Abstract
Background
Prostate cancer management involves a balance between the risks of cancer death against those from other causes. To evaluate the performance of several comorbidity indices in predicting comorbid death in a prostate cancer radiotherapy cohort.
Methods
2,131 men with localised prostate cancer treated with radical radiotherapy between 1999 and 2007 were studied. Tumour features, androgen deprivation usage, age, number of prescription medications (PMN) and Adult Comorbidity Evaluation-27 Index (ACE-27) were recorded. Death from prostate cancer (DPC) and death from other causes (DOC) were analysed as competing causes of death using a competing risks model, with discrimination assessed using the concordance index.
Results
ACE-27 scores correlated with patient’s PMN (median PMN = 2). Tumour features were independent of ACE-27 scores. Estimated cumulative incidences of DOC and DPC at 10 years were 16.4% and 7.7% respectively. In the low/intermediate risk group (n = 1026) there was a 3.4-fold predominance of DOC inside 10 years (cumulative incidence: 15.8% DOC vs 3.4% DPC). High-risk men had approximately equal rates of DPC and DOC at 10 years. Multivariable analysis showed age, ACE-27 score ≥ 1 and PMN to have significant associations with DOC (P < 0.002 for all). A multivariable model incorporating all 3 variables resulted in C-Index = 0.646.
Conclusion
Age, ACE-27 score and PMN act as independent prognostic factors for DOC in prostate cancer patients and can improve patient’s life expectancy prediction.
Keywords: Comorbidity, Prostate cancer, Radiotherapy
1. Introduction
Prostate cancer (PC) is the most prevalent malignancy in Australian males, with more than 17,000 new diagnoses each year. The risk of developing PC increases with age, with many of those diagnosed being 70 years of age or older. With increasing age comes an inevitable comorbidity burden in many PC patients.1, 2 The decision to treat PC in a patient can be complex when multiple comorbidities exist which can potentially impact his life expectancy. As PC has a long indolent natural history and its definitive treatment may cause morbidity, resulting in a significant quality of life burden,3 the decision to treat localized PC must be a balance between perceived risks related to cancer progression against those of comorbidities.
In maximizing life expectancy in PC, there needs to be a balance between not overtreating men with a substantial comorbid condition, and undertreating those with aggressive disease. Traditional doctrine has suggested that all men with < 10 years life expectancy from non-PC related conditions should not be offered curative therapy,4 bringing with it an assumption that intervention will never show an impact inside this time which may not always hold true. While prognostic features of PC are well described and give a good sense of possible rate of disease progression, comorbidity assessment is often performed irregularly or subjectively. Several retrospective studies3, 5, 6 concluded that using a comorbidity index can help optimize clinical decision making, as it provides a more accurate picture of mortality risk posed by patient’s comorbidities in a setting of newly diagnosed localized PC. Currently, there is no standard instrument used in a clinical setting to evaluate comorbidity burden in PC patients to predict mortality.
In this paper we investigate several indices of comorbidity burden in men with localized PC treated with curative intent radiotherapy in a large Australian cohort. We aim to assess the association and discriminatory impact of patient age, the number of prescription medications (PMN) the patient uses at the time of diagnosis,2 and the Adult Comorbidity Evaluation-27 Index (ACE-27) to comorbid death risk.7, 8 We make recommendations regarding the optimal use of these simple comorbidity indices clinically.
2. Materials and methods
Eligible cases had histologically confirmed adenocarcinoma of the prostate and were referred for consideration of radical radiation therapy at the Peter MacCallum Cancer Centre (PMCC). Cases were treated with radical radiotherapy consecutively between 1999 and 2007. Tumor features, androgen deprivation usage, age, PMN, and ACE-27 scores were recorded. Patients were grouped to have low, intermediate, and high risk PC according to the D’Amico risk classification.9 Death from PC (DPC) and death from other causes (DOC) were identified using a combination of medical records and death registry data. The study protocol was approved by the institutional Human Research and Ethics Committee.
2.1. Radiation therapy
External beam radiotherapy (EBRT) patients were simulated using computed tomography and planned for conformal radiation therapy. EBRT was administered using 18 MV beams generally with a 4-field or 5-field arrangement to a supine patient. Daily fractions of 2 Gy were delivered 5 days a week, to a median total dose of 74 Gy (prescribed to the International Commission on Radiation Units (ICRU) reference point). Image guided and intensity modulated radiation were not used. Low-dose-rate (LDR) brachytherapy was delivered using preplanning, and a modified peripheral weighting method to a planned dose of 145 Gy. High-dose-rate (HDR) brachytherapy was given as a boost to EBRT (46 Gy in 23 fractions), using a single implant to a typical dose of 19.5 Gy in three fractions over 24 hours.
2.2. Statistical analysis
Follow-up was calculated from the date of commencing radiation therapy. Descriptive data were expressed as a median value with associated 95% confidence interval (95%CI) based on the range from the 2.5th to the 97.5th centile values. Median potential follow-up was estimated using the methods of Schemper and Smith.10 A competing risks framework was used for all analyses using a subdistribution weighting method.11 DPC and DOC were treated as independent competing causes of death. First order interactions and nonlinearity of continuous covariable effects were examined. Discrimination assessment was performed using the c-index on both univariables and multivariable models of age, ACE-27 score, and PMN. The c-index is analogous to the area under a receiver operating characteristics curve, with a value of 1 indicating perfect discriminatory ability, and 0.5 indicating pure random chance. All analyses were performed using the R statistical language (R Foundation for Statistical Computing, Vienna, Austria) and estimated P values were two-sided and considered significant at a ≤ 0.05 level.
3. Results
In the years 1999–2007, 2,131 men treated with radical intent radiation therapy to the prostate at PMCC were eligible for analysis. Approximately 88% underwent EBRT, while 5% had LDR brachytherapy, with the remaining 7% having HDR brachytherapy, either as monotherapy or as a boost to EBRT. Some 21% of the EBRT cases had moderate or severe comorbidity score (ACE-27 ≥ 2), while 8% of those treated with HDR and LDR brachytherapy had a similarly high comorbidity burden, respectively. Summary baseline characteristics for the total cohort divided by ACE-27 are shown in Table 1. Median potential follow-up was 112.9 months.
Table 1.
Baseline characteristics of 2,131 radiotherapy patients analyzed grouped by ACE-27 score. Due to rounding, some sections may not add to exactly 100%.
| Variable | Group | ACE-27 score |
|||||
|---|---|---|---|---|---|---|---|
| 0 (n = 681) | 1 (n = 1,032) | 2 (n = 357) | 3 (n = 61) | Combined (n = 2,131) | P | ||
| Age | 67.6 (50.7–78.3) | 70.4 (55.3–79.7) | 70.3 (54.3–78.7) | 70.8 (58.9–78.9) | 69.7 (53.4 - 79.3) | < 0.001 | |
| Medication No. | 0 (0–3) | 3 (0–7) | 4 (0–9) | 5 (1–11) | 2 (0–7) | < 0.001 | |
| PSA (ng/mL) | < 10 | 312 (45.8%) | 486 (47.1%) | 160 (44.8%) | 22 (36.1%) | 980 (46%) | 0.059 |
| 10 – < 20 | 210 (30.8%) | 362 (35.1%) | 128 (35.9%) | 26 (42.6%) | 726 (34.1%) | ||
| ≥ 20 | 159 (23.3%) | 184 (17.8%) | 69 (19.3%) | 13 (21.3%) | 425 (19.9%) | ||
| T stage | 1 | 147 (21.6%) | 217 (21%) | 74 (20.7%) | 10 (16.4%) | 448 (21%) | 0.572 |
| 2 | 304 (44.6%) | 482 (46.7%) | 167 (46.8%) | 36 (59%) | 989 (46.4%) | ||
| 3 | 229 (33.6%) | 333 (32.3%) | 115 (32.2%) | 15 (24.6%) | 692 (32.5%) | ||
| Gleason score | ≤ 6 | 311 (45.7%) | 485 (47%) | 165 (46.2%) | 24 (39.3%) | 985 (46.2%) | 0.80 |
| 7 | 268 (39.4%) | 379 (36.7%) | 135 (37.8%) | 28 (45.9%) | 810 (38%) | ||
| 8–10 | 102 (15%) | 168 (16.3%) | 57 (16%) | 9 (14.8%) | 336 (15.8%) | ||
| Risk | Low | 116 (17%) | 155 (15%) | 51 (14.3%) | 4 (6.6%) | 326 (15.3%) | 0.095 |
| Intermediate | 243 (35.7%) | 421 (40.8%) | 138 (38.7%) | 31 (50.8%) | 833 (39.1%) | ||
| High | 322 (47.3%) | 456 (44.2%) | 168 (47.1%) | 26 (42.6%) | 972 (45.6%) | ||
| ADT given | 373 (54.8%) | 565 (54.7%) | 188 (52.7%) | 35 (57.4%) | 1,161 (54.5%) | 0.861 | |
| ADT duration (mo) | 3.7 (0–94.3) | 3.4 (0–68.96) | 2.9 (0–60.41) | 4.9 (0–106.25) | 3.2 (0–77.38) | 0.617 | |
| Radiation Modality | EBRT | 551 (80.9%) | 921 (89.2%) | 336 (94.1%) | 60 (98.4%) | 1,868 (87.7%) | < 0.001 |
| HDR | 74 (10.9%) | 66 (6.4%) | 12 (3.4%) | 1 (1.6%) | 153 (7.2%) | ||
| LDR | 56 (8.2%) | 45 (4.4%) | 9 (2.5%) | 0 (0%) | 110 (5.2%) | ||
ACE-27, Adult Comorbidity Evaluation-27; ADT, androgen deprivation therapy; EBRT, external beam radiotherapy; HDR, high-dose-rate; LDR, low-dose-rate; PSA, prostate specific antigen; T, tumor.
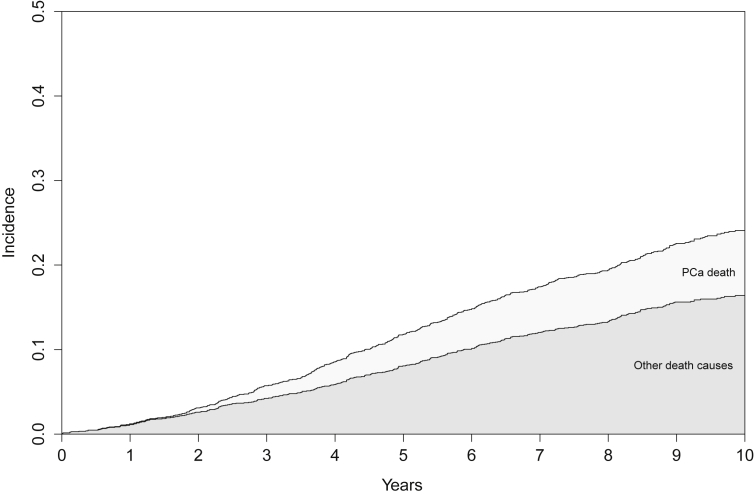
Overall, 483 deaths were recorded and 154 (32%) were from PC. Fig. 1 shows the relative survival states of the entire cohort for the first 10 years following radical radiation therapy for PC. The respective estimated cumulative incidence of DPC at 5 years and 10 years was 3.7% (95%CI 2.9–4.6%) and 7.7% (95%CI 6.5–8.9%). For DOC, 172 deaths were recorded within 5 years of RT, and 323 within 10 years. The cumulative incidence of DOC was 8.1% (95%CI 7–9.2%) and 16.4% (95%CI 14.7–18.1%), respectively.
Fig. 1.
Stacked cumulative incidence plot of prostate cancer (PCa death) and all other death causes for the total cohort (n = 2,131) over the 10 years following radiation therapy.
Subset analysis of men with low or intermediate risk cancer (n = 1,159) showed a 3.4% (95%CI 2.3–4.5%) risk of DPC at 10 years, which contrasted with a cumulative incidence of DOC of 15.7% (95%CI 13.4–18%). Some 80% of the cohort had low (ACE-27 score of 0 or 1), 17% had moderate (ACE-27 score of 2), and only 3% high (ACE-27 score of 3) scores. Grouped by ACE-27 score, the observed 10-year cumulative incidence of DOC was 9.6% (95%CI 7.3–12.1%) for ACE-27 = 0; 15.8% (95%CI 13.4–18.2%) for ACE-27 = 1; 27.4% (95%CI 22.2–32.3%) for ACE-27 = 2; and 37.7% (95%CI 25.2–52%) for the ACE-27 = 3 cohort. A subset analysis of men with low or intermediate risk cancer (n = 1,026) showed a 3.4% (95%CI 2.3–4.5%) risk of DPC at 10 years, which contrasted with a cumulative incidence of DOC of 15.7% (95%CI 13.3–18%).
Regression analysis of competing cause of death outcomes showed the tumor features of prostate specific antigen), Gleason score and tumor (T) stage to be significantly associated with risk of DPC, while only the comorbidity burden indices (ACE-27 score, age, and medication number) were associated with risk of DOC (Table 2). First order interactions showed no significant associations and no significant nonlinearity was demonstrable in the continuous covariates.
Table 2.
Results of a multivariate competing risks model testing for association between various covariates and the cumulative risk of death from prostate cancer, from other causes, or from any cause, and the cumulative risk of death from prostate cancer, from other causes, or from any cause.
| Factor | Death from other causes |
Death from prostate cancer |
Death from any cause |
||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | ||
| ACE 27 | 0 | Referent | |||||
| 1 | 1.24 (0.89–1.72) | 0.20 | 0.84 (0.57–1.24) | 0.37 | 1.05 (0.82–1.35) | 0.72 | |
| 2 | 2.12 (1.45–3.10) | < 0.001 | 1.43 (0.87–2.34) | 0.16 | 1.87 (1.39–2.53) | < 0.0001 | |
| 3 | 2.99 (1.72–5.21) | < 0.001 | 1.04 (0.37–2.89) | 0.94 | 2.41 (1.51–3.86) | 0.0002 | |
| Age | 1.05 (1.03–1.07) | < 0.001 | 1.01 (0.98–1.04) | 0.76 | 1.03 (1.01–1.05) | < 0.0001 | |
| No. of medications | 1.09 (1.03–1.15) | 0.005 | 1.02 (0.94–1.10) | 0.72 | 1.08 (1.03–1.13) | 0.003 | |
| Year of treatment | 0.85 (0.80–0.90) | < 0.001 | 0.87 (0.79–0.94) | < 0.001 | 0.86 (0.82–0.90) | < 0.0001 | |
| ADT | Nil | Referent | |||||
| ST | 1.06 (0.79–1.43) | 0.68 | 0.85 (0.51–1.43) | 0.54 | 0.94 (0.73–1.20) | 0.61 | |
| LT | 1.02 (0.69–1.50) | 0.93 | 1.11 (0.65–1.88) | 0.71 | 0.98 (0.73–1.32) | 0.89 | |
| GS | ≤ 6 | Referent | |||||
| 7 | 1.02 (0.80–1.30) | 0.88 | 2.12 (1.41– 3.47) | < 0.001 | 1.27 (1.02–1.57) | 0.028 | |
| 8–10 | 1.19 (0.83–1.70) | 0.35 | 5.68 (3.49–9.26) | < 0.001 | 2.30 (1.76–3.02) | < 0.0001 | |
| PSA | < 10 | Referent | |||||
| 10–<20 | 1.12 (0.88–1.43) | 0.36 | 2.16 (1.42–3.31) | < 0.001 | 1.40 (1.14–1.72) | 0.002 | |
| ≥ 20 | 1.15 (0.83–1.60) | 0.40 | 1.94 (1.19–3.15) | < 0.001 | 1.44 (1.11–1.87) | 0.005 | |
| Stage | 1 | Referent | |||||
| 2 | 1.00 (0.74–1.36) | 1.00 | 2.54 (1.21–5.33) | 0.014 | 1.22 (0.92–1.57) | 0.17 | |
| 3–4 | 1.06 (0.73–1.53) | 0.76 | 3.94 1.86–8.36) | < 0.001 | 1.57 (1.16–2.13) | 0.004 | |
ACE-27, Adult Comorbidity Evaluation-27; ADT, androgen deprivation therapy; CI, confidence interval; GS, Gleason score; HR, hazard ratio; LT, long term; PSA, prostate specific antigen; ST, short term.
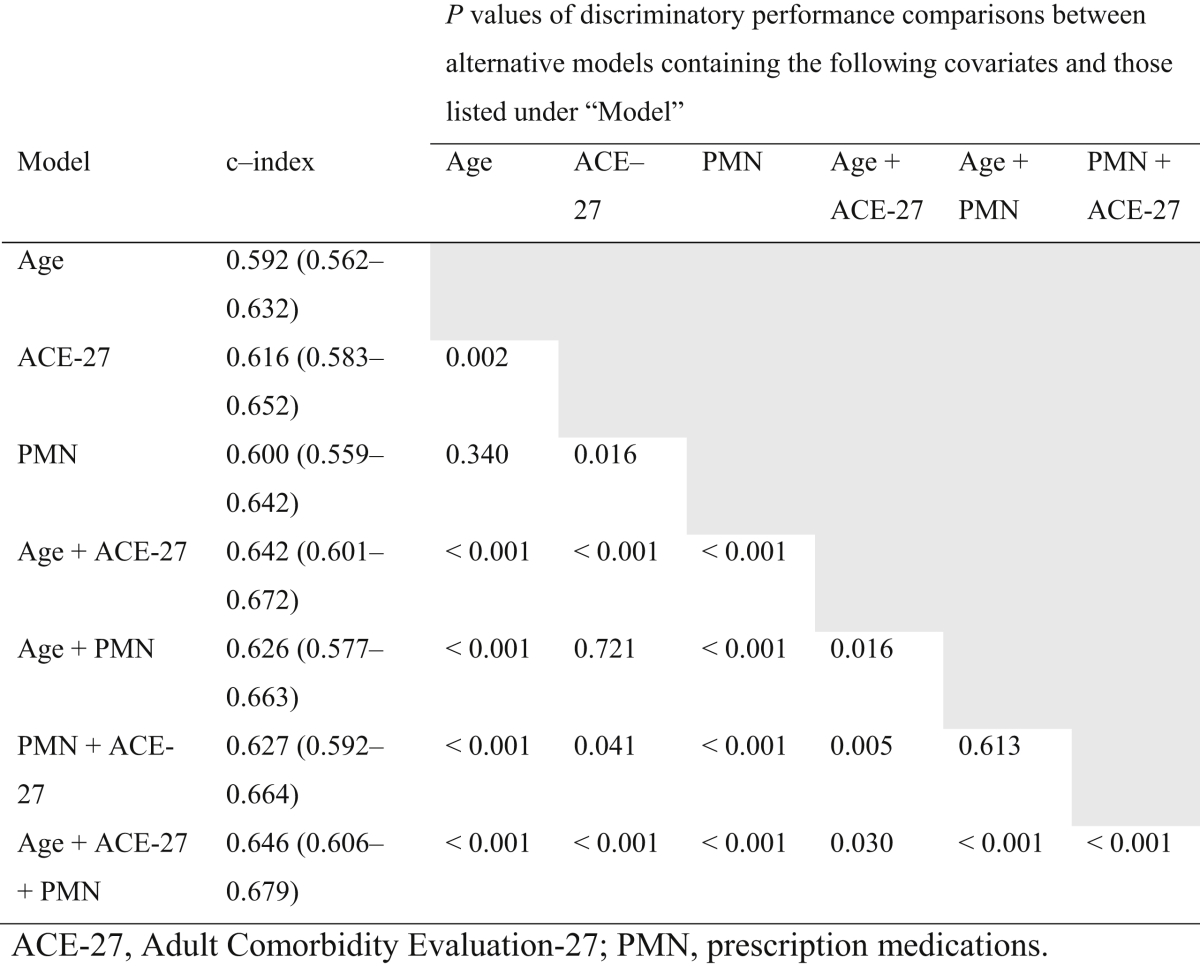
In univariate models of DOC, ACE-27 was the single most discriminating factor (c-index = 0.616), significantly bettering the performance of age or PMN alone (Table 3). For each of the age, PMN, and ACE-27 covariates, the addition of a second comorbidity covariate to the original univariate model resulted in significantly improved performance. This included the addition of age to other variables, despite it having weak univariate performance. Overall, a model of all three covariates was significantly more discriminating than all others (0.646).
Table 3.
Concordance indices for all univariate and multivariate models. P values shown are for the comparison of c-index between baseline and alternative models.
4. Discussion
With an increasing aging population and widespread use of early prostate specific antigen screening, the benefit to definitive treatment for localized PC is premised on the expectation of reasonable life expectancy. However, clinicians are known to be only modestly accurate at predicting patients’ life expectancy.6, 12 Our study shows that a higher comorbidity burden as assessed by either of three indices is associated with increased risk of DOC. This is consistent with previous studies of other comorbidity indices.3, 5, 13, 14, 15, 16
Patients are offered definitive treatment with an assertion that PC recurrence will not precede comorbidities as a cause of death. Even though our radically-treated cohort were preselected to have at least a 10-year life expectancy, the rate of DOC was consistently at a level approximately double that of DPC. The 10-year cumulative risk of DOC rises accordingly with ACE-27 scores, with 9.6% for ACE-27 score of 0, to 27.4% for ACE-27 score of 2. The rate of DOC at 10 years partly reflects clinicians’ limited ability to predict a patient’s 10-year life expectancy. This is in keeping with other studies that suggest that patients with low or intermediate risk PC should only be considered for definitive treatment if they have a low comorbidity burden.5, 16, 17
As with previous studies, we found that objective assessment of comorbidity burden was of greater value in predicting other cause mortality than age alone in PC patients.14, 15 Both ACE-27 and age are strongly associated with risk of DOC, however, ACE-27 score had a significantly higher concordance index than age alone, suggesting greater reliability in discriminating early from late comorbid deaths. Our suggestion to preferentially use a comorbidity score rather than patient age in selecting therapy contradicts what is known to happen in some clinical settings, where it has been shown that the use of conservative management for low-risk PC is much more likely when age is > 75 years rather than the presence of a severe comorbidity burden.14 This was despite the observation of a rate of comorbid death of 24% at 10 years for the > 75-year age group being eclipsed by a rate of 70% for those with a Charlson score ≥ 3.14 Furthermore, our finding that the combination of several indices of comorbidity always resulted in improved discriminatory performance suggests that different indices may be extracting unique data despite how similar they appear, and consideration should be given to always using combinations of measures.
We regard the discriminatory performance of the current model incorporating age, PMN and ACE-27 as modest. The implication from this is that current comorbidity assessment is rudimentary and needs to be greatly improved. Our decision to use the ACE-27 score was in part that it incorporates both the presence as well as the severity of medical conditions. This is in distinction to the commonly used Charlson index, which only accounts for the presence or absence of several conditions, but is well validated in PC.14 More complex measures which incorporate functional assessment also exist, such as the Total Illness Burden Illness,18 however, their complexity in administration must also be taken into account when they are aimed to be used in a clinic.
5. Conclusion
In conclusion, age, PMN, and ACE-27 score are all independently associated with death from non-PC causes in patients with localized PC. The ACE-27 comorbidity scoring system is an objective and simple way that can be used in a clinical setting to assist in predicting patients’ 10-year life expectancy and treatment decisions. With the addition of age and PMN to the ACE-27 scoring system, we can have a relatively more objective method of weighing comorbidity burden and predicting life expectancy. We recommend that patients with high comorbidity burden should be treated conservatively, whilst those with none/low comorbidity burden with high-risk PC should be considered for definitive treatment. For patients with intermediate comorbidity burden, tumor features should be taken into consideration and patients should be counselled regarding the benefits and possible side effects of definitive treatment. Ongoing work in improving comorbidity assessment will be crucial in reducing overtreatment in PC.
Conflicts of interest
None declared.
References
- 1.Lu C.Y., Barratt J., Vitry A., Roughead E. Charlson and Rx-Risk comorbidity indices were predictive of mortality in the Australian health care setting. J Clin Epidemiol. 2011;64:223–228. doi: 10.1016/j.jclinepi.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Vitry A., Wong S.A., Roughead E.E., Ramsay E., Barratt J. Validity of medication-based co-morbidity indices in the Australian elderly population. Aust N Z J Public Health. 2009;33:126–130. doi: 10.1111/j.1753-6405.2009.00357.x. [DOI] [PubMed] [Google Scholar]
- 3.Daskivich T., Sadetsky N., Kaplan S.H., Greenfield S., Litwin M.S. Severity of comorbidity and non-prostate cancer mortality in men with early-stage prostate cancer. Arch Int Med. 2010;170:1396–1397. doi: 10.1001/archinternmed.2010.251. [DOI] [PubMed] [Google Scholar]
- 4.Fowler F.J., Jr., McNaughton Collins M., Albertsen P.C., Zietman A., Elliott D.B., Barry M.J. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. J Am Med Assoc. 2000;283:3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 5.Albertsen P.C., Moore D.F., Shih W., Lin Y., Li H., Lu-Yao G.L. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walz J., Gallina A., Perrotte P., Jeldres C., Trinh Q.D., Hutterer G.C. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–1258. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]
- 7.Naqvi K., Garcia-Manero G., Sardesai S., Oh J., Vigil C.E., Pierce S. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011;29:2240–2246. doi: 10.1200/JCO.2010.31.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wo J.Y., Chen M.H., Nguyen P.L., Renshaw A.A., Loffredo M.J., Kantoff P.W. Evaluating the combined effect of comorbidity and prostate-specific antigen kinetics on the risk of death in men after prostate-specific antigen recurrence. J Clin Oncol. 2009;27:6000–6005. doi: 10.1200/JCO.2009.23.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amico A.V. Risk-based management of prostate cancer. New Engl J Med. 2011;365:169–171. doi: 10.1056/NEJMe1103829. [DOI] [PubMed] [Google Scholar]
- 10.Schemper M., Smith T.L. Efficient evaluation of treatment effects in the presence of missing covariate values. Stat Med. 1990;9:777–784. doi: 10.1002/sim.4780090707. [DOI] [PubMed] [Google Scholar]
- 11.Geskus R.B. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 12.Krahn M.D., Bremner K.E., Asaria J., Alibhai S.M., Nam R., Tomlinson G. The ten-year rule revisited: accuracy of clinicians' estimates of life expectancy in patients with localized prostate cancer. Urology. 2002;60:258–263. doi: 10.1016/s0090-4295(02)01712-0. [DOI] [PubMed] [Google Scholar]
- 13.Albertsen P.C., Hanley J.A., Gleason D.F., Barry M.J. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. J Am Med Assoc. 1998;280:975–980. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 14.Daskivich T.J., Chamie K., Kwan L., Labo J., Palvolgyi R., Dash A. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 15.Alibhai S.M., Naglie G., Nam R., Trachtenberg J., Krahn M.D. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003;21:3318–3327. doi: 10.1200/JCO.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Post P.N., Hansen B.E., Kil P.J., Janssen-Heijnen M.L., Coebergh J.W. The independent prognostic value of comorbidity among men aged < 75 years with localized prostate cancer: a population-based study. BJU Int. 2001;87:821–826. doi: 10.1046/j.1464-410x.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamstra D.A., Bae K., Pilepich M.V., Hanks G.E., Grignon D.J., McGowan D.G. Older age predicts decreased metastasis and prostate cancer-specific death for men treated with radiation therapy: meta-analysis of radiation therapy oncology group trials. Int J Radiat Oncol Biol Phys. 2011;81:1293–1301. doi: 10.1016/j.ijrobp.2010.07.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin M.S., Greenfield S., Elkin E.P., Lubeck D.P., Broering J.M., Kaplan S.H. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109:1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]