sandhofer F., Braunsteiner H. Umsatzraten für freie Fettsäuren und Triglyceride im Plasma bei essentieller Hyperlipämie. Klin Wochenschr. 1966 Sep 1;44(17):1032–1036. doi: 10.1007/BF01878782. [DOI] [PubMed] [Google Scholar]
- Sandhofer F., Bolzano K., Sailer S., Braunsteiner H. Quantitative Untersuchungen über den Einbau von Plasmaglucose-Kohlenstoff in Plasmatriglyceride und die Veresterungsrate von freien Fettsäuren des Plasmas zu Plasmatriglyceriden während oraler Zufuhr von Glucose bei primärer kohlenhydratinduzierter Hypertriglyceridämie. Klin Wochenschr. 1969 Oct 15;47(20):1086–1094. doi: 10.1007/BF01496641. [DOI] [PubMed] [Google Scholar]
- Stewart C. P., Hendry E. B. The phospholipins of blood. Biochem J. 1935 Jul;29(7):1683–1689. doi: 10.1042/bj0291683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYGSTRUP N., WINKLER K., LUNDQUIST F. THE MECHANISM OF THE FRUCTOSE EFFECT ON THE ETHANOL METABOLISM OF THE HUMAN LIVER. J Clin Invest. 1965 May;44:817–830. doi: 10.1172/JCI105194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., BODENLOS L. J. Urea metabolism in man. J Clin Invest. 1959 Sep;38:1617–1626. doi: 10.1172/JCI103940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]



