Abstract
A Ser105Ala mutant of the lipase B from Candida antarctica enables ‘perhydrolase-only’ reactions. At the example of the chemoenzymatic Baeyer-Villiger oxidation of cyclohexanone, we demonstrate that with this mutant selective oxidation can be achieved in deep eutectic solvent while essentially eliminating the undesired hydrolysis reaction of the product.
In 1899, Adolf von Baeyer and Victor Villiger first reported the transformation of ketones into esters or lactones using peracids1. Ever since then the Baeyer-Villiger (BV) oxidation represents one of the most-studied reactions in organic synthesis2,3. Particularly, the formation of ε-caprolactone is of interest due to its importance as polymer building block4. Commonly, peracids such as m-chloroberbenzoic acid or peracetic acid are used as stoichiometric reagents in BV-oxidation5,6,7.
Due to the poor atom efficiency of this methodology catalytic methods are now in focus of current research. Highly stereoselective BV-oxidations using so-called Baeyer-Villiger Monooxygenases (BVMO) are known8,9,10,11,12,13. However, due to their cofactor-dependency and sometimes low intrinsic stability, BVMOs are not yet practical catalysts14. In addition, BVMOs also can suffer from product inhibition impairing their catalytic efficiency. Bornscheuer and coworkers solved this by the lipase-catalyzed oligomerization of the corresponding lactones which may not always be the desired solution (product)3. Alternatively, chemoenzymatic BV-oxidations using hydrolases are gaining relevance in organic synthesis15. These systems utilize hydrolase-catalyzed formation of per acids (in a promiscuous ‘perhydrolase’ activity) followed by spontaneous BV-oxidation (Fig. 1).
Figure 1. The chemoenzymatic BV-oxidation.
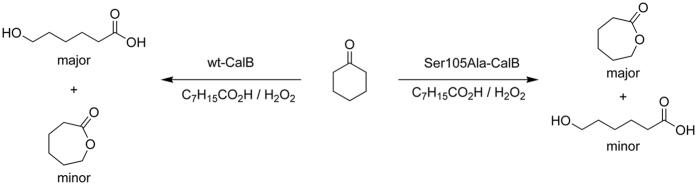
The hydrolase mediates the perhydrolysis reaction of octanoic acid to peroctanoic acid, which then spontaneously transforms cyclohexanone to ε-caprolactone (desired reaction). Hydrolysis of the lactone product, catalyzed by the hydrolase, represents the undesired side-reaction.
One major drawback of this approach however lies with the intrinsic activity of hydrolases with the products of interest. Esters are preferentially hydrolyzed by hydrolases leading to the undesired formation of ω-hydroxy acids. Hence, use of simple (aqueous) H2O2 is not possible but anhydrous reagents such as urea-H2O2 adducts have to be used16.
Recently, we have reported a mutant of the lipase from Penicillium camembertii where in the catalytic triad was disrupted by mutating Ser145 to an alanine17. Obviously this mutant showed no more hydrolase activity but some residual perhydrolase activity. The proposed mechanism for the Ser145Ala mutant involves activation of H2O2 by the remaining histidine17. In continuation of this work we suggested that a similar mutant of the well-known lipase B from Candida Antarctica (CalB) might exhibit a similar reaction pattern. This mutant has been investigated in detail by Hult and coworkers for various ‘unnatural’ reactions such as aldol18 and Michael-type reactions on α,β-unsaturated carbonyl groups19,20. Therefore, we synthesized the analogous CalB mutant missing the catalytic serine residue of the catalytic triad (Ser105) and replaced it by an alanine.
Results and Discussion
The mutant enzyme was heterologously expressed and purified to homogeneity (as judged by SDS gel analysis, Fig. SI). Then, we proceeded with the evaluation of Ser105Ala for selective BV-oxidation of cyclohexanone. It is worth mentioning that control experiments using a thermally inactivated Ser105Ala (under otherwise identical conditions) revealed no significant Baeyer-Villiger oxidation activity. Also, no conversion was observed in the absence of free carboxylic acids (data not shown).
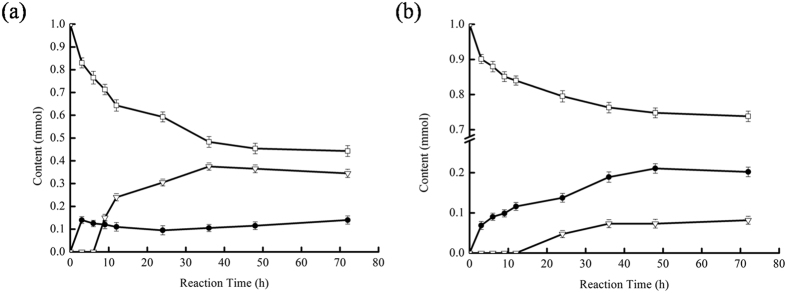
Quite expectedly, Ser105Ala exhibited almost no hydrolytic activity for example on ε-caprolactone whereas the wild type enzyme smoothly hydrolyzed this substrate (Fig. S2). Encouraged by this we proceeded to the chemoenzymatic BV-oxidation of cyclohexanone comparing both enzyme variants (Fig. 2).
Figure 2.
Products formation of ε-caprolactone (●) and 6-hydroxycaproic acid (△) and reducing of cyclohexanone (□) using wt-CalB (a) and Ser105Ala (b). General conditions: cyclohexanone (1 mmol), 30% aq. H2O2 (2 mmol), octanoic acid (1 mmol), 5 mg CalB (wild-type or S105A), H2O (1 mL), n-hexane (2 mL). T = 40 °C.
Surprisingly, the ε-caprolactone formation rate was only two times higher in case of wt-CalB as compared to the Ser105Ala mutant. Furthermore, accumulation of ε-caprolactone continued for at least 48 h in case of the mutant whereas it stopped after 3 h using the wt-CalB. One plausible explanation for this may be acidification of the reaction medium due to lactone hydrolysis.
More importantly, the acid to lactone ratio was inverted from 2:1 (in case of the wt-CalB) to 1:2.5 in case of the Ser105Ala-mutant. In other words, the selectivity of the overall reaction was improved about 5-fold. The hydrolysis product observed in case of the Ser105Ala-reaction can most likely be attributed to spontaneous hydrolysis of the ε-caprolactone under the current reaction conditions. Indeed, control reactions testing the stability of ε-caprolactone in the reaction medium gave essentially a comparable hydrolysis rate (Fig. S2). Therefore, we are confident that this issue can be overcome in future research by e.g. in situ extraction of the lactone into a suitable organic phase thereby preventing the undesired hydrolysis.
It should be mentioned that, both the wt-CalB and Ser105Ala exhibited comparably poor stability under the reaction conditions chosen in Fig. 1. Essentially, after 48 h no more product formation was observable, which we attribute to a loss in catalytic activity of the biocatalyst used.
Inspired by a recent contribution reporting the beneficial effect of deep eutectic solvent (DES) on the activity and stability of lipase21, we decided to evaluate a range of DES as alternative solvents for the chemoenzymatic BV-oxidation of cyclohexanone (Table 1).
Table 1. BV-oxidation of cyclohexanone by wt-CalB and Ser105Ala in various DESs.
| Entry | Solvent | Conversion (%) |
|
|---|---|---|---|
| wt-CalB | Ser105Ala | ||
| 1 | Water: n-hexane (1:2) | 55 | 24 |
| 2 | ChCl: urea (1:2) | 76 | 26 |
| 3 | ChCl: ethanediol (1:2) | 81 | 29 |
| 4 | ChCl: glycerol (1:2) | 85 | 33 |
| 5 | ChCl: xylitol (1:1) | 89 | 40 |
| 6 | ChCl: sorbitol (1:1) | 92 | 47 |
General conditions: cyclohexanone (1 mmol), 30% aq. H2O2 (2 mmol), octanoic acid (1 mmol), 5 mg CalB (wild-type or S105A), DES (1.2 g), H2O (0.3 mL). T = 40 °C, time 48 h.
Pleasingly, using DES generally improved the overall conversion of cyclohexanone. Particularly, ChCl/sorbitol increased the product formation by almost a factor of two, both for the wt- and the mutant enzyme. Again, control reactions with thermally inactivated enzymes yielded no detectable conversion of the starting material.
Encouraged by this, we further elucidated ChCl/sorbitol as ‘performance additive’ for a range of chemoenzymatic BV-oxidations (Table 2).
Table 2. Substrate Scope of wt-CalB and Ser105Ala catalyzed BV-oxidation.
| Entry | Substrate | Product | wt-CalB |
Ser105Ala |
||
|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | |||
| 1 | 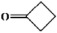 |
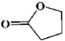 |
99 | 93 | 99 | 100 |
| 2 | 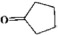 |
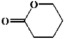 |
95 | 48 | 51 | 97 |
| 3 | 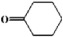 |
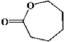 |
92 | 46 | 47 | 99 |
| 4 | 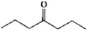 |
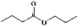 |
79 | 35 | 38 | 96 |
General conditions: ketone (1 mmol), 30% aq. H2O2 (2 mmol), octanoic acid (1 mmol), 5 mg CalB (wild-type or S105A), ChCl/sorbitol (1.2 g), H2O (0.3 mL). T = 40 °C, time 48 h.
The results shown in Table 2, confirm our previous observation that Ser105Ala enables significantly more selective reactions. In essence, hydrolysis was not observed with Ser105Ala. Interestingly, however, also the DES appeared to have a beneficial effect on the chemoselectivity by suppressing the undesired hydrolysis reaction. Currently, we are lacking a plausible explanation for this phenomenon. Possibly, the DES also influenced the water activity.
In conclusion, Ser105Ala indeed is a ‘perhydrolase only’ enzyme. Expectedly, removal of the catalytic triad Ser eliminated the enzyme’s hydrolytic activity. However, the perhydrolase activity was largely maintained. Admittedly, the current mutant is not suitable for large-scale preparative applications and further improvements of the specific enzyme activity via structure-guided protein engineering are currently underway in our laboratory. Despite the preliminary character of this study the product to enzyme ratio achieved was already roughly 10:1. Therefore, we are confident that a combination of enzyme- and reaction-engineering will result in a practical protocol for the synthesis of lactones.
Experimental Section
Material
Escherichia coli DH5α and plasmid pGAPZαA (Invitrogen, USA) were used as cloning host and vector, respectively. Pichia pastoris X-33 (Life technology, China) strain was used for expression. Polymerase and DNA restriction endonuclease were purchased from Takara biotechnology Co. Ltd (Dalian, China). Chemicals used in this study were purchased from Aladdin® Chemistry Co. Ltd (Shanghai, China) at the highest purity available.
CalB cloning and mutagenesis
The codon optimized the lipase B from Candida Antarctica (CalB) gene was synthesized by Shenggong biotechnology company (Shanghai, China). The resulted CalB gene was inserted into the pGAPZαA plasmid to get the expression vector pGAPZαA-CalB. Site-directed mutagenesis was carried out by site-directed mutagenesis following the QuikChange protocol (Stratagene, USA). Primers for mutant construction were designed by QuikChange Primer Design. The Ser105Ala mutant was confirmed by DNA sequencing in BGI (Shenzhen, China).
Protein Expression and Purification
Expression of CalB and mutant was performed as described previously17. They purified using Ion Exchange Chromatography (DEAE-Sephadex, GE Healthcare, China) and freeze dried. The purified proteins were analyzed by SDS-PAGE. Protein concentrations were determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc, USA).
Preparation of DESs
The deep eutectic solvents (DESs) were prepared according to the method22. The corresponding solid components of the desired DESs in the correct proportion were placed in a 250 mL round-bottom flask. The mixtures were heated at 100 °C under rotary evaporation until a homogeneous transparent liquid was formed.
Baeyer-Villiger oxidation reaction in water/n-hexane
Reactions mixture contained cyclohexanone (1 mmol), octanoic acid (1 mmol), n-hexane (2 mL), phosphate buffer (20 mmol pH 6.0, 1 mL) and lipase (wt-CalB, Ser105Ala or thermally inactivated CalB, 5 mg), 30% aq. H2O2 was added in 10 portions at 5 h intervals (total 2 mmol). The reaction was carried out at 40 °C for 72 h with magnetic stirring at 500 rpm. Extraction of the sample was done with ethyl acetate and removed water by anhydrous Na2SO4.
Baeyer-Villiger oxidation reaction in DESs
Reactions mixture contained ketone (1 mmol), octanoic acid (1 mmol), DES (1.2 g), H2O (0.3 mL) and lipase (wt-CalB or Ser105Ala, 5 mg), 30% aq. H2O2 was added in 10 portions at 5 h intervals (total 2 mmol). The reaction was carried out at 40 °C for 48 h. Other reaction conditions and the sample treated method as above.
Hydrolysis of ε-caprolactone in reaction medium
To verify whether Ser105Ala could hydrolyze ε-caprolactone in the reaction medium, ε-caprolactone (1 mmol), octanoic acid (1 mmol), n-hexane (2 mL), phosphate buffer (20 mmol pH 6.0, 1 mL) and lipase (wt-CalB, Ser105Ala or thermally inactivated CalB, 5 mg) were mixed in 10 mL conical flask. Then 30% aq. H2O2 was added in 10 portions at 5 h intervals (total 2 mmol). The mixture was carried out at 40 °C for 24 h with magnetic stirring at 500 rpm. The thermally inactivated CalB was prepared by boiling CalB in water for 2 h. Extraction of the sample was done with ethyl acetate and removed water by anhydrous Na2SO4.
Compounds Analysis
Gas chromatographic analyses were carried out with an Agilent Technology model 7890 GC-instrument equipped with a WAX 30 m × 0.25 mm × 2.0 μm column. A temperature program was used to keep the samples in a column oven at 60 °C for 1 min, then increased to 113 °C at 5 °C/min, increased to 190 °C at 20 °C/min, increased to 240 °C at 10 °C/min for 5 min. The split ratio was 30:1. The injector and the flame ionization detector temperatures were set at 250 and 280 °C, respectively. Peaks in GC chromatograms were identified by comparison of their retention times with reference standards.
Calculations
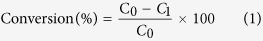 |
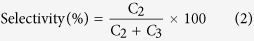 |
where C0 (mM) refers to the concentration of ketones (cyclobutanone, cyclopentanone, cyclohexanone and 4-heptanone) at t = 0 h; C1 (mM) refers to ketones at t = 48 h. C2 (mM) refers to the concentration of lactones and ester (γ-butyrolactone, δ-valerolactone, ε-caprolactone and propyl butyrate); C3 (mM) refers to the concentration of acids (6-hydroxycaproic acid, 5-hydroxyvaleric acid, 4-hydroxy-butanoic acid and butyrate).
Additional Information
How to cite this article: Wang, X.-P. et al. Engineering a lipase B from Candida antactica with efficient perhydrolysis performance by eliminating its hydrolase activity. Sci. Rep. 7, 44599; doi: 10.1038/srep44599 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank Professor Junpeng Zhao for the useful discussion for this manuscript. This work was supported by National Natural science foundation of China (31471690), National High Technology Research and Development Program of China (863 program, 2014AA093514, 2014AA093601) and Science and Technology Planning project of Guangdong province (2014B020204003, 2015B020231006).
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.-H.W. conceived the idea. X.-P.W. and P.-F.Z. performed the data collection. Z.-G.L. analyzed the results. F.H. wrote the manuscript. All authors participated in the revising of the manuscript.
References
- Renz M. & Meunier B. 100 Years of Baeyer-Villiger oxidations. European J. Org. Chem. 4, 737–750 (1999). [Google Scholar]
- ten Brink G. J., Arends I. W. C. E. & Sheldon R. A. The Baeyer-Villiger reaction: New developments toward greener procedures. Chem. Rev. 104, 4105–4123 (2004). [DOI] [PubMed] [Google Scholar]
- Schmidt S. et al. An enzyme cascade synthesis of ε-caprolactone and its oligomers. Angew. Chem. Int. Ed. 54, 2784–2787 (2015). [DOI] [PubMed] [Google Scholar]
- Woodruff Maria A. & Hutmacher D. W. The return of a forgotten polymer: Polycaprolactone in the 21st century. Prog. Polym. Sci. 35, 1217–1256 (2010). [Google Scholar]
- Okuno Y. Theoretical Investigations of the Mechanism of the Bayer-Villiger Reaction in Nonpolar Solvents. Chem. Eur. J. 3, 212–218 (1997). [DOI] [PubMed] [Google Scholar]
- Hickman Z. L., Sturino C. F. & Lachance N. A concise synthesis of 3-hydroxyindole-2-carboxylates by a modified Baeyer–Villiger oxidation. Tetrahedron Lett. 41, 8217–8220 (2000). [Google Scholar]
- Jiménez-Sanchidrián C. & Ruiz J. R. The Baeyer-Villiger reaction on heterogeneous catalysts. Tetrahedron 64, 2011–2026 (2008). [Google Scholar]
- Stewart J. D. Cyclohexanone Monooxygenase: A Useful Reagent for Asymmetric Baeyer-Villiger Reactions. Curr. Org. Chem. 2, 195–216 (1998). [Google Scholar]
- Fink M. J., Rudroff F. & Mihovilovic M. D. Baeyer-Villiger monooxygenases in aroma compound synthesis. Bioorganic Med. Chem. Lett. 21, 6135–6138 (2011). [DOI] [PubMed] [Google Scholar]
- Liu J. & Li Z. Cascade biotransformations via enantioselective reduction, oxidation, and hydrolysis: Preparation of (R)-δ-lactones from 2-alkylidenecyclopentanones. ACS Catal. 3, 908–911 (2013). [Google Scholar]
- Rodríguez-Mata M. et al. Baeyer-Villiger monooxygenase-catalyzed desymmetrizations of cyclobutanones. Application to the synthesis of valuable spirolactones. Tetrahedron 72, 7268–7275 (2016). [Google Scholar]
- Kara S., Bornadel A., Hatti-Kaul R. & Hollmann F. A bi-enzymatic convergent cascade for ε-caprolactone synthesis employing 1,6-hexanediol as a ‘double-smart cosubstrate’. ChemCatChem 7, 2442–2445 (2015). [Google Scholar]
- Parra L. P., Acevedo J. P. & Reetz M. T. Directed evolution of phenylacetone monooxygenase as an active catalyst for the baeyer-villiger conversion of cyclohexanone to caprolactone. Biotechnol. Bioeng. 112, 1354–1364 (2015). [DOI] [PubMed] [Google Scholar]
- Balke K., Kadow M., Mallin H., Saß S. & Bornscheuer U. T. Discovery, application and protein engineering of Baeyer–Villiger monooxygenases for organic synthesis. Org. Biomol. Chem. 10, 6249–6265 (2012). [DOI] [PubMed] [Google Scholar]
- Chávez G., Hatti-Kaul R., Sheldon R. A. & Mamo G. Baeyer-Villiger oxidation with peracid generated in situ by CaLB-CLEA catalyzed perhydrolysis. J. Mol. Catal. B Enzym. 89, 67–72 (2013). [Google Scholar]
- Ríos M. Y., Salazar E. & Olivo H. F. Baeyer-Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea-hydrogen peroxide in ethyl acetate. Green Chem. 9, 459–462 (2007). [Google Scholar]
- Tang Q. et al. Lipase-Driven Epoxidation Is A Two-Stage Synergistic Process. ChemistrySelect 1, 836–839 (2016). [Google Scholar]
- Branneby C., Carlqvist P., Magnusson A., Hult K. & Brinck T. Carbon - Carbon Bonds by Hydrolytic Enzymes. J. Am. Chem. Soc. 125, 874–875 (2003). [DOI] [PubMed] [Google Scholar]
- Svedendahl M., Hult K. & Berglund P. Fast Carbon - Carbon Bond Formation by a Promiscuous Lipase. J. Am. Chem. Soc. 127, 17988–17989 (2005). [DOI] [PubMed] [Google Scholar]
- Svedendahl M. et al. Direct epoxidation in Candida antarctica lipase B studied by experiment and theory. ChemBioChem 9, 2443–2451 (2008). [DOI] [PubMed] [Google Scholar]
- Kim S. H. et al. Effect of deep eutectic solvent mixtures on lipase activity and stability. J. Mol. Catal. B Enzym. 128, 65–72 (2016). [Google Scholar]
- Zeng C. X., Qi S. J., Xin R. P., Yang B. & Wang Y. H. Enzymatic selective synthesis of 1,3-DAG based on deep eutectic solvent acting as substrate and solvent. Bioprocess Biosyst. Eng. 38, 2053–2061 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.