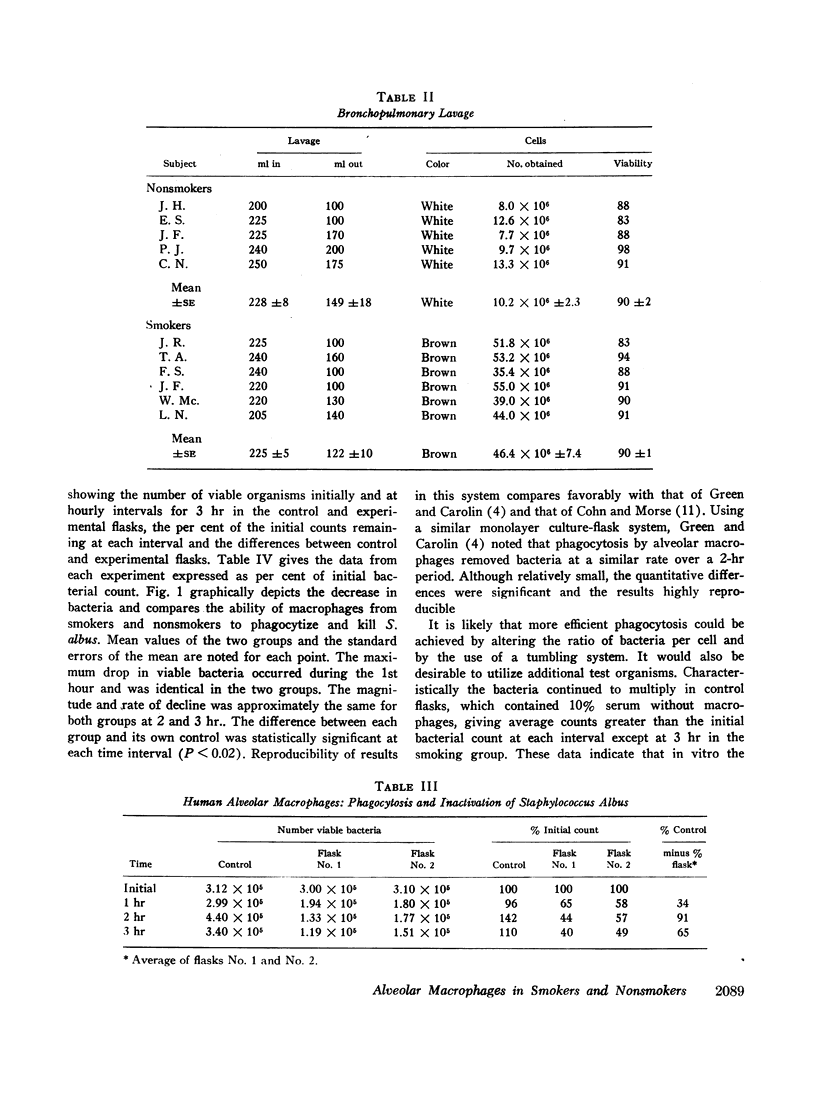
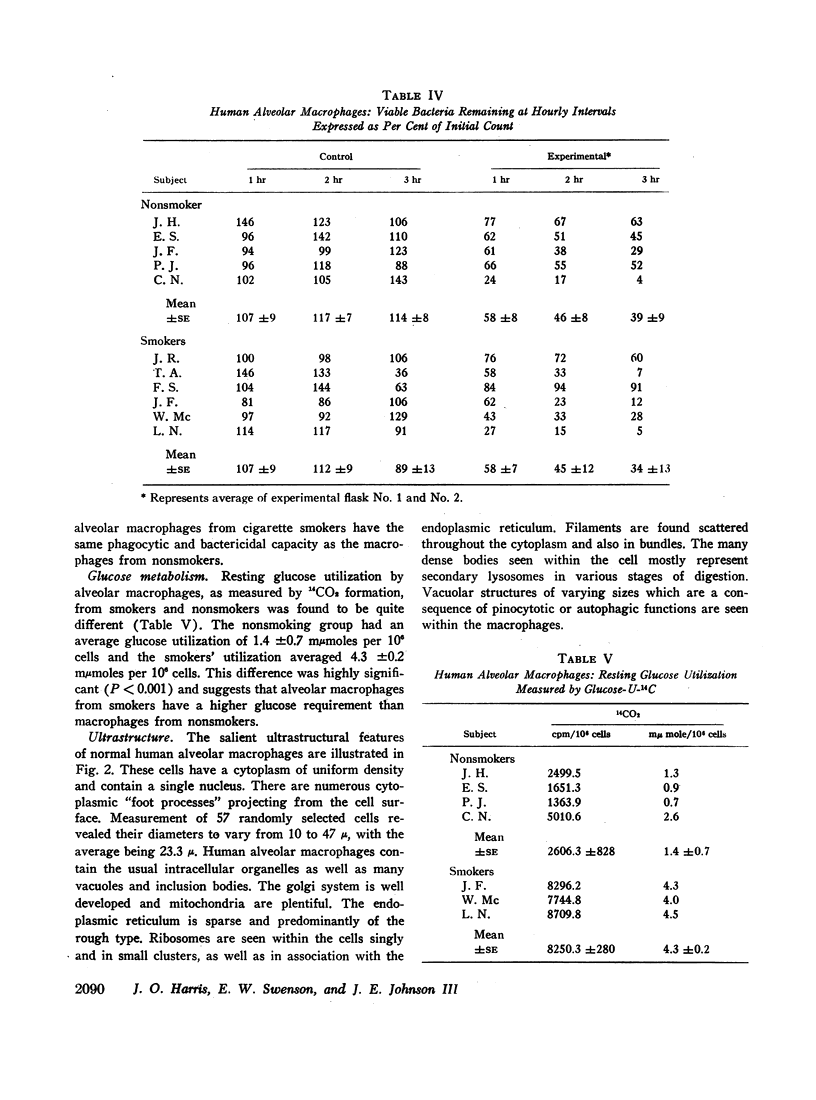
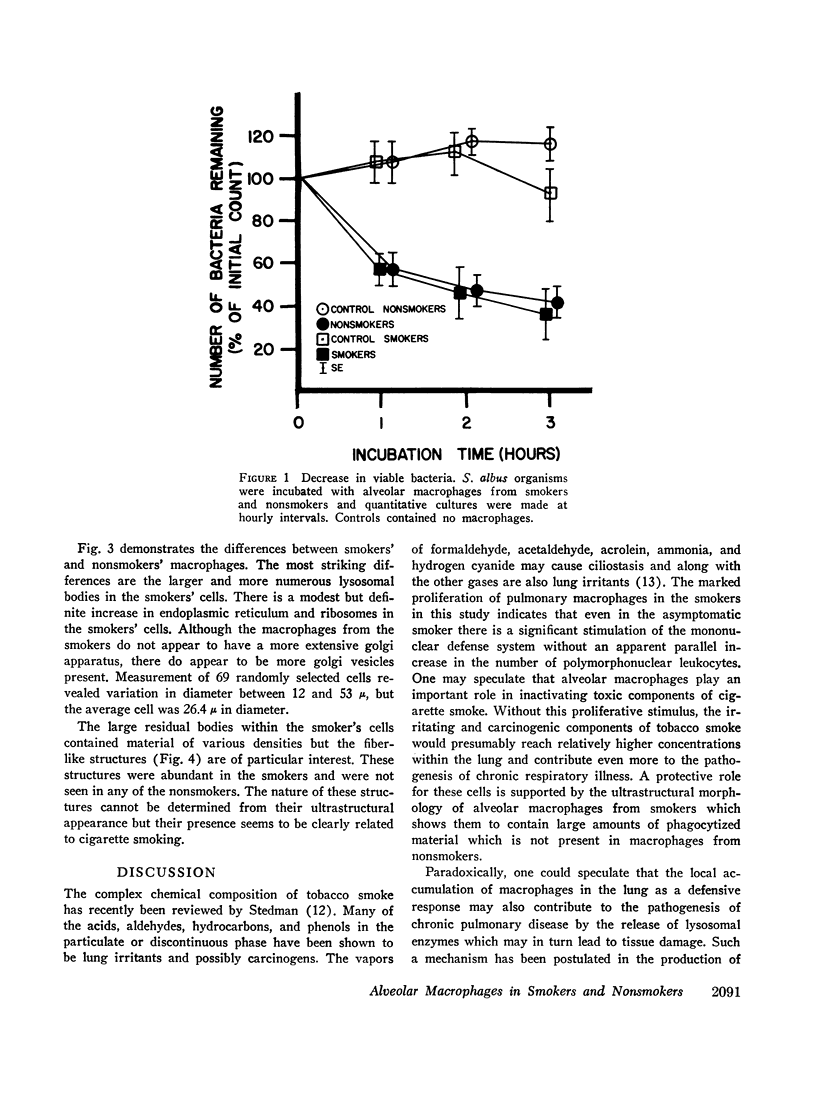
Abstract
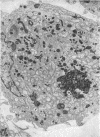
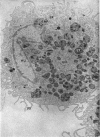
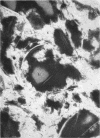
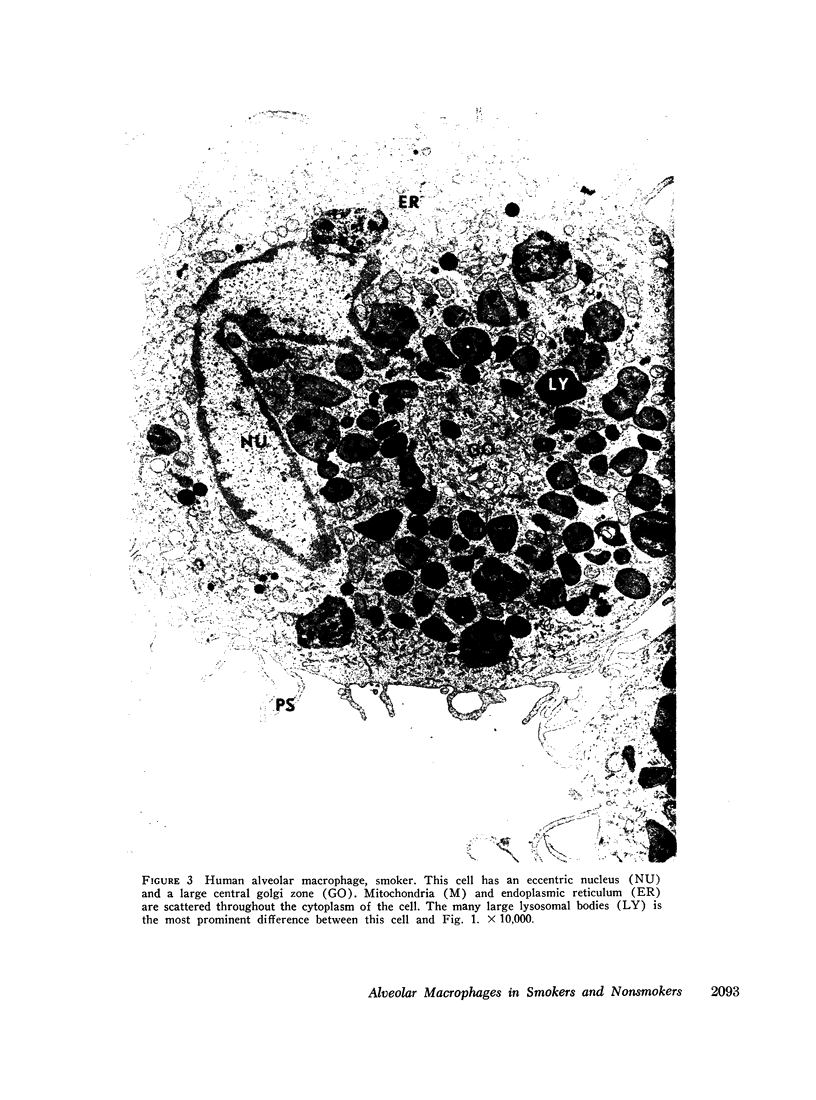
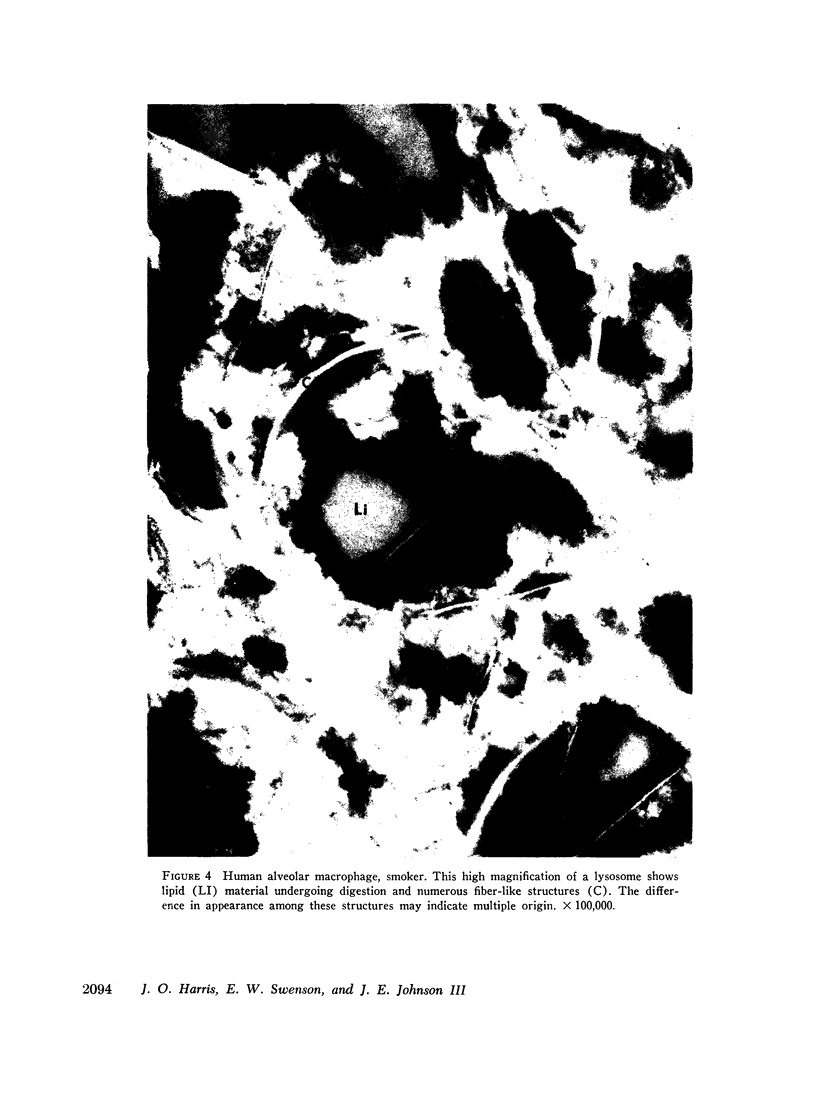
Phagocytic ability, glucose utilization, and ultrastructural morphology were studied in human alveolar macrophages in smokers and nonsmokers. The macrophages were obtained by bronchopulmonary lavage and the studies were carried out in vitro in the absence of smoke. Phagocytic ability was measured as the decrease in the number of viable Staphylococcus albus organisms incubated with the macrophages. Measurements of 14CO2 formation from glucose-U-14C were made in a resting state. 90-95% of the cells obtained by lavage were large mononuclear macrophages of which approximately 90% remained viable at the end of the experiment. Smokers yielded many more macrophages per lavage (mean 46.4 × 106 ±7.4) compared to the nonsmokers (mean 10.2 × 106 ±2.3). The decline in viable organisms was the same in each group, indicating phagocytic competence of alveolar macrophages removed from smokers. However, the mean glucose utilization for the smokers was 4.3 ±0.2 mμmoles/106 cells and 1.4 ±0.7 mμmoles/106 cells for the nonsmokers. This very significant difference (P < 0.0001) suggests that smokers' macrophages have a higher resting energy requirement than those of nonsmokers. Comparison of the ultrastructural morphology of the alveolar macrophages from each group reveals that the cells from smokers differ from those of nonsmokers in that they are slightly larger, and contain more golgi vesicles, endoplasmic reticulum, and residual bodies. The residual bodies in smokers' cells contain distinctive fiber-like inclusions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley T. N., Swenson E. W., Curran W. S., Huber G. L., Ladman A. J. Bronchopulmonary lavage in normal subjects and patients with obstructive lung disease. Ann Intern Med. 1967 Apr;66(4):651–658. doi: 10.7326/0003-4819-66-4-651. [DOI] [PubMed] [Google Scholar]
- Green G. M., Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967 Feb 23;276(8):421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Kass E. H., Green G. M., Goldstein E. Mechanisms of antibacterial action in the respiratory system. Bacteriol Rev. 1966 Sep;30(3):488–497. doi: 10.1128/br.30.3.488-497.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueppers F., Bearn A. G. A possible experimental approach to the association of hereditary alpha-1-antitrypsin deficiency and pulmonary emphysema. Proc Soc Exp Biol Med. 1966 Apr;121(4):1207–1209. doi: 10.3181/00379727-121-31006. [DOI] [PubMed] [Google Scholar]
- LAURENZI G. A., GUARNERI J. J., ENDRIGA R. B., CAREY J. P. CLEARANCE OF BACTERIA BY THE LOWER RESPIRATORY TRACT. Science. 1963 Dec 20;142(3599):1572–1573. doi: 10.1126/science.142.3599.1572. [DOI] [PubMed] [Google Scholar]
- LAURENZI G. A., POTTER R. T., KASS E. H. Bacteriologic flora of the lower respiratory tract. N Engl J Med. 1961 Dec 28;265:1273–1278. doi: 10.1056/NEJM196112282652601. [DOI] [PubMed] [Google Scholar]
- LEAKE E. S., GONZALEZ-OJEDA D., MYRVIK Q. N. ENZYMATIC DIFFERENCES BETWEEN NORMAL ALVEOLAR MACROPHAGES AND OIL-INDUCED PERITONEAL MACROPHAGES OBTAINED FROM RABBITS. Exp Cell Res. 1964 Feb;33:553–561. doi: 10.1016/0014-4827(64)90020-5. [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N., Evans D. G. Metabolic and immunologic activities of alveolar macrophages. Arch Environ Health. 1967 Jan;14(1):92–96. doi: 10.1080/00039896.1967.10664700. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S. A., Finley T. N., Smith M. H., Ladman A. J. A comparison of alveolar macrophages and pulmonary surfactant(?) obtained from the lungs of human smokers and nonsmokers by endobronchial lavage. Anat Rec. 1969 Apr;163(4):497–507. doi: 10.1002/ar.1091630402. [DOI] [PubMed] [Google Scholar]
- Stedman R. L. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968 Apr;68(2):153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]