Abstract
Cysticercosis is common in developing countries in which the combination of rural society, crowding, and poor sanitation facilities allows greater contact between humans and pigs and thus more opportunities for fecal contamination of food and water occurs. They are rarely located in oral and perioral tissues, particularly in the muscles of mastication, muscle of the facial expression, suprahyoid muscles, and postcervical musculature and also as in the tongue, buccal mucosa, and lip. Cysticercosis is a potentially fatal parasitic disease that rarely found in the maxillofacial region in humans. This paper reports the case of a young female patient presented with isolated lesion of cysticercosis involving buccinator muscle. In conclusion, we suggest that cysticercosis should be considered in the differential diagnosis of intraoral solitary nodules within the oral and maxillofacial region, especially in endemic areas. High-resolution ultrasonography is an excellent noninvasive and cost-effective modality for the diagnosis and also suggests that localized parasitic infections such as Cysticercus cellulosae can be successfully treated with conservative management using oral antiparasitic (antihelminthic) medication.
Key words: Cysticercus cellulosae, cysticidal, maxillofacial cysticercosis, Taenia solium, ultrasonography
INTRODUCTION
Cysticercosis was first described by Johannes Udalric Rumler in 1555. However, at that time, the connection between tapeworms and cysticercosis had not been recognized. By the middle of the 19th century, it was well established that cysticercosis was caused by the ingestion of the eggs of Taenia solium.[1,2]
The life cycle of Platyhelminthes is characterized by different stages of development and growth requiring various types of hosts which can appropriately harbor the eggs, oncospheres, larvae, and adults form. Usually, when the larvae infest the intermediate host tissue (pig), it results in cysticercosis; and when ingested by the definitive host (human), these larvae complete their life cycle by developing into adult worms [Figure 1].
Figure 1.
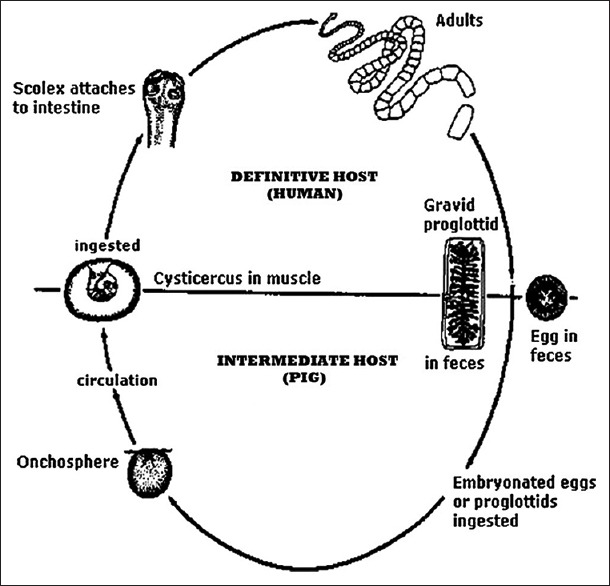
Life cycle of Taenia solium
Life cycle of Platyhelminthes is basically characterized by two stages:First as a larval stage (Cysticercus cellulosae), and then as an adult worm stage, besides an egg phase. Each of these phases requires a different type of host. The adult T. solium, or pork tapeworm, lives in the small intestine of man (definitive host). Infection by T. solium is common in areas where pig breeding is not controlled and where there is poor sanitation facilities and close interaction between humans and animals present. Latin America, Southern Africa, India, Southeast Asia, and Eastern Europe are the most frequent locations of occurrence of cysticercosis.[3]
The parasite can be ingested in the form of larvae from inadequately cooked pork meat containing cysticerci or in the form of eggs through fecally contaminated vegetables, food, or water or through the direct contact with another human carrier. The larva may develop into an adult tapeworm and colonize the human intestine. The larvae (C. cellulosae) penetrate the mucosa, enters into the blood vessels and lymphatics circulation, and are distributed in the tissues of the all over the body, but it preferentially located in the brain, muscle, skin, liver, lungs, and heart.[4] They are rarely located in oral and perioral tissues, particularly in the muscles of mastication, muscle of the facial expression, the suprahyoid muscles, and the postcervical musculature and also as in the tongue, buccal mucosa, and lip.[5,6,7] Cysticercosis is a potentially fatal parasitic disease that rarely found in the maxillofacial region in humans.[8]
According to the literature reports, the prevalence of oral cysticercosis is 4.1%.[9] A correct and precise clinical diagnosis is infrequently established. Usually, the disease is confused with other benign swellings. Diagnosis of cysticercosis can be confirmed by the detection of a cystic space containing the C. cellulosae histopathologically.[1,2]
This paper reports the case of a young female patient presented with isolated lesion of cysticercosis involving buccinator muscle.
CASE REPORT
A 14-year-old female patient reported to the Department of Oral and Maxillofacial Surgery at Sardar Patel Post Graduate Institute of Dental and Medical Sciences, Lucknow, complaining of painless swelling in the right cheek for 1 year. The patient was apparently alright 1 year back; then she noticed a small peanut-sized soft swelling accompanied with pain in the right inner cheek region which was slow growing. There was no history of trauma to the area, no history of discharge, and no other swelling in the body. The patient gave a history of mixed dietary habit and belongs to low socioeconomic status. Her medical history was noncontributory.
On extraoral examination, mild facial swelling present on right side of the cheek and the overlying facial skin appeared normal [Figure 2]. An intraoral examination revealed a well circumscribed with intact overlying mucosa, smooth submucosal, nodular swelling of approximately 1.5 cm × 1 cm in diameter in right buccal mucosa. Swelling was soft on palpation, nontender, and noncompressible [Figure 3]. No limitation in mandibular movement was noted. Neither any palpable lymph node was present in the head and neck nor any neurological deficit, or no history of seizures was present.
Figure 2.
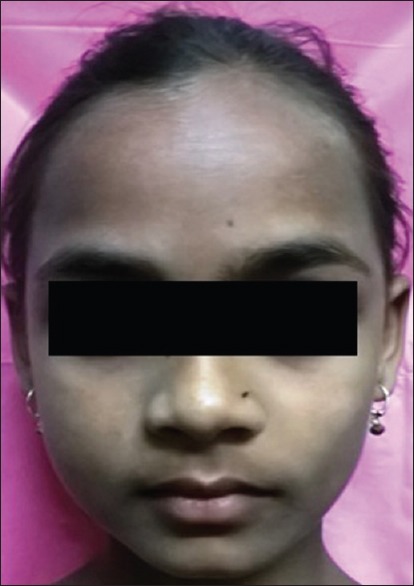
Extraoral photograph showing mild swelling present on the right cheek
Figure 3.
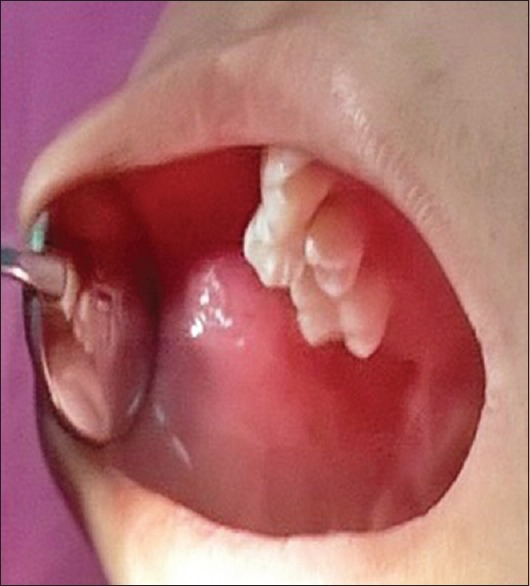
Intraoral photograph showing a smooth, nodular swelling in the right buccal
Differential diagnosis of fibroma, neurofibroma, focal fibrous hyperplasia, and mucocele was made. On further investigations, the orthopantomogram was found to be nonremarkable. Ultrasonography (USG) revealed well-defined oval-shaped intramuscular cystic lesion in muscle of right side of the cheek. It measures approximately 8.7 mm × 7.3 mm in dimension. A small echogenic eccentric mural nodule is seen within it. Surrounding muscle fibers are mildly thickened and inflamed. Findings were suggestive of cysticercosis [Figure 4].
Figure 4.
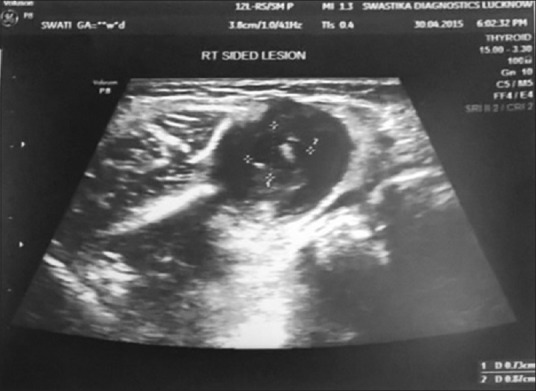
Ultrasonographic scan showing eccentric mural nodule in intramuscular cystic lesion
The patient was also referred for medical evaluation. Blood, urine, and stool examination were performed, which were in normal limit and did not showed any involvement of other areas. In our case, we instituted the conservative management with albendazole and prednisolone.
DISCUSSION
Cysticercosis is common in developing countries in which the combination of rural society, crowding, and poor sanitation facilities allows greater contact between humans and pigs and thus more opportunities for fecal contamination of food and water occurs.[4,5]
Maxillofacial cysticercosis is a rare infection with not more than 34 cases of oral involvement reported in the English literature.[10] There was no sex or age predilection in its occurrence. Risk factors for cycticercosis include frequent consumption of uncooked pork meat, poor sanitation facilities, and history of passing tapeworm proglottids (eggs) excreted with feces ingested by the pig or the intermediate host.[5] In the intermediate host (human), cysticercosis can develop in various organs and tissues [Figure 4]. The tissues most frequently affected by cysticercosis are subcutaneous tissue, brain, striated muscles of neck, heart, liver, lungs, orbit, peritoneum, and meninges, etc.[11] Neurocysticercosis is characterized by the signs of raised intracranial pressure, motor deficits, encephalitis, and other manifestations of cerebral involvement. It is the most common cause of acquired epilepsy in the developing countries. Magnetic resonance imaging (MRI) of the brain is the most important diagnostic tool in patients with suspected neurocysticercosis.[12]
There are various diagnosis aids necessary to confirm the diagnosis of cysticercosis which includes computerized tomography and MRI, USG, serology, and tissue biopsy (histopathological examination).[13] Parasitological examination is more reliable in revealing T. solium eggs in the collected stool sample. The immunodiagnosis of cysticercosis can be achieved by the serum, cerebrospinal fluid (CSF), and saliva examination for antibodies to cysticercus cellulose with the help of either enzyme-linked immune-sorbent assay (ELISA) or enzyme-linked immune-electrotransfer bolt (EITB).[14] EITB specificity and sensitivity are superior to ELISA for the diagnosis of cysticercosis.
Conventional radiographs are useful for the detection of muscles calcification.[15] Commonly radiodense images of the calcified cysticerci may appear on any radiograph of the soft tissues of the body. In the maxillofacial region, the locations of calcified cysticerci present on muscles of mastication and facial expression, the suprahyoid muscle, and the posterior cervical as well as the tongue, buccal mucosa, or lip.[16]
Oral cysticercosis is an unusual event and is usually asymptomatic. Furthermore, it is interesting to note that involvement of masseter is extremely rare. Reddi et al.[17] and Mittal et al.[18] reported similar cases of intramuscular cysticercosis in the masseter muscle, both of which were diagnosed by USG and treated conservatively. The most commonly involved intraoral sites include tongue (42.15%), lips (26.15%), and buccal mucosa (18.9%).[17,19] The most serious involvement is that of the central nervous system, followed by ocular involvement. Parasitic examinations with zinc sulfate flotation and formalin-ether sedimentation technique are also valuable in revealing T. solium eggs in stool samples.[19]
Basically, there are five treatment modalities recommended which can be offered to patients: (1) larvicidal agents (like praziquantel and albendazole) to kill the cystic larvae and/or tapeworm; (2) corticosteroids or other immunosuppressive agents to decrease or prevent inflammation; (3) anti-seizure medication to prevent or decrease the severity and number of seizures episodes; (4) surgical-based therapies to decrease the mass effect of cysts with or without accompanying inflammation; and (5) general supportive measures in impaired individuals or symptomatic treatments.[20]
Pharmacological management of cysticercosis with the cysticidal drugs such as praziquantel and albendazole help by reducing the parasite burden by facilitating the death of the cysts. The advantage of albendazole over praziquantel is that the albendazole also destroys subarachnoid and ventricular cysts because of its better penetration in CSF as well as the fact that it can be administered jointly with corticosteroid agents for anti-inflammatory therapy.[21]
However, cysticidal drugs may be associated with severe reactions such as local tissue swelling and generalized anaphylactic reaction due to massive release of antigens. The corticosteroids would help in decreasing the incidence of such complications if used before starting the cysticidal drug.[21] In cases of disseminated oral cysticercosis, albendazole is used in a dose of 15 mg/kg/day for 30 days and praziquantel in a dose of 50–75 mg/kg/day in three divided dosage for 15–20 days.
The prognosis in the oral cysticercosis is good with no recurrences. In contrast to the gravity of the disease in the cerebral, ocular, or cardiac sites, it is often well-tolerated. When associated with other localizations, the prognosis depends on site and number of other larval localizations and may be severe.[8]
CONCLUSION
We suggest that cysticercosis should be considered in the differential diagnosis of intraoral solitary nodules within the oral and maxillofacial region, especially in endemic areas. High-resolution USG is an excellent noninvasive and cost-effective modality for the diagnosis and also suggests that localized parasitic infections such as C. cellulosae can be successfully treated with conservative management using oral antiparasitic (antihelminthic) medication.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wadia NH, Singh G. Taenia solium Cysticercosis: From Basic to Clinical Science. Lucknow: CABI Publishing; 2002. Taenia solium: A historical note; pp. 157–68. [Google Scholar]
- 2.Cox FE. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612. doi: 10.1128/CMR.15.4.595-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhu SR. Oral Diseases in the Tropics. Lucknow: Oxford University Press; 1992. pp. 126–9. [Google Scholar]
- 4.Chandler AC. Introduction to Parasitology. New York, USA: John Wiley & Sons; 1958. [Google Scholar]
- 5.Timosca G, Gavrilita L. Cysticercosis of the maxillofacial region. A clinicopathologic study of five cases. Oral Surg Oral Med Oral Pathol. 1974;37:390–400. doi: 10.1016/0030-4220(74)90112-1. [DOI] [PubMed] [Google Scholar]
- 6.Kinnman J, Chi CH, Park JH. Cysticercosis in otolaryngology. Arch Otolaryngol. 1976;102:144–7. doi: 10.1001/archotol.1976.00780080066006. [DOI] [PubMed] [Google Scholar]
- 7.Tschen EH, Tschen EA, Smith EB. Cutaneous cysticercosis treated with metrifonate. Arch Dermatol. 1981;117:507–9. doi: 10.1001/archderm.1981.01650080061031. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh A, Avadhani A, Tupkari J, Sardar M. Cysticercosis of the upper lip. J Oral Maxillofac Pathol. 2011;15:219–22. doi: 10.4103/0973-029X.84509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigam S, Singh T, Mishra A, Chaturvedi KU. Oral cysticercosis – Report of six cases. Head Neck. 2001;23:497–9. doi: 10.1002/hed.1066. [DOI] [PubMed] [Google Scholar]
- 10.Wortman PD. Subcutaneous cysticercosis. J Am Acad Dermatol. 1991;25(2 Pt 2):409–14. doi: 10.1016/0190-9622(91)70217-p. [DOI] [PubMed] [Google Scholar]
- 11.Elhence P, Bansal R, Sharma S, Bharat V. Cysticercosis presenting as cervical lymphadenopathy: A rare presentation in two cases with review of literature. Niger J Clin Pract. 2012;15:361–3. doi: 10.4103/1119-3077.100652. [DOI] [PubMed] [Google Scholar]
- 12.Jay A, Dhanda J, Chiodini PL, Woodrow CJ, Farthing PM, Evans J, et al. Oral cysticercosis. Br J Oral Maxillofac Surg. 2007;45:331–4. doi: 10.1016/j.bjoms.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Jena A, Sanchetee PC, Tripathi R, Jain RK, Gupta AK, Sapra ML. MR observations on the effects of praziquantel in neurocysticercosis. Magn Reson Imaging. 1992;10:77–80. doi: 10.1016/0730-725x(92)90375-a. [DOI] [PubMed] [Google Scholar]
- 14.Del Brutto OH, Sotelo J, Roman GC. Therapy for neurocysticercosis: A reappraisal. Clin Infect Dis. 1993;17:730–5. doi: 10.1093/clinids/17.4.730. [DOI] [PubMed] [Google Scholar]
- 15.Khurana S, Dubey ML, Malla N. Association of parasitic infections and cancers. Indian J Med Microbiol. 2005;23:74–9. doi: 10.4103/0255-0857.16044. [DOI] [PubMed] [Google Scholar]
- 16.Carter CL. Soft tissue calcification and ossification. In: White SC, Pharoah MJ, editors. Oral Radiology Principles and Interpretation. 6th ed. Philadelphia: Mosby; 2009. p. 529. [Google Scholar]
- 17.Reddi SP, Morales MJ, Addante RR. Solitary lesion in the masseter muscle. J Oral Maxillofac Surg. 2001;59:71–5. doi: 10.1053/joms.2001.19294. [DOI] [PubMed] [Google Scholar]
- 18.Mittal A, Das D, Iyer N, Nagaraj J, Gupta M. Masseter cysticercosis – A rare case diagnosed on ultrasound. Dentomaxillofac Radiol. 2008;37:113–6. doi: 10.1259/dmfr/31885135. [DOI] [PubMed] [Google Scholar]
- 19.Romero de Leon E, Aguirre A. Oral cysticercosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:572–7. doi: 10.1016/s1079-2104(05)80098-8. [DOI] [PubMed] [Google Scholar]
- 20.Nash TE. Human case management and treatment of cysticercosis. Acta Trop. 2003;87:61–9. doi: 10.1016/s0001-706x(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Kong MH, Kim JH, Song KY. Disseminated cysticercosis. J Korean Neurosurg Soc. 2011;49:190–3. doi: 10.3340/jkns.2011.49.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


