Abstract
The aim of this study was to analyze the effects of oral consumption of curcumin and piperine in combination on the recovery kinetics after exercise-induced muscle damage. Forty-eight hours before and following exercise-induced muscle damage, ten elite rugby players consumed curcumin and piperine (experimental condition) or placebo. A randomized cross-over design was performed. Concentric and isometric peak torque for the knee extensors, one leg 6 seconds sprint performance on a non-motorized treadmill, counter movement jump performance, blood creatine kinase concentration and muscle soreness were assessed immediately after exercise, then at 24h, 48h and 72h post-exercise. There were moderate to large effects of the exercise on the concentric peak torque for the knee extensors (Effect size (ES) = -1.12; Confidence interval at 90% (CI90%): -2.17 to -0.06), the one leg 6 seconds sprint performance (ES=-1.65; CI90% = -2.51to -0.80) and the counter movement jump performance (ES = -0.56; CI90% = -0.81 to -0.32) in the 48h following the exercise. There was also a large effect of the exercise on the creatine kinase level 72h after the exercise in the control group (ES = 3.61; CI90%: 0.24 to 6.98). This decrease in muscle function and this elevation in creatine kinase indicate that the exercise implemented was efficient to induce muscle damage. Twenty four hours post-exercise, the reduction (from baseline) in sprint mean power output was moderately lower in the experimental condition (-1.77 ± 7.25%; 1277 ± 153W) in comparison with the placebo condition (-13.6 ± 13.0%; 1130 ± 241W) (Effect Size = -1.12; Confidence Interval 90%=-1.86 to -0.86). However, no other effect was found between the two conditions. Curcumin and piperine supplementation before and after exercise can attenuate some, but not all, aspects of muscle damage.
Key points.
When the recovery period between competitions was short, a curcumin and piperine supplementation could be an effective recovery strategy to attenuate muscle damage through limiting some loss of muscle function.
The recovery in sprint mean power output was moderately faster in the condition where the players consumed curcumin and piperine rather than placebo. This supplementation did not have any effect on muscle soreness neither on blood concentration in creatine kinase.
Key words: Anti-inflammatory, anti oxidant, team sport, nutrition
Introduction
An increased antioxidant status leads to the reduction of oxidative stress caused by the production of reactive oxygen species during the inflammatory process (Myburgh, 2014). Foods with high antioxidant or anti-inflammatory properties have been studied with conflicting results on recovery kinetics (Connolly et al., 2006; Howatson et al., 2009; Goldfarb et al., 2011). While some studies show a positive effect of the consumption of cherries and berries with a high content in polyphenols (Connolly et al., 2006; Bowtell et al., 2011; McLeay et al., 2012), others have failed to find significant effects of antioxidant supplementation on recovery kinetics (Bryer and Goldfarb, 2006; Bailey et al., 2011). These differences in results could be linked to the different polyphenol content of the supplementation used. A recent study showed that two supplements with the same antioxidant capacity, but with different polyphenol content, can exert contrasting effects following exercise-induced muscle damage (McLeay et al., 2012). Curcumin is rich in polyphenols and shows high anti-inflammatory and antioxidant abilities (Basnet and Shalko-Basnet, 2011) through a decrease in the expression of Nuclear Factor-Kb (NF-Kb) which is linked to the expression of pro-inflammatory genes (Singh and Aggarwal, 1995). Curcumin leads to a decrease in the activity of Cyclooxygenase-2 (COX-2) and 5-Lipoxygenase, which are pro-inflammatory enzymes (Menon and Sudheer, 2007). It also has an effect on the synthesis and the activity of the pro-inflammatory cytokine Tumor Necrosis Factor-Alpha TNF-α), and reduces the activity of m-prostaglandin E2 synthase-1 (Koeberle, 2009). Some studies have analyzed the effect of curcumin on the delayed onset of muscle soreness (Tanabe et al., 2015) or the effect of a highly bioavailable form of curcumin on the muscle function recovery kinetics (Drobnic et al., 2014) following exercise-induced muscle damage. The curcumin alone shows a very low bioavailability, however, piperine, the active component of black pepper, has been shown to enhance the bioavailability of curcumin by 2000% in humans (Shoba et al., 1998) and also presents antioxidant properties, with a radical oxygen species scavenging ability and through inhibition of lipid peroxidation (Srinivasan, 2014).
To our knowledge, no study has examined the effects of a mix of curcumin and piperine on the recovery kinetics following exercise-induced muscle damage. The aim of this study was to analyze the effects of combined curcumin and piperine supplementation on the muscle function recovery kinetics after exercise-induced muscle damage.
Methods
Participants
Sixteen elite level rugby players (age: 20.7 ± 1.4 y; height: 1.82 ± 0.06 m; mass: 89.4 ± 14.8 kg) with a weekly training volume based on rugby and gym training of 13 ± 4 h took part in this study. All participants were fully informed of the purpose, benefits and risks involved with participation before giving written informed consent. This study was conducted in accordance with the local ethical committee on biomedical research (N° 5915052012) and the standards of the declaration of Helsinki. Prior to the experimentation, players completed an assessment to verify the following inclusion criteria : (1) had not been injured during the previous 2 months, (2) were not taking any medication/drug, (3) were not participating in any session during the 2 days prior to and during the experiment, (4) were not using any recovery strategy, (5) respected the nutritional recommendations established by the investigators with the help of a dietician. According to these criteria, 6 players among the 16 initial were excluded, as they did not follow the inclusion criteria.
Protocol
A randomized, balanced cross-over design was used, which comprised two phases of 4 days ; these were separated by 15 days. The participants were divided into 4 groups: 1) dominant leg and curcumin + piperine supplementation, 2) non-dominant leg + curcumin + piperine supplementation, 3) dominant leg + placebo and 4) non-dominant leg + placebo in a randomized and balanced way (Table 1). Supplementation was blinded: placebo versus pills containing curcumin and piperine (Table 1).
Table 1.
Design of the randomized, balanced cross-over design according to the condition (curcumin + piperine versus placebo) and the legs (dominant versus non-dominant).
| First session | Second session | |
|---|---|---|
| Group 1 | Dominant leg–curcumin + piperine | Nondominant leg– placebo |
| Group 2 | Non dominant leg–curcumin + piperine | Dominant leg– placebo |
| Group 3 | Dominant leg– placebo | Nondominant leg– curcumin + piperine |
| Group 4 | Nondominant leg– placebo | Dominant leg–curcumin + piperine |
In the experimental condition, the participants consumed 2g of curcumin and 20mg of piperine, 3 times a day (MGD Nature, Brandérion, France), starting 48 h pre-exercise and continuing until 48 h post-exercise. This ratio enables the bioavailability of the curcumin to be increased (Shoba et al., 1998). In the control condition, the subjects were asked to take a placebo (glucose pills) at the same time of the day. The daily supplementation was divided into 3 intakes, every 6h between 8 am and 10 pm except on the exercise day. On the exercise day, the first intake was taken 45 min before the exercise, as it has been shown that with piperine, the highest curcumin availability is reached 45 min following consumption (Shoba et al., 1998) The second intake was taken immediately after the exercise, and the last intake was consumed 6h after the second one. Fifteen days following the first session, the participants were evaluated on the other leg and in the other condition, at the same time as during the first session.
Before each session, the participants performed a cycling warm up lasting 6 minutes, with an increasing intensity standardized using the Borg perceived intensity scale (Borg, 1982): 2 minutes at a perceived intensity of “9” (very light), 2 minutes at a perceived intensity of “11” (light) and 2 minutes at a perceived intensity of “13” (somewhat hard). On the first day of each period, the participants performed baseline testing, and then the exercise task, and were evaluated on the different tests immediately after, then 24h, 48h and 72h post-exercise.
The exercise inducing muscle damage task comprised 25 repetitions over 25m of one leg jumps on an 8% downhill slope. Each repetition was separated by 90 s. The participants were asked to cover the 25m as fast as possible and to stop in a pre defined zone of 3.5m at the end of the 25m slope.
The timing of the supplementation, the tests and the exercise task are summarized in Figure 1.
Figure 1.
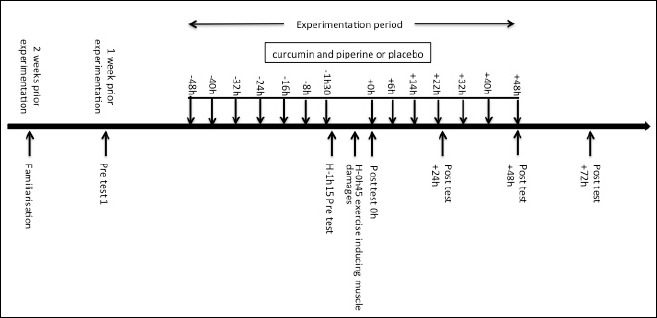
Timing of the supplementation, the exercise task and the pre- and post-tests.
For all tests, equipment calibration, participant posture, starting position and use of verbal encouragement was standardized.
Muscle function was assessed during the pre test familiarization session, just before exercise, then immediately, 24 h, 48 h and 72 h following exercise. The concentric peak torque of the knee extensors at 60°·s-1 (N·m-1) (CV=3.2%; ICC=0.98) and the isometric peak torque at 60° (N) (CV=5.5%; ICC=0.97) (Maffiuleti et al., 2007) were assessed on an isokinetic dynamometer (Con-Trex, Duebendorf, Switzerland). The position on the isokinetic dynamometer was standardized, the athltetes were seated with a hip flexion set at 75°. Full extension of the leg was considered as 0° and the range of motion for the test was set at 90° (0-90°). The position of the distal shin pad was standardized and set at 4 cm from the external malleolus. The isometric peak torque was performed at 60° of knee flexion, following the protocol of Maffiuletti et al. (2007). The mean power developed on a one leg 6 seconds sprint (W) on a calibrated non-motorized treadmill (Woodway Force 3.0, Waukesha, USA) was also measured (ICC = 0.94; CV = 7.9%). For this test, the subjects were asked to put the foot of the non tested leg on the side of the treadmill and to push rearward on the treadmill as fast as possible with the other leg during 6 seconds (exactly as a skateboarding movement). The height of a one leg counter movement jump (CMJ) (cm) was assessed with a force platform (1000 Hz sampling rate, Kistler 9260AA6, Wintherthur, Switzerland) (ICC = 0.98; CV = 6.3%). Each participant performed 3 jumps on each session and the best performance was retained. Total blood creatine kinase concentration was assessed by taking and analysing 30 µL of blood, using a Reflotron (Reflotron Plus, Roche, Rotkreuz, Switzerland) (CV = 3.1%) (Horder and Jorgensen, 1991). The general muscle soreness and specific quadriceps level of soreness were also assessed using the Hooper Scale from 0 to 7 (Hooper et al., 1995).
Statistical analyses
The results are presented as mean ± standard deviation (SD). Effect sizes (ES), intraclass correlation coefficients (ICC) and coefficients of variation (CV) were calculated to assess the magnitude of the differences between the two reliability tests and between the two conditions. A 95% confidence interval was used for the ES, CV, and ICC for the reliability tests. The ES was calculated for the effect of the exercise task on muscle function using the difference in the absolute results in pre-exercise and post-exercise tests, and for the effect of the condition on muscle function from differences in the relative loss in the two conditions. When the 95% confidence interval of the ES included 0, CV ≤ 15% and ICC ≥ 0.8, the reliability of the outcomes was considered as good (Hopkins, 2013, Atkinson and Nevill, 1998; Stokes and Abery, 1980). The criteria defined to analyze the ES magnitude were: 0.0 – 0.2 trivial; 0.2 – 0.6 small; 0.6 – 1.2 moderate; 1.2 – 2.0 large; 2.0 – 4.0 very large; >4.0 nearly perfect (Hopkins, 2013). To evaluate the differences between the placebo and experimental conditions, the ES was used only if the 90% confidence interval did not include 0 (Hopkins, 2001).
Results
Reliability
The reliability of each outcome measure was evaluated using the results of the two pre-tests. The concentric peak torque showed a good reliability (ES = 0.38, CI95%: -0.02 to 0.78; CV = 18% and CCI = 0.91), the isometric peak torque a moderate reliability (ES = 0.18, CI95%-0.41 to 0.76 ; CV = 16.5% and CCI = 0.75), the 6 s sprint mean power a good reliability (ES = 0.12 CI95% : -0.29 to 0.54 ; CV = 8.9% ; CCI = 0.87) and the CMJ height a good reliability (ES=0.30, CI95% = 0.12 to 0.47 ; CV = 14.3% and CCI = 0.97).
There was no effect of condition in the pre-test, for any of the outcomes measured.
Effect of time
The effects of time on the different outcomes are summarized in Table 2.
Table 2.
Effect of time on the different outcomes measured [Mean±SD and ES (CI95%)].
| POST-0h | POST-24h | POST-48h | POST-72h | |
|---|---|---|---|---|
| Concentric Peak Torque Experimental | -12.3 ± 10.5%* -.53 (-.84 to -.22) |
-6.8 ± 7.12%* -.46 (-.74 to -.18) |
- 7.2 ± 10.8%* -.25 (-1.26 to -.04) |
-5.3 ± 9.0% -.41 (-.86 to .04) |
| Concentric Peak Torque Control | -8.1 ± 10.9%* -.55 (-.96 to -.15) |
-9.0 ± 13%* -.66 (-1.15 to -.17) |
-8.2 ± 16.9%* -.94 (-1.89 to .00) |
-3.4 ± 16.9% -.75 (-2.14 to 0.63) |
| Isometric Peak Torque Experimental | -15.9 ± 11%* -1.34 (-1.90 to -0.77) |
-5.5 ± 5.9%* -.51 (-.82 to -.19) |
-5.9 ± 11.3%* -.70 (-1.43 to .03) |
-2.3 ± 9.6% .14 (-.26 to .54) |
| Isometric Peak Torque Control | -15.0 ± 7.9%* -1.29 (-1.78 to -0.80) |
-4.7 ± 6.6%* -.41 (-.78 to -.04) |
-4.5 ± 11.1% -.44 (-0.99 to0.11) |
-0.1 ± 11 % -.09 (-.65 to .47) |
| CMJ Experimental | -15.0 ± 12,1 %* -1.11 (-1.83 to -0.81) |
-7.9 ± 14.2 %* -.36 (-.70 to -.03) |
-8.87 ± 17.2 % -.44 (-.94 to .06) |
-4.28 ± 14.9 % -.18 (-.56 to .20) |
| CMJ Control | -16.7 ± 10.3 %* -.57 (-.76 to -.39) |
-12.2 ± 8.1 %* -.46 (-.65 to -.26) |
-13.9 ± 10.1 %* -.51 (-.75 to -.27) |
-8.9 ± 17.4 %* -.39 (-.66 to -1.12) |
|
Mean Power Output Experimental |
-4.1 ± 12.6% -.36 (-.96 to .23) |
-1.8 ± 7.3 % -.19 (-.52 to .15) |
-16.2 ± 14 %* -1.25 (-1.89 to -.62) |
-1.6 ± 16.1 -.17 (-.94 to .59) |
|
Mean Power Output Control |
-2.6 ± 8.1 % -.29 (-.75 to .16) |
-13.6 ± 13 %* -1.03 (-1.58 to -.48) |
-22.9 ± 24.6 % -1.73 (-.80 to 2.51) |
-5.6 ± 15.1 % -1.61 (-3.67 to 0.44) |
| Muscle Soreness Experimental | +151.5 ± 165 %* 3.39 (.92 to 5.87) |
+168.8 ± 179%* 2.06 (.96 to 3.16) |
+83.2± 129 % .89 (-.32 to 2.11) |
|
|
Muscle Soreness Control |
+143.3 ± 159.5 %* 1.43 (.55 to 2.18) |
+106.7 ± 106.0 %* 2.26 (.66 to 3.87) |
+68.3 ± 101.7 % 1.48 (-.42 to 3.38) |
|
|
Creatine Kinase Exprimental |
+132.3 ± 191.5 %* 3.39 (.92 to 5.87) |
+81.1 ± 117 %* 2.06 (.96 to 3.16) |
+77.4 ± 121 % .89(-.32 to 2.11) |
|
|
Creatine Kinase Control |
+ 211.3 ± 331.3 %* 1.43 (.55 to 2.18) |
+221.2 ± 496.1%* 2.26 (.66 to 3.87) |
+559.4 ± 1131 % -.42 to 3.38) |
* Effect of the time on the outcome measured with a confidence interval which does not include 0.
There was a moderate loss of performance in concentric peak torque up to 48h after exercise in both control (ES = -1.12; CI90%: -2.17 to -0.06) and experimental (ES = -0.74; CI90%: -0.74 to -0.04) conditions, but no effect of the exercise was noted at 72h post-exercise.
There was also a large decrement in isometric peak torque immediately post-exercise in both control (ES = -1.35; CI90%: -1.91 to -0.79) and experimental conditions (ES = -1.42; CI90% = -2.07 to -0.78), but no effect was seen at 24h, 48h and 72h post-exercise.
Counter movement jump height was lower 24h after exercise for both control (ES = -0.48; CI90% = -0.69 to -0.26) and experimental (ES = -0.40; CI90%: -0.77 to -0.03) conditions, and at 48h (ES = -0.56; CI90% = -0.81 to -0.32) and 72h (ES = -0.44; CI90% = -0.73 to -0.15) post-exercise for the control only.
The mean power output during the one leg 6s sprint was largely impaired up to 48h after the exercise in the control (ES = -1.65; CI90% = -2.51to -0.80) and experimental conditions (ES = -1.37; CI90% = -2.06 to -0.67).
Muscle soreness ratings were largely greater up to 48h after exercise in both control (2.3 ± 1.2 A.U. at pre, 4.4 ± 1.7 A.U. at post 24h (ES = 1.43, CI90%: 0.55 to 2.18), and 4.1 ±2.2 A.U. at post 48h (ES = 2.26, CI90%:0.66 to 3.87)) and experimental conditions (2.4 ± 1.7 at pre, 4.0±1.2 at post 24h (ES = 3.39, CI90%: 0.92 to 5.87), 4.7 ± 2.0 at post 48h (ES = 2.06; CI90%: 0.96 to 3.16)); there was also a moderate effect of the exercise on quadriceps soreness up to 48h after the exercise in the control (1.6 ± 1.3 A.U. at pre, 3.9 ± 1.9 A.U. at post 24h (ES = 1.10, CI90%: 0.12 to 1.99), and 3.5 ± 2.1 A.U. at post 48h (ES = 0.91; CI90%: 0.1 to 1.64)) and experimental conditions (1.8 ± 0.8 A.U. at pre, 3.3 ± 1.8 A.U. at post 24h (ES = 5.54, CI90%: 1.70 to 9.21)), and 3.2 ± 2.0 A.U. at post 48h (ES = 2.40; CI90%: 0.66 to 4.14)). Creatine kinase concentration was largely higher 72h after exercise in the control group (ES = 3.61; CI90%: 0.24 to 6.98), but there was no effect of exercise before 72h in the control group and no effect at any time point in the experimental group.
Effect of condition
There was no effect of condition on isometric peak torque, concentric peak torque, jump performance, creatine kinase concentration and muscle soreness at any time point.
There was a moderately smaller loss of mean power output during the 6s sprint 24h after the exercise in the experimental condition only (ES = -1.12; CI90% = -1.86 to -0.29). The evolution of the mean power developed during the 6s sprint in the two conditions are summarized in Figure 2.
Figure 2.
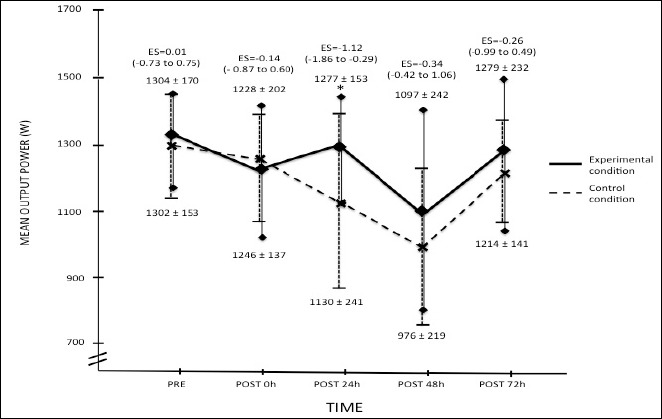
Mean ± SD power developed during the 6s one leg sprint. ES: Effect of the supplementation on the loss of mean power output. (): 90% Confidence interval of the effect size. (*) = Moderate effect in favor of the supplementation on the loss of mean power output.
Discussion
The main aim of this study was to analyze the effect of a curcumin and piperine supplementation on the muscle function recovery kinetics after exercise-induced muscle damage.
As the exercise task used to induce muscle damage was new and has never been used before with this population, it is required to check that this exercise induced muscle damage before analysing the effect of the supplementation. The measurements to quantify typical symptoms (muscle weakness, muscle pain, increased muscle stiffness, muscle swelling) of muscle damage are commonly used as markers of muscle damage (Nosaka, 2011). A loss of muscle function is considered to be the best marker to quantify muscle damage (Warren et al. 1999). In the current study, the muscle function was impaired up to 48h following the exercise. There was a moderate effect on the concentric peak torque up to 48h (-8.2%, ES = -1.12; CI90%: -2.17 to -0.06), there was a small effect on the CMJ up to 48h after the exercise (-13.9%, ES = -0.44; CI90% = -0.73 to -0.15) and a large effect on the mean power developed during the one leg 6s sprint up to 48h after the exercise (-22.9%, ES = -1.65; CI90% = -2.51to -0.80). Some other indirect markers of muscle damage are also used: delayed onset muscle soreness (DOMS) and an increase in blood markers such as creatine kinase. In the curent study, there was a large effect of the exercise on the muscle soreness up to 48h (+106,7%, ES = 3.0 ; CI90% = 0.57 to 5.58). The blood content in CK was largely higher up to 72h in the control group (+221,2%, ES = 3.61; CI90%: 0.24 to 6.98). According to Nosaka et al. (2011) criteria, the loss of muscle function up to 48h after the exercise combined with elevated muscle soreness 2 days after the exercise and the elevation in creatine kinase up to 72h after the exercise in the control condition tend to indicate that the exercise task chosen induced muscle damage.
The main results show that there was a moderate effect in favor of the supplementation of the curcumin and piperine combination on the loss of one leg 6 s sprint mean power output 24h after the exercise (ES = -1.12; CI90% = -1.86 to -0.29).
However, this result is counterbalanced by the absence of effect of the supplementation on muscle force outcomes measured with an isokinetic dynamometer and the absence of effect of the supplementation on the blood creatine kinase level. This may be explained by the fact that the exercise-induced muscle damage here was from a ‘global’ exercise, which involved a larger neuromuscular recruitment pattern than was required for the isokinetic and isometric tests. This could explain why the only positive effect of supplementation was detected on the 6s one leg sprint test, as the movement pattern needed for this test was the closest to the movement performed during the exercise task inducing muscle damage. During a downhill sprint, there is a strong eccentric activity of the hip extensors and knee extensors (Eston et al., 1995), which are the most activated parts of the lower limb during a sprint (Simonsen et al., 1985). The results concerning this one leg sprint could reflect better than an isokinetic test the effects of the exercise and of the supplementation on the loss of muscle function. Moreover, the 6s one leg sprint test showed the highest level of reproducibility, as calculated in this study, which allows to be confident in the results found with this test.
The results of this study confirm those found by Tanabe et al. (2015) where the muscle damage induced by exercise was attenuated with curcumin supplementation. Tanabe et al. (2015) showed that after 50 eccentric contractions of the elbow flexors, the use of Theracurmin before and 12h after the exercise significantly reduced the loss of strength of the elbow flexors immediately post-exercise, although there was no effect on creatine kinase blood concentration measured at 48h, 72h and 96h after the exercise.
One of the limitations of this study was the muscle damage recovery kinetics, which was relatively quick. Some of the muscle damage markers, such as the muscle function, were not impaired anymore 72h after the exercise. These results could come from the level of the population tested (i.e. elite rugby players). However, it also corresponds to the time course of muscle damage recovery markers obtained after some soccer matches or simulated matches (Nédelec et al., 2012). Consequently, it is difficult to draw conclusions about the effect of the supplementation 3 days after exercise induced muscle damage. The confidence intervals in the results recorded here are very large and may be linked to the small sample size of the study. The statistical power was calulated at posteriori and reached 80% only on the sprint mean power output, but was slightly below 80% on all the other outcomes measured. It is possible that with a larger sample size, other positive effects of supplementation could have been detected. Moreover, as this study was a cross over study, the participants were evaluated on one leg in each condition and there may have been a repeated bout effect with a faster recovery in the second condition in comparison with the first one. To counteract this possible effect, the period between the two conditions should have been longer, but as this study was conducted with elite athletes, it was not possible to ask them to wait 6 weeks or more between the two conditions. However, the design of the study limits the impact of this possible repeated bout effect on the results, as the number of participants was equal in each group and was balanced by starting an equal number with the curcumin and piperine or the placebo in the first phase. The one leg 6s sprint test has never been validated as a test to evaluate muscle damage. However, as explained above, it was necessary to use a test that allows to sollicitate knee and hip extensor of the evaluated leg, as these muscle groups are the ones which were eccentrically sollicitated during the downhill exercise (Eston et al., 1995). Even if this test has never been validated, the performance on the one leg 6s sprint was altered by the muscle damage inducing exercise during 48h after the exercise, and this alteration was reduced by the supplementation. It means that even if it is not possible to be sure that this supplementation acted on the muscle damage, this supplementation is efficient to hasten the recovery after an exercise sollicitating knee and hip extensors eccentrically.
Although highly speculative, the theorical explanation could be that curcumin coupled with piperine, by inhibiting NF-Kb and COX-2 (Basnet and Shalko-Basnet, 2011; Singh and Aggarwal, 1995; Koeberle et al., 2009) could limit post-exercise inflammation. As curcuminoids are also strong free radicals scavengers (Basnet and Shalko-Basnet, 2011) when coupled with their anti-inflammatory action, the hypothesis was that curcumin could also reduce secondary muscle damage. While some strategies used more specifically target the recovery process following muscle damage, (e.g. protein consumption) (Etheridge, 2008), it seems the supplementation used in the present study could attenuate muscle damage before it occurs, especially the secondary muscle damage linked to the inflammatory process. As a perspective, it could be interesting in a new study to analyze the effect of this supplementation on the inflammation level and the oxidative stress, with the help of blood level in inflammatory markers that could be influenced by the curcumin and piperine supplementation (IL-6, C-recative protein, TNF-α) and oxidative stress markers in urine (F2-isoprostanes).
As this study is the first to evaluate the effect of curcumin and piperine supplementation on muscle function, it is unknown what the ideal dose to efficiently attenuate muscle damage may be. It would be useful to compare the effect of varied doses of this combined supplement on the muscle function and recovery kinetics following the same, high damaging exercise task. Moreover, several strategies have been studied separately after exercise-induced muscle damage, such as cold water immersion (Poppendieck et al., 2013), protein (Etheridge at al., 2008) or berry consumption (Connolly et al., 2006, Bowtell et al., 2011; McLeay et al., 2012) but how effective these strategies may be when they are implemented together has not been studied. In a sporting context, several strategies are often implemented without knowing if there is a potential conflicting effect between them. For example, a recent study showed that the antioxidant power of red fruit is reduced when it is consumed with milk, a high protein content drink (Serafini et al., 2009). Therefore, it would be interesting to evaluate the effect of the implementation of several recovery strategies together on muscle function and recovery kinetics followed exercise-induced muscle damage. It has been shown that the post-exercise oxidative and inflammatory stress are highly involved in the training adaptation process, and that the use of antioxidant supplementation could limit these adaptations (Slattery et al., 2015). Whether a combined supplementation of curcumin and piperine would have a detrimental effect on chronic muscle adaptations following damaging exercise (Nosaka et al., 1991) is unclear and warrants further investigation.
Conclusion
In conclusion, supplementation with 6g of curcumin and 60mg of piperine each day between 48h before and 48h after exercise induced muscle damage shows an effect on the recovery of some aspects of the muscle function 24h and 48h after the exercise, however this effect is limited to the loss of power during the one leg 6s sprint, without any effect on other aspects of muscle damage, neither on muscle soreness.
Acknowledgment
The experiments comply with the law of the country in which they were performed. The authors declare that no financial assistance was associated with the project. The authors declare no conflict of interest. MGD nature (Brandérion, France) supplied the supplementation for free. The authors would like to thank Florence Mullie and Martin Lainé for their help during the experiments, and all the subjects who took part in the study.
Biographies
Barthélémy DELECROIX
Employment
PhD Student at University of Lille, EA 7369. Sport scientist and Strength and conditioning coach at Lille Olympique Sporting Club (LOSC).
Degree
MSc
Research interests
Injury Prevention, Sport Nutrition, Recovery, Training, Team sports.
barthelemy.delecroix@etu.univ-lille2.fr
Abd-Elbasset ABAÏDIA
Employment
Sport scientist at Lille Olympique Sporting Club (LOSC).
Degree
PhD
Research interests
Recovery, team sports, training, sports physiology, Sport analysis.
abd-elbasset.abaidia@etu.univ-lille2.fr
Cedric LEDUC
Employment
Sport scientist at Fédération Française de Rugby (FFR).
Degree
MD
Research interests
Training, recovery, fatigue, Team sports, Mental fatigue.
cedric.leduc1@gmail.com
Brian DAWSON
Employment
Professor at School of Sport Science, Exercise and Health, The University of Western Australia, Perth, Australia.
Degree
PhD
Research interests
Sport performance analysis, Sports physiology, Team sports, Sports training and recovery, heat and altitude
brian.dawson@uwa.edu.au
Gregory DUPONT
Employment
Head of Performance at Lille Olympique Sporting Club (LOSC).
Degree
PhD
Research interests
Sports physiology, Injury prevention, Nutrition, Recovery, Sports training, Team sports.
gregory.dupont@univ-lille2.fr
References
- Atkinson G., Nevill A.M. (1998) Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Medicine 26, 217-38. [DOI] [PubMed] [Google Scholar]
- Bailey D.M., Williams C., Betts J.A., Thompson D., Hust T.L. (2011) Oxidative stress, inflammation and recovery of muscle function after damaging exercise: effect of 6-week mixed anti-oxidant supplementation. European Journal of Applied Physiology 111, 925-936. [DOI] [PubMed] [Google Scholar]
- Basnet P., Skalko-Basnet N. (2011) Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 16, 4567-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G.A.V. (1982) Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercice 14, 377-381. [PubMed] [Google Scholar]
- Bowtell J.L., Sumners D.P., Dyer A., Fox P., Mileva K. N. (2011) Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Medicine and Science in Sports and Exercise 43,1544-1551. [DOI] [PubMed] [Google Scholar]
- Bryer S.C., Goldfarb A.H. (2006) Effect of a high dose of vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. International Journal of Sport Nutrition and Exercice Metabolism 16, 270-280. [DOI] [PubMed] [Google Scholar]
- Connolly D.A.J., McHugh M.P., Padilla-Zakour O.I. (2006) Efficacy of tart cherry juice blend in preventing the symptoms of muscle damage. British Journal of Sports Medicine 40, 679-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnic F., Riera J., Appendino G., Togni S., Franceshi F., Valle X., Pons A., Tur J. (2014) Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): a randomised, placebo-controlled trial. Journal of the International Society of Sports Nutrition 18, 11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge T., Philp A., Watt P.W. (2008) A single protein meal increases recovery of muscle function following an acute eccentric exercise bout. Applied Physiology Nutrition and Metabolism 33, 483-488. [DOI] [PubMed] [Google Scholar]
- Goldfarb A.H., Garten R.S., Cho C., Chee P.D.M., Chambers L.A. (2011) Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Medicine and Science of Sports and Exercise 43, 501-508. [DOI] [PubMed] [Google Scholar]
- Hooper S.L., Mackinnon L.T., Howard A., Gordon R.D., Bachmann A.W. (1995) Markers of monitoring overtraining and recovery. Medicine and Science in Sports and Exercise 27, 106-112. [PubMed] [Google Scholar]
- Hopkins W.. (2001) A new view of statistics : The confidence interval and statistical significance. Available form URL: http://www.sportsci.org/resource/stats/
- Hopkins W.G. (2013) A new view of statistics: Summarizing data, simple statistics & effects statistics. Available form URL: http://www.sportsci.org/resource/stats/
- Horder M, Jorgensen PJ. (1991) Creatine kinase determination: A European evaluation of creatine kinase determination in serum, plasma and whole blood with the Reflotron system. European Journal of Clinical Chemistry and Clinical Biochemistry 29, 691-696. [PubMed] [Google Scholar]
- Howatson G, McHugh MP, Hill JA, Brouner J., Jewell A. P., van Someren K. A., Shave R. E., Howatson S. A. (2009) Influence of tart cherry juice on indices of recovery following marathon running. Scandinavian Journal of Medicine and Science in Sports 20, 843-852. [DOI] [PubMed] [Google Scholar]
- Koeberle A., Northoff H., Werz O. (2009) Curcumin blocks prostaglandin E2 biosynthesis through direct inhibition of the microsomal prostaglandin E2 synthase-1. Molecular Cancer Therapeutics 8, 2348-2355. [DOI] [PubMed] [Google Scholar]
- Maffiuletti N.A., Bizzini M., Desbrosses K., Babault N., Munzinger U. (2007) Reliability of knee extension and flexion measurements using the Con-Trex isokinetic dynamometer. Clinical Physiology and Functional Imaging 27, 346-353. [DOI] [PubMed] [Google Scholar]
- McLeay Y., Barnes M.J., Mundel T. (2012) Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. Journal of the International Society of Sports Nutrition 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Sudheer A. R. (2007) Antioxidant and anti-inflammatory properties of curcumin. Advances in Experimental Medicine and Biology 595, 105-125. [DOI] [PubMed] [Google Scholar]
- Myburgh K.H. (2014). Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Medicine 44, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka K., Clarkson P.M., McGuiggin M.E., Byrne J.M. (1991) Time course of muscle adaptation after high force eccentric exercise. European Journal of Applied Physiology 63, 70-76. [DOI] [PubMed] [Google Scholar]
- Nosaka K., Aldayel A., Jubeau M., Chen T.C. (2011) Muscle damage induced by electrical stimulation. European Journal of Applied Physiology 111, 2427-2437. [DOI] [PubMed] [Google Scholar]
- Poppendieck W., Faude O., Wegmann M., Meyer T. (2013) Cooling and performance recovery of trained athletes: A meta-analytical review. International Journal of Sports Physiology and Performance 8, 227-242. [DOI] [PubMed] [Google Scholar]
- Serafini M., Testa M.F., Villano D. (2009) Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radical Biology and Medicine 46, 769-774. [DOI] [PubMed] [Google Scholar]
- Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas S. (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica 64, 353-356. [DOI] [PubMed] [Google Scholar]
- Singh S., Aggarwal B. (1995) Activation of transcription factor NF-kB is suppressed by curcumin (Diferulolymethane). The Journal of Biological Chemistry 270, 24995-25000. [DOI] [PubMed] [Google Scholar]
- Slattery K., Bentley D., Coutts AJ. (2015) The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Medicine 45, 453-471. [DOI] [PubMed] [Google Scholar]
- Srinivasan K. (2014) Antioxidant potential of spices and their active constituents. Critical Review of Food Science and Nutrition 54, 352-372. [DOI] [PubMed] [Google Scholar]
- Stokes I.A., Abery J.M. (1980) Influence of the hamstring muscles on lumbar spine curvature in sitting. Spine 5(6), 525-28. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Maeda S., Akazawa N., Zempo-Miyaki A., Choi Y., Ra S.-G., Imaizumi A., Otsuka Y., Nosaka K. (2015) Attenuation of indirect markers of eccentric exercise induced muscle damage by curcumin. European Journal of Applied Physiology 115, 1949-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G.L., Lowe D.A., Armstrong R.B. (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Medicine 27(1), 43-59. [DOI] [PubMed] [Google Scholar]


