Abstract
Aims and Objectives:
Successful preservation of the edentulous ridge after extraction may eliminate or reduce the need for ridge augmentation procedures. It is proved that grafting of fresh extraction sockets with bone grafts promotes ridge preservation. An objective method of maintaining height and width of alveolar ridge using mesenchymal stem cells (MSCs) seeded on collagen membrane was implemented in this study.
Methodology:
Ten bilaterally symmetrical extraction sockets scheduled for extraction were selected for this study. Involved teeth were extracted atraumatically and the sockets were curetted. MSCs seeded on collagen membrane were placed in the extracted socket on one side. On the other side, only collagen membrane was placed inside the socket. Both the sockets were closed primarily with nonresorbable sutures. Buccolingual and mesiodistal widths of the ridges at three different levels (2 mm below cementoenamel junction [CEJ], 5 mm below CEJ, and 8 mm below CEJ) were assessed immediately after extraction and postoperatively at 3 and 6 months.
Results:
There was statistically significant observation in maintaining the alveolar ridge width in the grafted site when compared to the nongrafted site.
Conclusion:
Socket healing procedure using MSCs and collagen membrane was successful in maintaining width of alveolar socket.
Key words: Cementoenamel junction, collagen membrane, mesenchymal stem cells, ridge preservation technique, stromal stem cell
INTRODUCTION
Extraction of teeth is the most routinely performed oral surgical procedure. Undisturbed extraction sockets heal uneventfully with bone tissue within 1–2 months following extraction. With healing process, there is some loss of the alveolar bone height and width, which in some cases may esthetically compromise an implant-supported prosthesis.[1] Immediate bony augmentation is done at the time of tooth extraction to improve ridge form and periodontal health of the adjacent teeth.[2]
Bone is a unique biological tissue which has the ability to heal itself when gets fractured. When part of bone is lost or excised, a defect is formed. In these cases, the bone does not heal itself but need bone reconstruction to prevent a nonunion.[3] Bone grafting to promote healing has become one of the most common surgical procedures in recent decades. Ideal bone graft substitute should not be toxic and carcinogenic, easily available in sufficient quantity, and should be easy to use. Various bone grafting materials used in alveolar bone grafting procedures having varying degrees of success include autogenous bone from patient's iliac crest, rib, mandibular symphysis and retromolar region, and tuberosity of maxilla. Allogenic bone graft substitutes such as tricalcium phosphate and hydroxyapatite and a combination of autogenous and allgenic grafts. There are few limitations of these bone grafts such as insufficient autogenous sources, donor-site morbidity, contour dissimilarities, pathology transmission, graft versus host response, unpredictable outcome for bone formation, immunosuppression, and infection of foreign material. The limitations also include inadequate blood supply and prolonged healing.
Recent development in concept of stem cell-based tissue regeneration provides a reliable approach and therapeutic strategy for osseous repair.[4] Stem cells are characterized by property of pluripotent (capable of giving rise to all three germ layers) and with each cell division, they generate two cells of equally pluripotency, allowing self-renewal consistently. Adult stem cells are multipotent progenitor cells found in infants to adults and live within organs to repair cells during normal physiologic damage and repair. These cells present in adipose tissue, bone marrow, brain, skin, skeletal muscle, testes, and umbilical cord. Adult stem cells derived from mesodermal derivatives are known as mesenchymal stem cells (MSCs).[5]
The application of MSC increases angiogenesis and osteogenesis in the damaged bone after extraction. MSCs are capable of promoting repair and regeneration of bone defects. MSCs release cytokines and growth factors such as vascular endothelial growth factor and transforming growth factor, which are important for therapeutic angiogenesis and wound healing.[6] The present study evaluates the healing of extraction sockets with MSCs seeded on collagen membrane.
METHODOLOGY
Patients in age group of 18–35 with 10 bilateral symmetrical extraction sockets were selected for the study. After complete a history and examination, patients underwent routine preoperative hematological investigations and viral markers. The surgical procedure was carried out after obtaining an informed consent from the patient. Preoperative orthopantomography was taken to assess the alveolar bone height and root form of the teeth. Following a standard basic surgical preparation, local anesthetic with adrenaline was injected to attain local anesthesia. Flap was reflected using periosteal elevator and tooth was extracted atraumatically. On one side, socket was filled with collagen membrane seeded with 1 million MSCs and sutured. On the other side, socket was filled with collagen membrane without MSCs and sutured [Figure 1]. Patients were advised to take tablet diclofenac sodium 50 mg and amoxicillin 500 mg capsules for 5 days. Cone-beam computed tomography (CBCT) of the extracted sockets was taken on the same postoperative day after the placement of stem cells. Further, patients were recalled at regular intervals of 3 months and 6 months for reevaluation of alveolar bone width on CBCT. Radiographic alveolar buccolingual (BL) and mesiodistal (MD) bone width was measured at three different levels (2 mm below cementoenamel junction [CEJ], 5 mm below CEJ, and 8 mm below CEJ) on CBCT immediately after extraction, postoperatively after 3 months and 6 months. The measurements were compared and evaluated [Figures 2 and 3].
Figure 1.
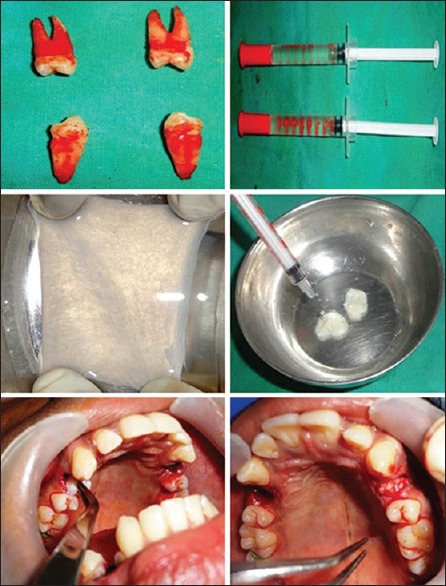
Intraoperative view showing extracted teeth, stem cells in saline, collagen membrane, seeding of stem cells on collagen, placement of collagen with mesenchymal on right side, and placement of only collagen on stem cells
Figure 2.
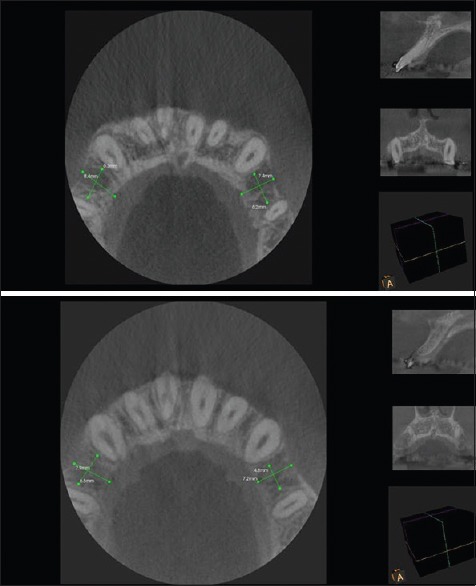
Cone-beam computed tomography images at 3 months and 5 months postoperatively (5 mm below cementoenamel junction)
Figure 3.
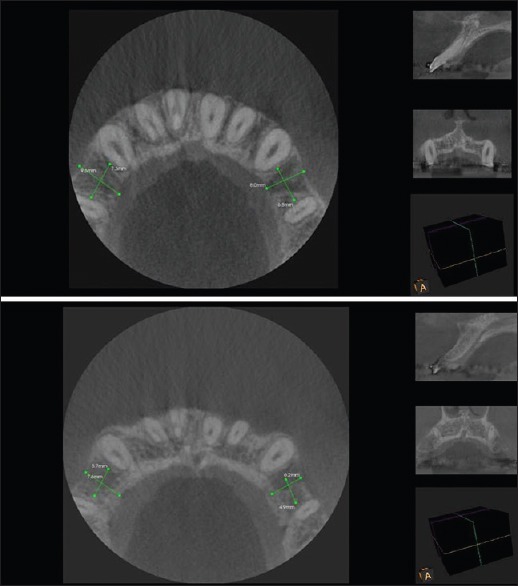
Cone-beam computed tomography images at 3 months and 6 months postoperatively (8 mm below cementoenamel junction)
Stem cell harvesting
Healthy donors according to the donor inclusion criteria were included and had both the sexes of age group of 18–35 years and wished to get in study voluntarily with written informed consent. Donors with autoimmune disorders, tuberculosis, malaria, and any other infection, history of malignancy, diabetes, hypertension, significant heart disease, hemoglobin <10, and pregnant women were excluded from the study. Routine investigations such as complete blood count, renal function test, liver function test blood glucose, chest X-ray, echocardiogram, and electrocardiogram were done.
Bone marrow-derived MSCs (BMMSCs) were isolated and expanded. Sixty milliliters of bone marrow was taken under strict aseptic condition from the iliac crest of 5 voluntary donors under deep sedation. All processing of the samples were done inside a class 100 biosafety hood in class B cGMP facility. Dulbecco's modified Eagle's medium was used to dilute bone marrow with ratio of 1:1 and anticoagulants were removed by centrifuging. Mononuclear cells which were present in the buffy coat were isolated and washed with culture medium (density gradient method). The mononuclear fraction which also contains MSCs were plated onto tissue culture plates and cultured.
Subculturing and expansion of mesenchymal stem cells
Once the cells became confluent, they were dissociated with 0.25% trypsin/0.53 mM ethylenediaminetetraacetic acid. These cells were then upscaled and expanded to provide the required number of cells. After 10 days in culture, the cells reached 90% confluency and ready for transplantation.
Quality control
MSCs were tested using flow cytometry for quality control such as mycoplasma, endotoxin, sterility, and cell surface markers such as CD34, CD45, CD73, CD90, and CD105. They were positive (>80%) for CD73, CD90, and CD105 but negative (<10%) for CD34 and CD45. Karyotyping was done by visualizing chromosomes by G-banding technique. Evaluation of endotoxin level was done using Limulus amebocyte lysate test and mycoplasma using polymerase chain reaction-ELISA, respectively.
End product testing
The final cell suspension which was provided to us for transplantation was again tested for cell surface marker analysis. Viability of cells was evaluated by flow cytometry through 7-aminoactinomycin D. One million stem cells were dispensed in 1 ml normal saline in a 1 cc syringe and were delivered. Certificate of analysis was prepared and cells were released for transplantation.
RESULTS
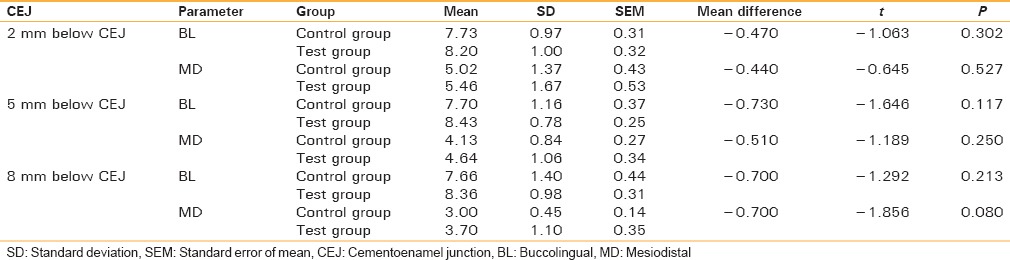
We analyzed the BL and MD width of bone at three different levels (2 mm below CEJ, 5 mm below CEJ, and 8 mm below CEJ) on the CBCT immediately after extraction and at regular intervals of 3 and 6 months. The measurements on test and control side were compared and evaluated. The difference in mean value was not statistically significant between control group in BL and MD at 2 mm below CEJ (P > 0.05) or at 5 mm below CEJ (P > 0.05) or at 8 mm below CEJ (P > 0.05). This signifies that immediately after extraction, there is no bone formation between test and control side [Table 1 and Graph 1].
Table 1.
Analysis of the values immediately after extraction
Graph 1.
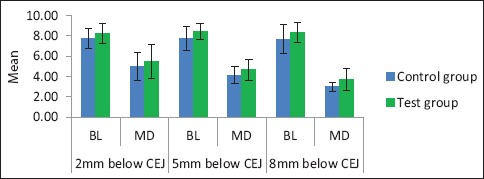
The difference in mean values between control group and test group immediately after extraction
Analysis of the values after 3 months
The difference in mean values between control group and test group was found to be statistically significant in MD at 2 mm below CEJ (P < 0.05), in BL as well as MD at 5 mm below CEJ (P < 0.05) and in BL at 8 mm below CEJ (P < 0.05). This signifies that there is more bone formation on the test side as compared to the control side after 3 months [Table 2 and Graph 2].
Table 2.
The difference in mean values between control group and test group after 3 months
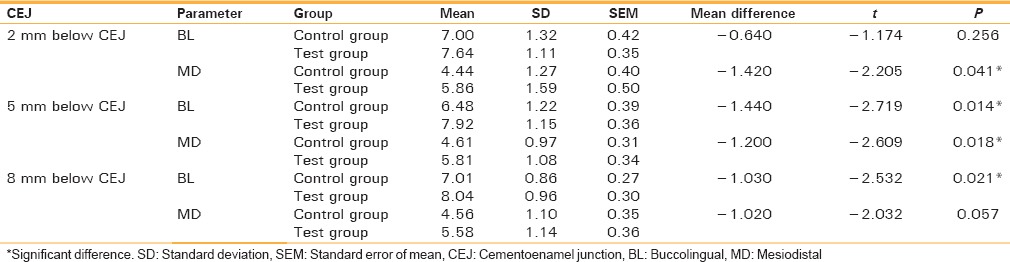
Graph 2.
The difference in mean values between control group and test group after 3 months
Analysis of the values after 6 months
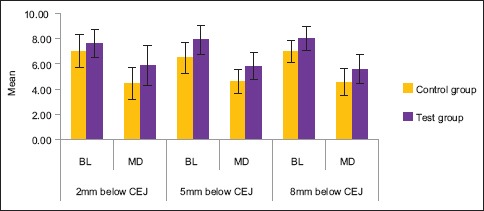
The difference in mean values between control group and test group was found to be statistically significant in MD at 2 mm below CEJ (P < 0.05), in BL at 5 mm below CEJ (P < 0.05), and in MD at 8 mm below CEJ (P < 0.01). This signifies that there is more bone formation on the test side as compared to the control side after 6 months [Table 3 and Graph 3].
Table 3.
The difference in mean values between control group and test group after 6 months
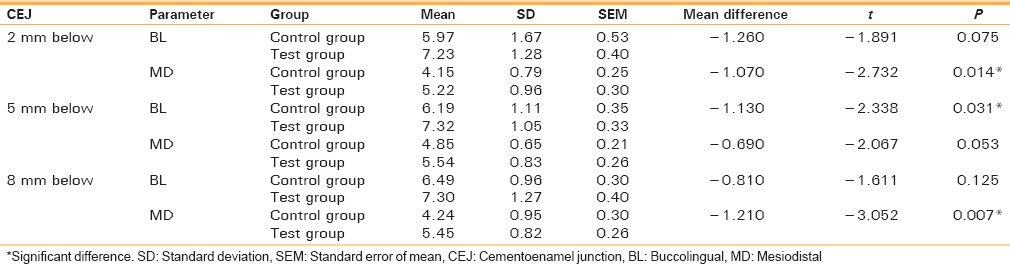
Graph 3.
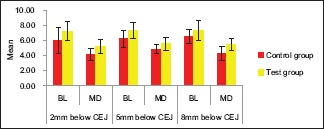
The difference in mean values between control group and test group after 6 months
DISCUSSION
Stem cells are one of the most growing arenas of biological tissue today. These cells are found in most multicellular organisms. Two types of stem cells include embryonic stem cells, which are found in the inner cell mass of blastocysts and can differentiate into all types of the specialized embryonic tissues and adult stem cells, which are found in adult tissues which are actually progenitor cells which help regulate the normal turnover of regenerative system such as blood, skin, or intestinal tissues. Today, stem cells can be cultivated through cell culture techniques and can be transformed into specialized cells such as muscles or nerves. Adult stem cells from umbilical cord blood and bone marrow are now commonly used in various medicinal and surgical therapies.
Embryonic stem cells
Embryonic stem cells are originated from the epiblastic tissue of the inner cell mass of a blastocyst. These cells are pluripotent and can develop into the all three primary germ layers ectoderm, endoderm, and mesoderm.
Adult stem cells
The adult stem cell is found in a developed organism. They have ability to divide and form similar cells and also to form more differentiated cells. Most adult stem cells are multipotent and are usually designated by their tissue origin such as endothelial stem adipose-derived stem cell and MSC.
Fetal stem cells
They are primitive cell types found in the organs of fetuses. Their classification is still unclear and is currently grouped into an adult stem cell. However, a more clear demarcation between the two cell types is required.
Amniotic stem cells
Amniotic stem cells are multipotent cells found in amniotic fluid and can differentiate into adipogenic, osteogenic, myogenic, and neuronal tissues. They are highly active, expand rapidly without feeders, and are not tumorigenic.
Induced pluripotent stem cells
These are not truly adult stem cells but are infact reprogrammed cells having pluripotent tendency using genetic reprogramming with protein transcription factors.
Unique properties of stem cells includes self-renewal
It is the capability to undergo various cell division cycles and maintaining its undifferentiated state. Potency: it is the ability to differentiate into all specialized cell types. Potency means the differentiation potential of the stem cell. Totipotency, pluripotency, multipotency, or unipotency are few terminology describing potency of stem cells. Expanded BMMSCs are pluripotent stem cells and can differentiate into a nearly all cells, i.e., cells derived from any of the three germ layers.
Healing of extraction sockets
The removal of tooth induces inflammation, epithelization, fibroplasia, and remodeling. When a tooth is extracted, the socket fills with blood that clots and seals off the socket from the oral environment. The 1st week of healing is characterized by inflammatory stage. Blood cells enter the socket to remove contaminating bacteria from the socket. During 1st week, there is growth of fibroblasts and capillaries. The epithelium migrates down the socket wall until it encounters the bed of granulation tissue. At the end of 1st week, osteoclasts survive along the crest of alveolar bone surrounding the socket. The 2nd week is potentiated by the large amount of granulation tissue that fills the socket. Collection of osteoid cells takes place along the alveolar bone proper in the socket. The process starts during the 2nd week and continues till the 3rd and 4th weeks of healing process and epithelization of most of the socket gets completed till this time.[7] There is continuous resorption of cortical bone from the crest and walls of the socket and new trabecular bone is formed down across the socket. Normally, extraction sockets heal uneventfully with bony tissue within 1–2 months after extraction. This healing process leads to reduction of the original height and width of the alveolar bone proper. This is evident by our results shown on the control side.
There are many bone grafting materials used in alveolar bone grafting procedures having different success prospective today. These include autogenous bone grafts such as iliac crest, rib, mandible chin and retromolar area, or maxillary tuberosities. Allogenic bone, bone graft substitutes such as tricalcium phosphate and porous hydroxyapatite. The limitations of bone grafting are limited resources, donor-site morbidity, irregular contour, transmission of disease, graft versus host response, suppression of immunity, and prolonged healing. To overcome these limitations and to preserve the socket width and height, stem cell-based tissue regeneration is an evolving and a promising technique for osseous repair.
Cells needed for bony healing are derived from periosteum, endosteum, and mesenchymal cells. Actions of bone forming (osteoblasts) and bone resorbing cells (osteoclasts) are coupled and regularized by molecular interactions between through the actions of the precursor cells of the osteoblast lineage and MSCs.[8]
MSCs are present in sufficient quantity in the skeletal tissues, but damaged bone may fail to heal spontaneously. Hence, a working MSC transplantation system should have a carrier with osteoconductive potential and an inductive microenvironment to support the natural property of bone regeneration.[9] MSCs from human adult bone marrow is a reliable source for skeletal regeneration due to their capacity for osteogenic differentiation potential through a well-defined pathway as suggested by Rebekka et al. in 2010. For stem cells to survive in the socket, the socket should be uninfected. For this reason, we selected patients requiring extraction for orthodontic purposes. In all the cases, selected tooth was uninfected and did not have periapical or periodontal pathology.
Three-dimensional scaffolds give support for cells attachment, growth, and differentiation. Collagen is one of the most promising biomaterials for bone tissue engineering and is the most abundant osteoinductive protein in the osteocyte environment. There is excellent cytocompatibility of collagen and BMMSC with collagen scaffolds. Collagen matrices have antigenic properties, smooth microgeometry, and transmural permeability along with easy application.[10] Statistically significant (P < 0.05) difference in bone formation between the test and control site 3 and 6 months postoperatively, buccolingually, and mesiodistally at three different levels (2 mm below CEJ, 5 mm below CEJ, and 8 mm below CEJ) was seen in our study. Our results suggest that there is more bone formation on the test side as compared to the control side. Our results support the data of Kakudo et al. who suggested that collagen is a suitable carrier for stem cell and bone formation.[11]
Anatomical origin of MSC induces the osteogenic potential of the cells. Zhang et al. demonstrated that bone marrow MSCs have more osteogenic potential as compared to MSC from umbilical cord and adipose tissue. For this reason, we selected expanded BMMSC for transplantation into extraction sockets.
After extraction, collagen membrane seeded with MSC was transplanted into the socket. MSCs are low-immunogenic both in vitro and in vivo, suggesting their utility for autologous as well as allogeneic transplantations. We selected allogenic transplantation in this study. MSCs undergo osteogenic differentiation, using osteoblastic markers, and secreting extracellular matrix and calcium deposits. MSC takes part in remodeling of matrix and has intrinsic paracrine activity, especially in wound environment These characteristics make them an interesting tool for wound healing applications. The osteogenic differentiation properties of MSC increase with the contact of collagen. MSC and osteoblast can develop bony matrix when cultured or implanted in suitable conditions. MSC releases proangiogenic factors that promotes new blood vessel formation and accelerates wound healing by promoting angiogenesis and cell differentiation. These properties of stem cells explain the statistically significant (P < 0.05) difference in bone formation between the test and control site 3 months and 6 months postoperatively. We found more bone formation on test side as compared to control side after 3 months and 6 months. Our results support the study of Riccardo d'Aquino who demonstrated that stem cells and collagen biocomplex can fully restore the third molar extraction bony defects.[12] Our results correlate with the study of ex vivo cultured and autologous bone marrow derived cell product and showed accelerated bone formation induced by autologous bone marrow cells.[13] The potential of the seeded cells to take host endothelial cells directly affects the vascularization of the scaffold and the mobilization of host progenitor cells toward the graft site. Our results are in conjunction with the review stated that critical-sized bone defects can be repaired by MSC.[14] Although various theories and studies have been given in medical literature which increases our understanding of bone biology, there is little data about the role of MSCs in bone turnover and fracture repair in vivo. Hence, it is important to study the in vitro nature of the stem cells. So as prediction for in vivo integration of the hybrid into the injured tissue through migration of cells and remodeling of matrix can be made.
CONCLUSION
Extracting socket healing process usually proceeds with substantial reduction of the original height and width of the alveolar bone proper, which in some cases may be compromised condition for an implant supported prosthesis. An objective method of maintaining width of alveolar ridge is using MSCs seeded on collagen membrane. Socket healing procedure using MSCs and collagen membrane was successful in maintaining width of alveolar socket.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Serino G, Biancu S, Iezzi G, Piattelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: A clinical and histological study in humans. Clin Oral Implants Res. 2003;14:651–8. doi: 10.1034/j.1600-0501.2003.00970.x. [DOI] [PubMed] [Google Scholar]
- 2.Throndson RR, Sexton SB. Grafting mandibular third molar extraction sites: A comparison of bioactive glass to a nongrafted site. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:413–9. doi: 10.1067/moe.2002.127582. [DOI] [PubMed] [Google Scholar]
- 3.Lucarelli E, Donati D, Cenacchi A, Fornasari PM. Bone reconstruction of large defects using bone marrow derived autologous stem cells. Transfus Apher Sci. 2004;30:169–74. doi: 10.1016/j.transci.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006;12:514–22. doi: 10.1111/j.1601-0825.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 5.Runyan CM, Taylor JA. Clinical applications of stem cells in craniofacial surgery. Facial Plast Surg. 2010;26:385–95. doi: 10.1055/s-0030-1265017. [DOI] [PubMed] [Google Scholar]
- 6.Zografou A, Tsigris C, Papadopoulos O, Kavantzas N, Patsouris E, Donta I, et al. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J Plast Reconstr Aesthet Surg. 2011;64:1647–56. doi: 10.1016/j.bjps.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Peterson LJ, Ellis E, III, Hupp JR, Tucker MR. Contemporary oral and maxillofacial surgery. 4th ed. London: St. Louis Mosby, Peterson; 2003. p. 55. [Google Scholar]
- 8.Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(Suppl 1):S26–32. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Ciapetti G, Ambrosio L, Marletta G, Baldini N, Giunti A. Human bone marrow stromal cells: In vitro expansion and differentiation for bone engineering. Biomaterials. 2006;27:6150–60. doi: 10.1016/j.biomaterials.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467–80. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 11.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84:191–7. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 12.d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 13.Gutwald R, Haberstroh J, Kuschnierz J, Kister C, Lysek DA, Maglione M, et al. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: Comparison with augmentation by autologous bone in adult sheep. Br J Oral Maxillofac Surg. 2010;48:285–90. doi: 10.1016/j.bjoms.2009.06.226. [DOI] [PubMed] [Google Scholar]
- 14.Tortelli F, Tasso R, Loiacono F, Cancedda R. The development of tissue-engineered bone of different origin through endochondral and intramembranous ossification following the implantation of mesenchymal stem cells and osteoblasts in a murine model. Biomaterials. 2010;31:242–9. doi: 10.1016/j.biomaterials.2009.09.038. [DOI] [PubMed] [Google Scholar]


