Abstract
Context:
Septic shock can rapidly evolve into multiple system organ failure and death. In the recent years, hyperlactatemia has been found to be a risk factor for mortality in critically ill adults.
Aims:
To evaluate the predictive value of lactate clearance and to determine the optimal cut-off value for predicting outcome in children with septic shock.
Settings and Design:
A prospective observational study was performed on children with septic shock admitted to pediatric Intensive Care Unit (PICU).
Subjects and Methods:
Serial lactate levels were measured at PICU admission, 24 and 48 h later. Lactate clearance, percent decrease in lactate level in 24 h, was calculated. The primary outcome measure was survival or nonsurvival at the end of hospital stay. We performed receiver operating characteristic analyses to calculate optimal cut-off values.
Results:
The mean lactate levels at admission were significantly higher in the nonsurvivors than survivors, 5.12 ± 3.51 versus 3.13 ± 1.71 mmol/L (P = 0.0001). The cut-off for lactate level at admission for the best prediction of mortality was determined as ≥4 mmol/L (odds ratio 5.4; 95% confidence interval [CI] =2.45–12.09). Mean lactate clearance was significantly higher in survivors than nonsurvivors (17.9 ± 39.9 vs. −23.2 ± 62.7; P < 0.0001). A lactate clearance rate of <10% at 24 h had a sensitivity and specificity of 78.7% and 72.2%, respectively and a positive predictive value of 83.1% for death. Failure to achieve a lactate clearance of more than 10% was associated with greater risk of mortality (likelihood ratio + 2.83; 95% CI = 1.82–4.41).
Conclusions:
Serial lactate levels can be used to predict outcome in pediatric septic shock. A 24 h lactate clearance cut-off of <10% is a predictor of in-hospital mortality in such patients.
Key Words: In-hospital mortality, lactate clearance, Pediatric Risk of Mortality III score, septic shock, serial lactate
INTRODUCTION
Septic shock is one of the major causes of admission and death in intensive care units. Prompt identification of inadequate tissue perfusion and its aggressive management is essential in treating patients with septic shock, particularly with the increasing incidence and burden of managing the morbidity and mortality.[1] Many critically ill patients, who are normotensive and have adequate urine output, may remain in a state of compensated shock.[2] Hence, relying solely on the normalization of vital signs and urine output may be inadequate.
In the state of shock anaerobic metabolism ensues, releasing lactate into the bloodstream. Elevated blood lactate levels provide an insight into the presence of impaired tissue perfusion. In the recent years, lactate has been studied as a biomarker for sepsis and septic shock.[3,4,5,6] In addition, reduced lactate clearance may reflect globally impaired renal and hepatic metabolic function, both of which normally contribute to systemic lactate disposal. Thus, lactate clearance biologically reflects homeostasis of the host and provides more meaningful data about the overall adequacy of the resuscitative processes.[7,8] The Surviving Sepsis Campaign recommends lactate normalization as a target of resuscitation for patients with severe sepsis and septic shock with a recent update.[9]
However, majority of research with serum lactate in sepsis and septic shock has been conducted in adults. Pediatric data on association of lactate levels with mortality in sepsis and septic shock are scarce.[10,11,12,13] The present study was aimed to ascertain whether lactate clearance predicts outcome of children with septic shock admitted to pediatric Intensive Care Unit (PICU) and to determine the optimal cut-off value for in-hospital mortality prediction.
SUBJECTS AND METHODS
This study was a hospital-based, prospective observational study conducted at the PICU of Department of Pediatrics of a tertiary care center in North India, over 1 year from December 2011 to November 2012. The study was approved by the Ethics Committee of the institute.
All PICU patients were assessed for inclusion, inclusion criteria were patients with septic shock aged between 1 month and 12 years. Septic shock was defined as confirmed or presumed infection, having two or more systemic inflammatory response criteria,[14] and hypoperfusion evidenced by either a systolic blood pressure lower than – 2 standard deviation (SD) adjusted for age or at least one manifestation of inadequate organ perfusion, that is, altered mentation, hypoxia (PaO2 <45 mmHg while breathing room air or PaO2/FiO2 <350), metabolic acidosis (arterial pH <7.35 or base deficit >5), or oliguria (i.e., urine output <0.5 ml/kg/h), along with signs of poor peripheral perfusion. The exclusion criteria were patients with other causes of shock not due to sepsis itself, malignancies and immunosuppressive treatment and those with conditions known to cause elevated lactate, for example, chronic medical illnesses and inborn errors of metabolism.
Assessments and outcome measures
Patients' demographics were collected. Initial work up consisted of detailed general and systemic examination, an active search for the underlying infection, routine hematological, and biochemical profile. Record of requirement of ventilator support, fluid boluses, and inotropes received was kept and monitoring of clinical status was done at regular intervals until discharge or death. To assess the severity of illness and organ dysfunction, the Pediatric Risk of Mortality III (PRISM III) score was calculated.
Arterial blood gas analysis was performed at 0–3 h of PICU admission, 24 h and 48 h and then as and when required. Lactate levels were measured in arterial blood by the NADH-dependent kinetic method using a blood gas analyzer (Cobas 221, Roche). The initial arterial blood lactate was done within 3 h of PICU admission and again at 24 h and 48 h. Serial lactate levels were labeled as lactate 1, lactate 2, and lactate 3, respectively. Lactate clearance was defined as the percent change in lactate level from PICU admission (0–3 h) to hour 24, calculated as (Lactate1 − Lactate2)/Lactate1 × 100.
The primary outcome measure of patients was recorded as survival or nonsurvival at the end of hospital stay. The secondary outcome measurements were the length of hospital stay and requirement of mechanical ventilation. All patients were treated according to local protocol. Written consent from the parents was obtained for inclusion in the study.
Statistical analysis
Continuous normally distributed variables were expressed as mean and SD or when not normally distributed as median and their interquartile ranges (IQRs). To test group differences, the Student's t-test was used; if continuous data were not normally distributed the Mann–Whitney U-test was used. Categorical variables were expressed as n (%) and the Pearson's Chi-square test and Fisher's exact test were used to analyze differences in proportions. Analysis of the area under the curve (AUC) of the receiver operating characteristic (ROC) curve was constructed to assess the predictive strength. Cut-off points for initial lactate level and lactate clearance were obtained from the ROC curves at the best sensitivity and specificity, along with the positive predictive value (PPV), negative predictive value (NPV), and positive and negative likelihood ratios and odds ratio (OR) for the prediction of death. Differences with P < 0.05 were considered statistically significant. Appropriate statistical uncertainty was expressed by the 95% confidence levels.
RESULTS
Of the total 713 patients admitted to PICU, 161 fulfilled the study criteria. Thirteen patients who died before 24 h were excluded from the study, and hence the subjects of this study comprised 148 patients. Table 1 describes clinical characteristics of the study population. Most common underlying infective pathology was pneumonia in 48 (32.4%) cases. Twenty patients (13.5%) had culture positive sepsis, of these nine survived. Most common pathogen was coagulase positive staphylococci (9) followed by enterobacter (3). Mechanical ventilation was required in 107 (72.3%) cases. The overall mortality in the study population was 63.5%.
Table 1.
Clinical characteristics of the subjects (n=148)
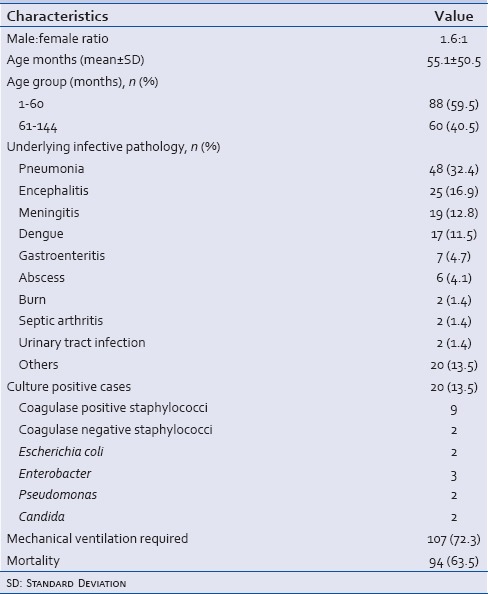
A comparison of clinical and laboratory parameters among survivors and nonsurvivors is shown in Table 2. Sex, duration of illness, total leucocytes count and initial pH were not significantly different among the two groups. The median duration of hospital stay was significantly higher in survivors, 12 (IQR: 8–15) days than 5 (IQR: 3–9) days in nonsurvivors (P < 0.0001). The mean lactate levels at admission were significantly higher in the nonsurvivors than survivors (5.12 ± 3.51 vs. 3.13 ± 1.71 mmol/L; P = 0.0001). The subsequent lactate levels after 24 and 48 h of admission and PRISM III score were also higher in nonsurvivors. The mean lactate clearance rate was 17.9% ± 39.9% in survivors and -23.2% ± 62.7% in nonsurvivors (P < 0.0001). The relationship of initial lactate with the PRISM III score was determined by calculating the Spearman rank order correlation coefficient and two-tailed significance. A positive correlation existed between the PRISM III score and lactate level (rho = 0.370; P < 0.0001). The initial lactate level and lactate clearance were further analyzed using ROC curves and cut off points were obtained at best sensitivity and specificity [Figure 1]. The AUC for initial lactate was 0.697 (95% confidence interval [CI] = 0.612–0.781) indicating a fair correlation between initial lactate and mortality. The cut-off point for initial lactate was obtained as ≥4 mmol/L. A lactate level of ≥4 mmol/L at 0–3, 24, 48 h of admission had a sensitivity of 57%, 62%, 67%, and specificity of 82%, 87%, 92% with a PPV of 84%, 89%, 94%, and a NPV of 51%, 56% and 61%, and an OR for death as 5.4, 10.8, 25.4 (95%CI = 2.453–12.099, 4.414–26.510, 8.409–76.736), respectively [Table 3].
Table 2.
Comparison of demographic and clinical characteristics and laboratory findings on admission day between survivors and nonsurvivors
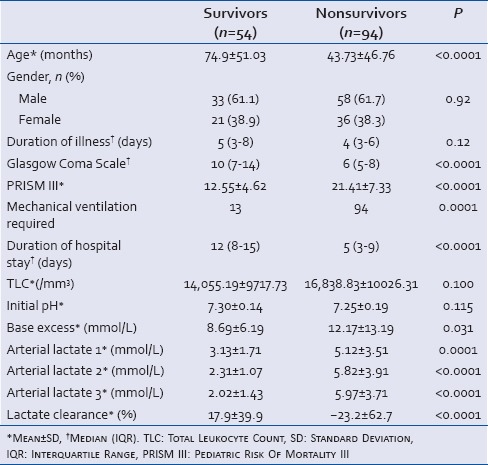
Figure 1.
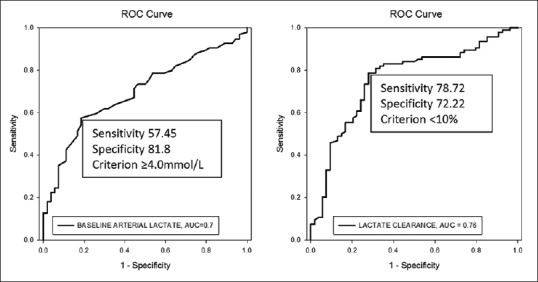
Receiver operating characteristic curves for the ability of blood lactate at admission and lactate clearance to predict in-hospital mortality in children with septic shock (n = 148). The area under the receiver operating characteristic curve for blood lactate and lactate clearance were 0.697 and 0.755, respectively
Table 3.
Odds ratio, sensitivity, and specificity for serial blood lactate to predict in-hospital mortality in children with septic shock

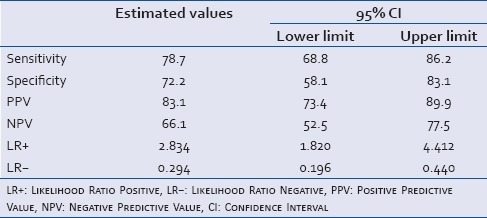
The area under ROC curve for lactate clearance was 0.755 (95% CI = 0.673–0.837) indicating a fair correlation between lactate clearance and mortality [Figure 1]. The cut-off point for lactate clearance for best prediction of mortality was determined as <10% from the ROC curve. The patients who had a lactate clearance of <10% from baseline had a significantly higher mortality than the mortality in those who had lactate clearance of more than 10% (83.1% vs. 33.9%; P < 0.0001) [Table 4]. Sensitivity, specificity, positive and NPVs of lactate clearance rate of <10% are shown in Table 5.
Table 4.
Distribution of survivors and nonsurvivors according to lactate clearance

Table 5.
Sensitivity, specificity, positive predictive value and negative predictive value of lactate clearance <10%
DISCUSSION
Severe sepsis and septic shock are medical emergencies which have a significant and increasing impact on public health. Intensivists agreed that traditional markers, such as blood pressure or urine output, are not sufficient indicators of adequate global perfusion.[15] Studies have found lactate levels as a useful parameter indicating sepsis induced hypoperfusion.[16,17] Previous studies in this area support the use of serum lactate in both the diagnostic and treatment phases for sepsis and septic shock in adults.[7,18,19]
However, the studies on lactate as the predictor of mortality in children have shown varied results. Koliski et al. concluded that lactate levels on admission and after 12 h of treatment were not efficient in predicting the risk of death among the patients and only after 24 h of treatment the lactate level can predict survival and risk of death with the best sensitivity (55.6%) and specificity (97.2%).[10] Balasubramanyan et al. in their study compared lactate levels with other parameters, and found that lactate levels did not correlate with mortality. Mortality was more strongly related to base excess < or = −5 mEq/L (relative risk of death = 10.25; P = 0.002) than to lactate > or = 45 mg/dL (relative risk of death = 2.35; P = 0.04).[11] Hatherill et al. found that the initial lactate concentration in children submitted to cardiac surgery cannot predict death.[20]
This study was designed to determine the optimal relationship between the sensitivity and specificity of blood lactate assessment in predicting mortality and to set appropriate cut-off values for predicting in-hospital mortality. Data from the present study of 148 children with septic shock shows that the mean lactate levels at admission as well as after 24 and 48 h of admission were significantly higher in the nonsurvivors thansurvivors (P = 0.0001). The cut-off point for initial lactate was ≥4 mmol/L as obtained from the ROC curves at the best sensitivity (55%) and specificity (82%). In two previous studies,[10,12] a lactate level of >3 mmol/L significantly predicted mortality. In their study of 65 patients, Kim et al. demonstrated that patients with initial lactate levels higher than 5 mmol/L showed a significantly higher mortality rate and also concluded that lactate area, which includes the exposure time of hyperlactatemia as well as the actual lactate concentrations, has a strong predictive value for mortality in pediatric septic shock patients.[21] Bai et al. studied 1109 critically ill children and found an optimal cut-off of lactate at admission for predicting in-hospital mortality to be 5.55 mmol/L, which had a sensitivity of 61% and a specificity of 86%.[22] In both the studies, serial changes in the lactate levels during the first 24 h of admission were not evaluated.
The overall mortality in the study group was 63.5%. In various studies, the mortality in pediatric septic shock varied from 9.8% to 50%.[10,11,12,13,23] The high mortality in our study may be due to the reason that most patients who died belonged to younger age group. Pneumonia was the most common underlying infection in our study, accounting for 32.4% of total cases. Infection was proved in 13.5% of cases as blood culture positive. Most common pathogen was coagulase positive staphylococci followed by enterobacter. Sáez-Llorens et al. reported the mortality rate as 39% in pediatric sepsis with bacteriologically proven infection in 26% cases; Staphylococcus aureus and Neisseria meningitidis being the most frequent responsible organisms.[23]
Several studies in adults have shown that changes in serial lactate concentrations may be more useful marker than a single lactate level estimation.[7,8,24] In our study, the patients whose lactate levels declined more than 10% within 24 h of PICU admission had low in-hospital mortality when compared to those whose 24 h lactate clearance was <10%. This is in line with a study of 111 adult patients with sepsis and septic shock where low lactate clearance (<10%) within the first 6 h predicted death two-thirds of the time.[7] In another study lactate clearance at 24 h after admission had a major statistically significant association than that found at 6 and 12 h, showing that sustained time of clearance lactate predicts mortality better.[24] However, Walker et al. demonstrated the optimal cut-off to be 36% for the prediction of 30-day mortality in septic patients, which is much higher than our results.[25]
This is the first available discussion looking into lactate clearance in septic shock in pediatric population, and is likely to prove useful in patients with varied etiology and management protocols since the study population has been subjected to different treatment protocols prior to shifting to PICU, which is frequently observed in referrals to tertiary care centers. There are some limitations to our study. The first lactate level was measured in PICU; therefore pre-Intensive Care Unit management of these patients may have influenced the lactate values. Because of the lack of complete information regarding interventions during PICU transfers, the data regarding use of epinephrine, which are known to affect lactate levels[26,27] could not be included in analyses.
CONCLUSIONS
Our study indicates that all the three serial blood lactate levels, that is, on admission to the PICU and after 24 and 48 h were significantly associated with mortality in children with septic shock. Based on the findings of this study, we conclude that rising or persistently high lactate levels as shown by <10% lactate clearance at 24 h is a predictor of mortality in such patients. These findings suggest an important role for serial lactate sampling rather than isolated measurement for prediction of outcome in children with septic shock.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Scalea TM, Maltz S, Yelon J, Trooskin SZ, Duncan AO, Sclafani SJ. Resuscitation of multiple trauma and head injury: Role of crystalloid fluids and inotropes. Crit Care Med. 1994;22:1610–5. [PubMed] [Google Scholar]
- 3.Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221–6. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 4.Levy B, Sadoune LO, Gelot AM, Bollaert PE, Nabet P, Larcan A. Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine-treated septic shock. Crit Care Med. 2000;28:114–9. doi: 10.1097/00003246-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 6.Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33:970–7. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs. central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA. 2010;303:739–46. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koliski A, Cat I, Giraldi DJ, Cat ML. Blood lactate concentration as prognostic marker in critically ill children. J Pediatr (Rio J) 2005;81:287–92. [PubMed] [Google Scholar]
- 11.Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl-Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive care unit. Crit Care Med. 1999;27:1577–81. doi: 10.1097/00003246-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997;23:684–92. doi: 10.1007/s001340050394. [DOI] [PubMed] [Google Scholar]
- 13.Jat KR, Jhamb U, Gupta VK. Serum lactate levels as the predictor of outcome in pediatric septic shock. Indian J Crit Care Med. 2011;15:102–7. doi: 10.4103/0972-5229.83017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 15.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–92. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Poeze M, Solberg BC, Greve JW, Ramsay G. Monitoring global volume-related hemodynamic or regional variables after initial resuscitation: What is a better predictor of outcome in critically ill septic patients? Crit Care Med. 2005;33:2494–500. doi: 10.1097/01.ccm.0000185642.33586.9d. [DOI] [PubMed] [Google Scholar]
- 17.Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettilä V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31:1066–71. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 18.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Hatherill M, Sajjanhar T, Tibby SM, Champion MP, Anderson D, Marsh MJ, et al. Serum lactate as a predictor of mortality after paediatric cardiac surgery. Arch Dis Child. 1997;77:235–8. doi: 10.1136/adc.77.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YA, Ha EJ, Jhang WK, Park SJ. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013;39:1818–23. doi: 10.1007/s00134-013-2959-z. [DOI] [PubMed] [Google Scholar]
- 22.Bai Z, Zhu X, Li M, Hua J, Li Y, Pan J, et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr. 2014;14:83. doi: 10.1186/1471-2431-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sáez-Llorens X, Vargas S, Guerra F, Coronado L. Application of new sepsis definitions to evaluate outcome of pediatric patients with severe systemic infections. Pediatr Infect Dis J. 1995;14:557–61. doi: 10.1097/00006454-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Montiel- Jarquin A, Lascarez-Lagunas I, Sanchez-Gasca L, Garcia-Cano E, Gomez-Conde E, Garcia-Carrasco M, et al. Lactate clearance is a prognostic factor in patients on shock state. Eur J Gen Med. 2012;9:98–103. [Google Scholar]
- 25.Walker CA, Griffith DM, Gray AJ, Datta D, Hay AW. Early lactate clearance in septic patients with elevated lactate levels admitted from the emergency department to intensive care: Time to aim higher? J Crit Care. 2013;28:832–7. doi: 10.1016/j.jcrc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–23. doi: 10.1016/s0140-6736(96)09096-4. [DOI] [PubMed] [Google Scholar]
- 27.Levy B. Bench-to-bedside review: Is there a place for epinephrine in septic shock? Crit Care. 2005;9:561–5. doi: 10.1186/cc3901. [DOI] [PMC free article] [PubMed] [Google Scholar]


