Abstract
Deforestation in the tropics is not only responsible for direct carbon emissions but also extends the forest edge wherein trees suffer increased mortality. Here we combine high-resolution (30 m) satellite maps of forest cover with estimates of the edge effect and show that 19% of the remaining area of tropical forests lies within 100 m of a forest edge. The tropics house around 50 million forest fragments and the length of the world's tropical forest edges sums to nearly 50 million km. Edge effects in tropical forests have caused an additional 10.3 Gt (2.1–14.4 Gt) of carbon emissions, which translates into 0.34 Gt per year and represents 31% of the currently estimated annual carbon releases due to tropical deforestation. Fragmentation substantially augments carbon emissions from tropical forests and must be taken into account when analysing the role of vegetation in the global carbon cycle.
Vast quantities of carbon stored in tropical forests are threatened by deforestation. Here, using high resolution satellite data, Brinck et al. examine how edge effects influence carbon emissions and they find an additional 10.3 Gt of carbon are released by deforestation when including fragmentation effects.
Tropical forests are the largest terrestrial component of the global carbon budget1,2. They account for 50% of the carbon stored in the global vegetation (350–600 Gt C)3,4,5, rivalling the amount of carbon in the atmosphere (750 Gt C)6. Carbon losses from tropical forests due to forest loss and degradation are currently estimated to be 1.1 Gt C per year7,8,9. However, quantification of carbon fluxes of tropical forests suffer uncertainties in the measurement of stored biomass10,11 and rates of forest loss12. Moreover, deforestation alters the structure of the remaining forest—a process referred to as fragmentation13,14,15,16,17,18.
Increased wind speed, vapour pressure deficit and disturbance pressure in the vicinity of forest edges increase tree mortality15, resulting in persistent carbon emissions long after deforestation occurs. Fire, wind and desiccation can penetrate up to kilometres into the forest15,19,20, altering microclimate within 100–300 m of the forest edge13,21,22,23. Field studies have estimated relative carbon losses in forest edges (e) averaging 11% (ref. 24) and up to 36% (ref. 22) and 50% (ref. 25) of aboveground biomass within the 100 m edge zone, and modelling studies have shown biomass reductions of up to 70% in small fragments26.
Although regional estimates of fragmentation state and carbon losses have been obtained by combining local findings and modelling results27, a comprehensive pan-tropical analysis using high-resolution maps is missing. A recent pan-tropical analysis based on coarse-resolution data (500 m) found reductions of biomass extending 1.5 km from the forest edge20. Another analysis estimated the distance from all forests to forest edge using remote sensing data18.
Here we analysed the extent of tropical forest fragmentation and its effects on the global carbon balance of the terrestrial biosphere. We quantified the total number of forest fragments and the area of their edges across the tropics using a global, high-resolution (30 m) tree-cover data set from the year 2000 (ref. 28). We estimated biomass loss in the edge area based on a biomass reduction factor and biomass values of the forest core areas that we extracted from a published aboveground biomass map29. Then we overlaid the high-resolution forest edge analysis with this biomass loss assumption to incorporate the edge effect into the global carbon balance of tropical forests. We find that fragmentation substantially increases carbon emissions from tropical forests by 31% (of the currently estimated annual carbon losses due to tropical deforestation) and is hence relevant for the global carbon cycle.
Results
Analysis of tropical forest fragmentation
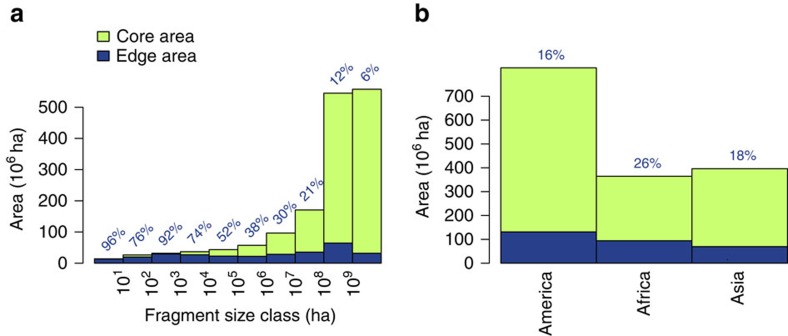
The tropics house >50 million forest fragments with a mean fragment area of 29 ha and total area >1.5 billion ha (Table 1). Size–frequency distributions of forest fragments are similar across continents (see Supplementary Fig. 1). The length of the world's tropical forest edges sums to nearly 50 million km, and a total of 295 million ha (19%) of tropical forest area is <100 m from the forest edge. Depending on the complexity of each fragment's shape, the proportion of a fragment's edge area decreases with increasing fragment size (Fig. 1a). In tropical America and Asia, the fraction of edge area lies between 16% and 18% of total forest area and is much lower than in Africa (26%) (Fig. 1b).
Table 1. Forest fragmentation across tropical regions.
| Unit | America | Africa | Asia | Total | |
|---|---|---|---|---|---|
| Number of fragments | 23,491,573 | 22,894,239 | 7,593,226 | 53,979,038 | |
| Total forested area | (106 ha) | 819 | 364 | 396 | 1,579 |
| Average fragment size | (ha) | 35 | 16 | 52 | 29 |
| Total edge length | (106 km) | 22 | 18 | 10 | 50 |
| Total edge area | (106 ha) | 131 | 94 | 70 | 295 |
| Total edge area/total forested area | (%) | 16 | 26 | 18 | 19 |
| Average aboveground carbon stock | (t C ha−1) | 101 | 90 | 114 | 102 |
| Aboveground carbon stock in edge area | (Gt C) | 8.72 | 5.20 | 6.66 | 20.58 |
| Total aboveground carbon stock | (Gt C) | 83 | 33 | 45 | 161 |
| Total carbon losses | (Gt C) | 4.36 | 2.60 | 3.33 | 10.29 |
Figure 1. Fragment size distribution for tropical forest.
Distribution of total forested area, core area (green) and edge area (blue; edge depth=100 m, blue numbers: edge area/total area (%)) for different fragment size classes (a) and different continents (b).
Additionally, we found that 84% of the edge area is anthropogenically created (see Supplementary Table 1). In Asia, the ratio of anthropogenic to total edge area is 95%—higher than in Africa (63%) and South America (83%). Only 1% of the total forest-edge area in the tropics represents the tree line (America 1.4%, Africa 0.5%, Asia 0.4%). Analysing how much forest fragments are created due to corridor-like clear cuts (for example, road building), we found that 21% of total edge area has a characteristic similar to a corridor (America 20%, Africa 20%, Asia 22%).
Impact of tropical fragmentation on the global carbon cycle
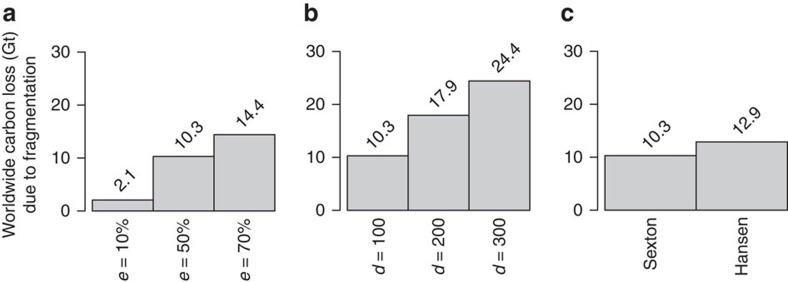
Aboveground carbon storage of tropical forests worldwide amounts to 161 Gt C, of which 21 Gt C are located within 100 m of the forest edge (Table 1). Assuming edge penetration depth of 100 m (ref. 30) and carbon losses of e=50% (refs 25, 26) in the edge areas leads to a release of 10.3 Gt C due to tropical fragmentation (Table 1, Fig. 2a,b). We compared different scenarios of edge depth and fraction of lost biomass to explore the sensitivity of the results. Varying fraction of lost biomass e from 10% to 70% (for a 100 m wide edge depth), results in total carbon losses between 2.1 and 14.4 Gt C (Fig. 2a). Comparing carbon-loss scenarios for edge depths from 100 to 300 m while assuming carbon loss of e=50% in the edge areas leads to a release of 10.3 to 24.4 Gt C from the fragment edges (Fig. 2b). Carbon-loss estimates are robust to the choice of underlying forest cover map (Fig. 2c). The 30 m resolution forest cover maps from Sexton et al.28 and Hansen et al.31 yield similar results for carbon losses from forest fragment edges, with the Sexton data set providing the more conservative estimate (10.3 Gt resp. 12.9 Gt; compare Table 1 and Supplementary Table 2). Vegetation cover maps with lower resolution (GlobCover map32 300 m) give lower estimates for carbon losses (5.42 Gt) as many of the small forest fragments and fine-scale edge structures are neglected (Supplementary Table 1).
Figure 2. Total carbon losses due to tropical forest fragmentation.
Comparing carbon-loss scenarios assuming different assumptions for relative carbon losses e in the forest edge area, for different edge penetration distances d and different underlying forest cover maps. The numbers represent the total carbon loss due to fragmentation for different scenarios. (a) Carbon loss for different assumptions of relative carbon losses in forest edges e assuming edge depth d=100 m; (b) carbon loss for different assumptions of edge depth d assuming relative carbon losses in forest edges of e=50%; (c) comparison of results based on different forest cover maps28,32 assuming d=100 m and e=50% (for additional details, see Supplementary Tables 2 and 3).
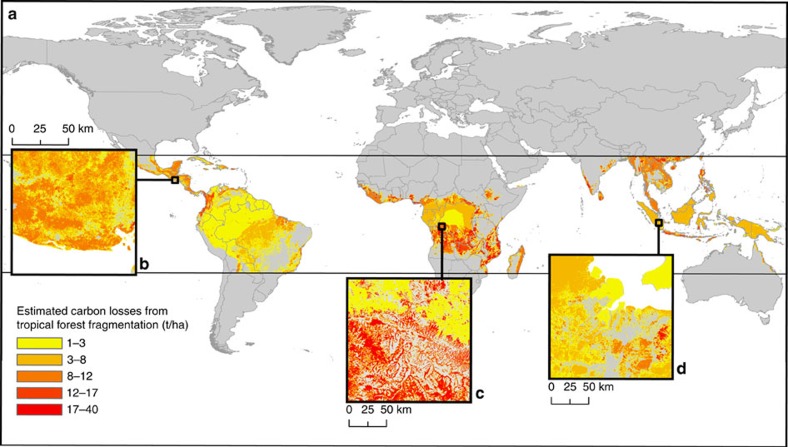
The global distribution of carbon losses from tropical forest fragmentation is shown in Fig. 3. Although the Amazon houses the world's largest area of intact tropical forests, the prevalence of deforestation and fragmentation across Central and South America results in South America leading the world in total carbon emissions. The large, intact forests of the Congo basin are also abutted by highly fragmented forests, leading to more than a fourth of Africa's total forested area lying within 100 m of the forest edge. Asia accounts for the second largest share of carbon emissions among continents (see Table 1), explained by the very high fragmentation due to topography and small-scale fragmentation.
Figure 3. Worldwide carbon emissions due to fragmentation of tropical forests.
(a) Colours represent the estimated carbon losses for each fragment, setting edge depth d to 100 m and relative carbon losses in forest edges e to 50%. Insets illustrate exemplary regional carbon emissions for (b) tropical America (89.752 W, 13.515 N), (c) tropical Africa (17.206 E, 4.499 S) and (d) tropical South-East Asia (103.898 E, 3.091 S).
Previous studies estimated a lag of 10–50 years for newly created forest edges to reach new, postdisturbance equilibria24,26. Assuming that the majority of biomass is lost during the first 30 years27 and setting edge depth to 100 m (ref. 30) and e to 50% of standing biomass25,26, the overall 10.3 Gt C lost from fragmentation would contribute 0.34 Gt C per year to the annual global carbon cycle.
Discussion
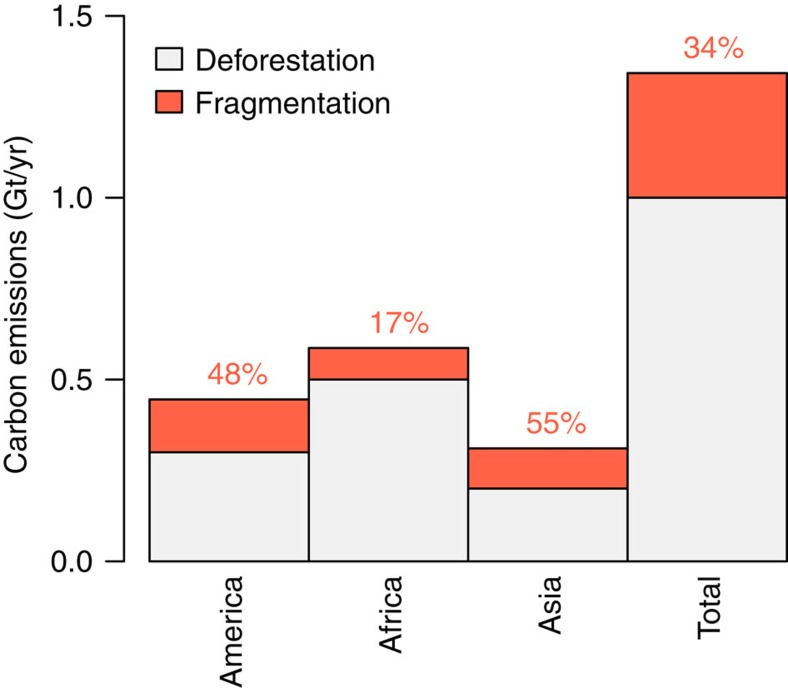
This estimated carbon losses due to tropical forest fragmentation (0.34 Gt C per year) equals 31% of the direct carbon emissions due to land-use changes in tropical forest regions7 globally (1.1 Gt C per year) and 8.5% of the overall annual atmospheric carbon gain5 (Fig. 4). Whereas many studies have concluded the intact tropical biome are carbon sinks4,33, if land-use change is considered tropical forests are a carbon source8. Our results corroborate the latter and indicate that additional carbon emissions from tropical forest fragmentation increase overall carbon emissions to the atmosphere to be higher values than previously assumed. The estimated absolute carbon losses due to tropical fragmentation (10.3 Gt C) are higher than estimated values in a previous study (6.7 Gt C, Pütz et al.27) and are within the same order as another recent study based on coarser-resolution maps (9.4 Gt C, 500 m resolution, Chaplin-Kramer et al.20). The study by Pütz et al.27 have calculated lower carbon losses compared to this study, as they did a detailed analysis only for South America. However, the fragment counting and forest edge area is different for the continents (Table 1). As fragmentation effects occur on small spatial scales (<100 m), our study benefits from the high (30 m) resolution of the satellite-based tree-cover map, which avoided mixing of forest edges with unaffected forests and other land-cover types within pixels.
Figure 4. Annual carbon emissions from tropical forests.
Annual carbon emissions from tropical deforestation2 (grey bars) and additional emissions from tropical forest edges due to fragmentation from this study (orange bars). The percentages represent the fraction of tropical fragmentation carbon emissions and tropical deforestation carbon emissions.
Our assumption for the distance of edge-depth penetration (d=100 m) and the resulting estimates of carbon loss are conservative; other studies have shown reductions of biomass extending up to 1.5 km from the forest edge20. To investigate the effect of the parameter for biomass reduction within the edge area, we investigated the range of lower (e=10%) to higher values (e=70%) and in our standard assumption e=50% (refs 20, 25, 26). Incorporating the shape and edge-length of forest fragments from satellite-based maps overcomes the potential limitation imposed by incorporating only estimates of area and simplified shapes. However, the assumption of the rate of edge creation over time depends on harvesting strategies12 and history34, so conclusions are challenging with the current state of knowledge and open up further questions in the field of dynamic landscape development. Our analyses show that forest fragmentation augments carbon emissions beyond those caused by deforestation. Although we expect that our study bases on conservative assumptions, they are already substantial enough to highlight the importance of forest fragmentation in the global carbon balance.
Methods
Methods summary
Our analysis is based on the GLCF (Global Land Cover Facility) tree-cover data set, a global, 30 m resolution map of fractional tree-canopy cover in the year 2000 (ref. 28). For the analyses of forest patches, we developed a C++ program that implements an adapted Hoshen–Copelman algorithm35. Core and edge areas of forest patches were calculated from fragment size and perimeter36. Carbon losses were estimated based on a range of biomass-reduction factors and carbon densities of tropical forest from a global aboveground biomass map29 by calculating a mean aboveground biomass for each fragment. It was thereby assumed, conservatively, that no root or soil carbon contributes to the emissions from the edge areas. To account for uncertainties in the parameters, scenarios of edge depth and the fraction of carbon loss in edge areas were compared. The influence of the choice of underlying tree-cover data was assessed by comparing results between the GLCF global tree-cover map28 (30 m resolution), the Hansen global map of forest cover change31 (30 m resolution) and the GlobCover land cover map32 (300 m resolution) (Table 1, Supplementary Tables 2 and 3). We also compared results based on different biomass maps, including the biomass map by Saatchi et al.29 (1,000 m), the biomass map by Baccini et al.2 (500 m), the biomass map by Avitabile et al.37 (1,000 m) and mean values from the global biomass data set compiled from Pan et al.4 (see Supplementary Table 3). The time span after edge creation during which carbon is emitted from the edge areas was taken from previous studies (suggesting 10–50 years24,26) for estimating annual carbon emission rates. A detailed description of the methods can be found below. The data of the findings (Fig. 3) of this study are available from the corresponding author upon request.
Forest cover and biomass data
The forest-fragment distribution is based on the global tree-cover map by Sexton et al.28 for the year 2000. This map has a 30 m spatial resolution. The tree-cover threshold to define forest cover is set to 30%.
To compare the effects of different maps, results based on the Sexton tree-cover map are compared with the Hansen global map of twenty-first century forest cover change31 and the GlobCover land cover map32. The Hansen global map is based on Landsat data and infers forest-cover loss between 2000 and 2012 at 30 m resolution. Forested areas are defined as having a tree cover of >30%. The GlobCover land cover map (http://due.esrin.esa.int/page_globcover.php) from the year 2009 was produced by the ESA GlobCover 2009 Project (300 m resolution). The land-cover map is based on a time series of global MERIS mosaics for the year 2009 and is classified into 22 land cover classes defined according to the United Nations Land Cover Classification System.
Biomass per hectare is taken from the map of forest carbon stocks in tropical regions by Saatchi et al.29. For comparison, carbon-loss estimates are also calculated based on the carbon map by Baccini et al.2, the biomass map by Avitabile et al.37 and alternatively based on continent-wise average biomass densities derived from forest inventory data from Pan et al.4 (see Supplementary Table 3).
Connected-component analysis
The binary forest/non-forest image is considered a graph, in which each forest pixel is a single vertex. The connectivity of a pixel is determined by its four-pixel neighbourhood in cardinal directions, N/S/E/W. A forest pixel is considered an ‘edge' if two pixels along that side are non-forest pixels (two pixels represent a distance of around 60 m near the equator).
A classic two-pass algorithm to determine the connected components is impractical; the entire labelled image would need to be stored in memory. For example, South America (+23.5 N, −23.5 S) would consume about ∼300 GB memory with 64-bit labels. We therefore modified the classic Hoshen–Kopelman algorithm35 to estimate the most relevant information in a single pass using a tree structure that consumes about 1 GB of memory. As the image was traversed row by row, 2P+1 rows of the image were held in memory (here, P=2). After one pass, we have a structure, where every fragment has an entry consisting of its area, edge length and biomass.
The biomass of each pixel was retrieved from the biomass map29, with a fall-back to a mean value given by Pan et al.4 when no valid biomass estimate was available for a given pixel. The different map resolutions were combined via nearest neighbour interpolation.
The size of a pixel in the WGS-84 projection is dependent on its geographical position; sizes were corrected using the Haversine formula38. As the evaluation for every pixel would be too costly, we linearly interpolated the area along 256 latitudes.
Estimation of edge and core area
Which proportion of a forest fragment is affected by edge effects depends largely on the shape of the patch: the higher the perimeter-to-core ratio, the higher the fraction of edge-affected area. We applied the Didham and Ewers core area model36 to each fragment separately in order to estimate its amount of core and disturbed edge area, respectively. From the size index
 |
calculated via the fragment's perimeter P (m) and its total area A (m2), the edge-affected area Aedge (m2) is estimated, depending on the depth of the edge effects d (m) using the following formula:
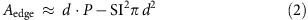 |
The model is, even for circles, only valid for d≤r, where r is the radius of a circle with the respective size36. Therefore, for a given fragment area size A, the formula for Aedge was only used for 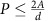 (for a circle if
(for a circle if 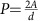 , in this case r equals d) and for
, in this case r equals d) and for  we assume Aedge=A. The size index SI is designed such that it yields 1 for circular fragments and larger values for other shapes.
we assume Aedge=A. The size index SI is designed such that it yields 1 for circular fragments and larger values for other shapes.
Estimates of carbon emissions from forest fragment edges
Carbon losses per fragment (t) are calculated as
 |
where e (%) is the fraction of biomass in the edge area that is lost due to edge effects. Aedge is the edge-affected area (m2) (calculated from equation (2)). C (t m−2) stands for the amount of carbon stored in the forest (here, 50% of aboveground biomass). Mean aboveground biomass for each fragment were calculated by averaging the biomass values (from the underlying biomass map) for the whole fragment. We assume that this mean biomass values represents potential biomass of the local forest before fragmentation. Additional analyses in Supplementary Discussion 1 confirm that this original analysis (averaging the biomass values) is conservative.
The estimated 0.34 Gt annual carbon losses reflect 31% of the annual carbon emissions due to land-use change in tropical forest regions, based on annual emissions (2000–2009) of 1.1±0.11 Gt C reported by Houghton et al.7. The annual total carbon flux to the atmosphere is estimated to equal 4 Gt per year (2000–2009)5. The carbon emissions due to tropical forest fragmentation found in this study of 0.34 Gt correspond to 8.5% of those total emissions.
Natural and anthropogenic edges
Natural transition from tropical forest to other biomes (for example, open forests and savannahs) or landscape features such as water bodies most probably have existed since centuries and therefore do not contribute to any additional carbon emissions today, whereas edges joining anthropogenically created land-use covers such as cropland are thought to have once been part of the interior forest. For the GlobCover 2009 map, we distinguish natural edges from non-natural anthropogenically created edges (see Supplementary Table 1). We assume that natural edges occur where tropical forest lies next to moist savannahs (the climatically successive vegetation zone), flooded mangrove forests and the coast, there we expect the vegetation to have already reached an equilibrium state centuries ago and therefore additional carbon losses do not contribute to our balance. Edges are classified as anthropogenic if forest is neighboured by grassland, cropland or artificial surfaces.
Natural edges are defined by a border between tropical forest and one of the following land cover classes: 50: ‘Closed (>40%) broadleaved deciduous forest (>5 m)', 60: ‘Open (15–40%) broadleaved deciduous forest/woodland (>5 m)', 170: ‘Closed (>40%) broadleaved forest or shrubland permanently flooded—saline or brackish water', 180: ‘Closed to open (>15%) grassland or woody vegetation on regularly flooded or waterlogged soil—fresh, brackish or saline water', and 210: ‘Water bodies'.
Treeline edges
To estimate forest edges that represent the natural tree line, we summed up all forest edges located in regions with elevation >3,000 m according to Körner39.
Corridor edges
We analysed how much non-forest areas have the character of a corridor (for example, due to roads). A forest fragment edge belongs to the corridor class if the distance between two forest fragments is less than two pixels (∼60 m). That means that a forest pixel is followed by two non-forest pixels and then again a forest pixel occurs. The connectivity of a pixel is determined by its four-pixel neighbourhood in cardinal directions, N/S/E/W.
Data availability
The used input data (global forest cover maps and biomass maps) can be found in the corresponding references. The resulting data set of our analysis—worldwide carbon emissions due to fragmentation of tropical forests with a resolution of 30 m (Fig. 3)—can be requested from the corresponding author (size ∼900 GB). The same map with a coarser resolution of 300 m is downloadable from the following link https://oc.ufz.de/index.php/s/fOvlXYUQqByxJgH (password: fragmentation).
Additional information
How to cite this article: Brinck, K. et al. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 8, 14855 doi: 10.1038/ncomms14855 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figure, supplementary tables, supplementary discussion and supplementary references
Acknowledgments
We thank UC Louvain for access to the land cover map (Globcover), Raphael Didham for additional comments on the use of the core area model, Justin Kitzes for helpful discussions on the manuscript and Amanda H. Armstrong and Thorsten Wiegand for proof-reading and comments. We thank Sassan S. Saatchi, Alessandro Baccini and Valerio Avitabile for providing access to their digital biomass maps. We thank the anonymous reviewers for their careful reading of our manuscript and their insightful comments and suggestions. R.F., S.L. and A.H. were supported by the Helmholtz-Alliance Remote Sensing and Earth System Dynamics. S.L., S.P. and A.H. were supported by the ERC advanced grant 233066.
Footnotes
The authors declare no competing financial interests.
Author contributions K.B., R.F. and A.H. conceived research. S.L. designed and implemented the cluster analysis software. All authors contributed extensively to the work presented in this paper.
References
- Bonan G. B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008). [DOI] [PubMed] [Google Scholar]
- Baccini A. et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2, 182–185 (2012). [Google Scholar]
- Houghton R. A., Hall F. & Goetz S. J. Importance of biomass in the global carbon cycle. J. Geophys. Res. 114, G00E03 (2009). [Google Scholar]
- Pan Y. et al. A large and persistent carbon sink in the world's forests. Science (80-.) 333, 988–993 (2011). [DOI] [PubMed] [Google Scholar]
- Ciais P. et al. in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change eds Stocker T. F.et al. 465–570Cambridge University Press (2013). [Google Scholar]
- Grace J. Understanding and managing the global carbon cycle. J. Ecol. 92, 189–202 (2004). [Google Scholar]
- Houghton R. A. et al. Carbon emissions from land use and land-cover change. Biogeosciences 9, 5125–5142 (2012). [Google Scholar]
- Mahli Y. The carbon balance of tropical forest regions. Curr. Opin. Environ. Sustain. 2, 237–244 (2010). [Google Scholar]
- Grace J., Mitchard E. & Gloor E. Perturbations in the carbon budget of the tropics. Glob. Ecol. Biogeogr. 20, 3238–3255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S. J. et al. Measurement and monitoring needs, capabilities and potential for addressing reduced emissions from deforestation and forest degradation under REDD+. Environ. Res. Lett. 10, 123001 (2015). [Google Scholar]
- Houghton R. A., Lawrence K., Hackler J. & Brown S. The spatial distribution of forest biomass in the Brazilian Amazon: a comparison of estimates. Glob. Chang. Biol. 7, 731–746 (2007). [Google Scholar]
- Houghton R. A. Aboveground forest biomass and the global carbon balance. Glob. Chang. Biol. 11, 945–958 (2005). [Google Scholar]
- Laurance W. F. et al. The fate of Amazonian forest fragments: A 32-year investigation. Biol. Conserv. 144, 56–67 (2011). [Google Scholar]
- Laurance W. F., Delamonica P., Laurance S. G., Vasconcelos H. L. & Lovejoy T. E. Rainforest fragmentation kills big trees. Nature 404, 836 (2000). [DOI] [PubMed] [Google Scholar]
- Laurance W. F. Do edge effects occur over large spatial scales? Trends Ecol. Evol. 15, 134–135 (2000). [DOI] [PubMed] [Google Scholar]
- Robinson G. R. et al. Diverse and contrasting effects of habitat fragmentation. Science (80-.) 257, 524–526 (1992). [DOI] [PubMed] [Google Scholar]
- Ewers R. M. et al. A large-scale forest fragmentation experiment: the Stability of Altered Forest Ecosystems Project. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366, 3292–3302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad N. M. et al. Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv. 1, e1500052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant G., Gond V. & Laurance S. G. Habitat fragmentation and the desiccation of forest canopies: a case study from eastern Amazonia. Biol. Conserv. 143, 2763–2769 (2010). [Google Scholar]
- Chaplin-Kramer R. et al. Degradation in carbon stocks near tropical forest edges. Nat. Commun. 6, 10158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance W. F. Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biol. Conserv. 141, 1731–1744 (2008). [Google Scholar]
- Laurance W. F. et al. Biomass collapse in Amazonian forest fragments. Science 278, 1117–1118 (1997). [Google Scholar]
- Broadbent E. N. et al. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol. Conserv. 141, 1745–1757 (2008). [Google Scholar]
- Laurance W. F., Laurance S. G. & Delamonica P. Tropical forest fragmentation and greenhouse gas emissions. For. Ecol. Manage. 110, 173–180 (1998). [Google Scholar]
- Dantas de Paula M., Alves Costa C. P. & Tabarelli M. Carbon storage in a fragmented landscape of Atlantic forest: the role played by edge-affected habitats and emergent trees. Trop. Conserv. Sci. 4, 349–358 (2011). [Google Scholar]
- Pütz S., Groeneveld J., Alves L. F., Metzger J. P. & Huth A. Fragmentation drives tropical forest fragments to early successional states: a modelling study for Brazilian Atlantic forests. Ecol. Modell. 222, 1986–1997 (2011). [Google Scholar]
- Pütz S. et al. Long-term carbon loss in fragmented Neotropical forests. Nat. Commun 5, 5037 (2014). [DOI] [PubMed] [Google Scholar]
- Sexton J. O. et al. Global, 30-m resolution continuous fields of tree cover: Landsat-based rescaling of MODIS vegetation continuous fields with lidar-based estimates of error. Int. J. Digit. Earth 6, 427–448 (2013). [Google Scholar]
- Saatchi S. S. et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl Acad. Sci. 108, 9899–9904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance W. F. et al. Ecosystem decay of amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618 (2002). [Google Scholar]
- Hansen M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013). [DOI] [PubMed] [Google Scholar]
- Arino O., Ramos P., Jose J., Kalogirou V., Bontemps S. & Defourny P. et al. Global Land Cover Map for 2009 (GlobCover 2009). doi:10.1594/PANGAEA.787668 (European Space Agency (ESA) & Université catholique de Louvain (UCL), 2012).
- Lewis S. et al. Increasing carbon storage in intact African tropical forests. Nature 457, 1003–1006 (2009). [DOI] [PubMed] [Google Scholar]
- Numata I., Cochrane M. A., Souza C. M. Jr & Sales M. H. Carbon emissions from deforestation and forest fragmentation in the Brazilian Amazon. Environ. Res. Lett. 6, 44003 (2011). [Google Scholar]
- Hoshen J. & Kopelman R. Percolation and cluster distribution. I. Cluster multiple labeling technique and critical concentration algorithm. Phys. Rev. B 14, 3438–3445 (1976). [Google Scholar]
- Didham R. K. & Ewers R. M. Predicting the impacts of edge effects in fragmented habitats: Laurance and Yensen's core area model revisited. Biol. Conserv. 155, 104–110 (2012). [Google Scholar]
- Avitabile V. et al. An integrated pan-tropical biomass map using multiple reference datasets. Glob. Chang. Biol. 22, 1406–1420 (2016). [DOI] [PubMed] [Google Scholar]
- Robusto C. C. The Cosine-Haversine formula. Am. Math. Monthly 64, 38–40 (1957). [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 115, 445–459 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure, supplementary tables, supplementary discussion and supplementary references
Data Availability Statement
The used input data (global forest cover maps and biomass maps) can be found in the corresponding references. The resulting data set of our analysis—worldwide carbon emissions due to fragmentation of tropical forests with a resolution of 30 m (Fig. 3)—can be requested from the corresponding author (size ∼900 GB). The same map with a coarser resolution of 300 m is downloadable from the following link https://oc.ufz.de/index.php/s/fOvlXYUQqByxJgH (password: fragmentation).