Sumary
Objectives
This study examines the hypothesis that lower adipose tissue lipoprotein lipase (LPL) activity and a limited capacity for subcutaneous adipocyte expansion will be associated with metabolic syndrome (MSyn) in postmenopausal women who are overweight and obese.
Methods
Women (N = 150; age 60 ± 1 year; BMI: 31.5 ± 0.3 kg m−2; mean ± standard errors of the means [SEM]) with and without MSyn had dual‐energy X‐ray absorptiometry scans for total body fat, CT scans for visceral and subcutaneous abdominal adipose tissue areas, lipid and glucose metabolic profiles, and abdominal and gluteal fat aspirations for subcutaneous fat cell weight (FCW; N = 150) and LPL activity (N = 100).
Results
Women with MSyn had similar total body fat, but 15% larger abdominal and 11% larger gluteal FCWs and more visceral fat (179 ± 7 vs. 134 ± 6 cm2) than women without MSyn (P's < 0.05). Abdominal LPL activity was 13% (P = 0.18) lower in women with than without MSyn and correlated with abdominal FCW (r = 0.49, P < 0.01) only in those without MSyn. Visceral fat and abdominal and gluteal FCWs correlated with MSyn components, and subcutaneous adipose tissue correlated with abdominal FCW (r = 0.43, P < 0.01) and LPL activity (r = 0.18, P < 0.05), independent of total body fat.
Conclusions
These results show that women with MSyn have lower LPL activity, limited capacity for subcutaneous adipocyte lipid storage and greater ectopic fat accumulation in viscera than women without MSyn of comparable obesity. This suggests that the development of novel therapies that would enhance adipocyte expandability might prevent the accumulation of ectopic fat and reduce the risk for MSyn in postmenopausal women with obesity.
Keywords: Adipocyte hypertrophy, LPL activity, metabolic syndrome, visceral adipose tissue
Introduction
Menopause is commonly associated with weight gain, and the accumulation of body fat in the abdominal region, which independently increases risk for type 2 diabetes mellitus (T2DM), metabolic syndrome (MSyn) and subsequent cardiovascular disease (CVD) 1. While weight gain and ageing are strong risk factors for MSyn, not all overweight and obese individuals develop these metabolic abnormalities. A range of metabolically healthy and unhealthy phenotypes was shown to exist across the continuum of obesity, with some classified as having metabolic healthy obesity or obesity in the absence of metabolic dysfunction and others as having unhealthy obesity 2, 3. Analysis of NHANES data found that the age‐standardized prevalence of the metabolically abnormal phenotype [defined by ≥2 of the criteria for MSyn, including elevated waist circumference, blood pressure (BP), triglycerides (TG), glucose (FPG) and reduced high‐density lipoprotein cholesterol (HDL‐C) 4, as well as high‐sensitive C‐reactive protein and homeostasis model assessment‐estimated insulin resistance (HOMA‐IR)] was 65% in obese, 43% in overweight and 21% in normal women, whereas 35% of obese, 57% of overweight and 79% of normal weight women were metabolically healthy, as defined by <2 risk factors 5. The presence of ≥3 or more of five of these metabolic abnormalities constitutes MSyn, a clustering of risk factors that enhances risk for CVD mortality in postmenopausal women to a greater extent than in men or premenopausal women 6; therefore, identifying potential mechanisms other than obesity, sedentary behaviour and ageing that result in metabolic dysfunction may provide insight into therapeutic strategies to prevent the development of T2DM and CVD complications in older women with obesity 4.
The limited capacity to expand subcutaneous adipose tissue (SAT), or the ‘adipose tissue expandability hypothesis’ as proposed by Danforth 7, posits that there is a finite limit to which an individual's adipose tissue can expand to fulfil its role as an organ for lipid storage. Once this capacity is exceeded, any excess circulating triacylglycerol (TG‐FFA) will be deposited in undesirable or ectopic fat depots, including visceral adipose tissue (VAT), liver and skeletal muscle, which are associated with insulin resistance and metabolic dysfunction 7, 8, 9. Lipoprotein lipase (LPL) is the rate‐limiting enzyme for the uptake and storage of TG‐FFAs in adipose tissue, and genes related to fat cell expansion and lipid storage such as LPL, PPARɣ and fatty acid synthase are higher in SAT of insulin sensitive than resistant subjects 9, 10. Intervention studies show that thiazolidinedione (TZD) administration, by upregulating PPARɣs causes SAT hypertrophy and reduces VAT and intrahepatic fat to improve lipid and glucose metabolism in patients with obesity and T2DM 11, and that PPARɣ2 activation increases adipogenesis and insulin sensitivity 12. This supports the hypothesis that the pathways involved in lipid storage in subcutaneous and ectopic sites play a role in the insulin resistance‐associated metabolic consequences of obesity. This is inferred from the work of others 9, 13, 14, 15, 16, and our recent research that showed in a select cohort of sedentary, older women with obesity higher adipose tissue LPL activity (AT‐LPLA) in those with a low, compared with a higher ratio of VAT/VAT + SAT area is associated with larger adipocyte size and SAT area, as well as fewer metabolic abnormalities 17. It is the aim of this study to extend the generalizability of our prior finding to MSyn by testing the hypothesis that lower AT‐LPLA will be associated with a limited capacity for subcutaneous adipocyte expansion, greater visceral fat accumulation and MSyn in postmenopausal women who are overweight and obese.
Methods
Subjects
One hundred and fifty sedentary, postmenopausal (age: 45–78 years) women who were overweight and obese (body mass index (BMI): 25–41 kg m−2; body fat: 36–59%) and had previously provided informed consent 18, 19 were selected for inclusion if their MSyn status was known, exercised <20 min twice/week and had waist circumference, dual‐energy X‐ray absorptiometry and computerized axial tomography (CT) scan for analysis of body composition. We only studied Caucasian women to obviate the race differences in adipocyte metabolism and insulin resistance 19. The presence of three or more of the following criteria was used to diagnose MSyn: waist circumference >88 cm, impaired glucose metabolism (fasting glucose >5.6 mmol L−1), elevated BP (>130/85 mmHg or anti‐hypertensive treatment), and dyslipidemia (TG >1.7 mmol L−1, HDL‐C <1.3 mmol L−1 or hypolipidemic treatment) 4. Women with diabetes (FPG >7 mmol L−1 or 2‐h glucose tolerance test glucose >11 mmol L−1) or on oral agents, TG >400 mg dL−1, overt cardiovascular, renal, or liver disease or unstable medical conditions that would affect outcomes were excluded. The women with MSyn were on more lipid lowering (22% vs. 3%; P < 0.01) and anti‐hypertensive (30% vs. 15%; P < 0.05) medications. The 150 women were divided into four groups based on their BMI and MSyn status: 39 women who were overweight without MSyn, 21 women who were overweight with MSyn, 40 women who were obese without MSyn and 50 women who were obese with MSyn.
Body composition
Height and body weight were measured, and BMI (weight [kg]/height [m2]) were calculated. Waist circumference was measured at the midpoint between the inferior border of the ribcage and the superior aspect of the iliac crest using an inelastic measuring tape. A total body dual‐energy X‐ray absorptiometry scan (DPX‐IQ or Prodigy; LUNAR Radiation Corp., Madison, Wisconsin, USA) determined body fat percentage. A single CT scan was taken using a PQ 6000 scanner (Marconi Medical Systems, Cleveland, OH, USA) at the L4–L5 region to determine VAT and SAT area and analysed using Medical Image Processing, Analysis and Visualization, version 7.0.0. A second scan at the mid‐thigh was used to quantify muscle low‐density lean adipose tissue area by Hounsfield units (−190 to −30 HU); values of the right leg were used in the statistical analyses 20.
Metabolic testing
All women received education in a weight maintaining step I American Heart Association (AHA) diet 18 by a registered dietitian for 6–8 weeks prior to metabolic testing to minimize the effects of inter‐individual differences in dietary macronutrients on the metabolic outcomes. Dietary compliance was verified by food records, and women received extra counselling, as needed, to reach compliance within 5% of AHA recommendations and weight stability. Women were provided an isocaloric diet composed of 50–55% carbohydrate, 15–20% protein and 30–35% fat for two days prior to the adipose tissue biopsies to insure weight stability and diet consistency. Body weight was stable (± < 0.5 kg) for two weeks during metabolic and body composition testing. Resting BP was measured in the sitting position after 10 min on three occasions and averaged. Blood for fasting (overnight for 10–12 h) lipid profiles was obtained on two separate mornings, and results were averaged. Plasma triglyceride and cholesterol levels were analysed using enzymatic methods (UniCelDxC880i; Beckman Coulter, Inc., Brea, CA), HDL‐C measured in the supernatant after precipitation with dextran sulphate, and low‐density lipoprotein cholesterol (LDL‐C) calculated as LDL‐C = total cholesterol − (HDL‐C − TG/5) 18. A 2‐h oral glucose tolerance test was performed to measure glucose by glucose oxidase method (2300‐STAT Plus; YSI, Yellow Springs, OH, USA) and insulin by radioimmunoassay (Linco Research Inc., St. Charles, MO, USA) during fasting and every 30 min following ingestion of 75‐g glucose to exclude diabetes 21.
Adipocyte weight and lipoprotein lipase activity
After an overnight fast, abdominal (ABD) and gluteal (GLT) subcutaneous aspirates were taken with a cannula under local lidocaine anaesthesia and immediately transported to the laboratory on ice. Adipocytes were isolated, fat cell size measured by microscopy and FCW were calculated 22. AT‐LPLA was measured in ~50‐mg ABD and GLT adipose tissue as the heparin elutable LPLA (nmol FFA/min/106cells) on a 14C triolein substrate as previously described 23 in 52 women with and 48 without MSyn. A human postheparin plasma control pool stored frozen in 0.2‐mLaliquots at −80 °C was thawed and included in each assay to insure the quality of the 14C triolein substrate. The inter‐assay pool activity was calculated as the % of 14C‐FFA counts hydrolysed, and the interassay CV was 12.0 ± 0.5%. Assays with a pool activity <5% were excluded. AT‐LPLA was not corrected for the pool activity because all postheparin plasma hydrolysed 5–20% of the triolein substrate.
Statistical analysis
Data were analysed using spss Version 20 (PAWS Statistics, Version 20, Chicago, IL). Standard methods were used to compute means and SEM, and data normalized as appropriate by log transformation. One‐way analysis of variance and standard post‐hoc analyses were used to compare differences in variables of interest across obesity and MSyn status. X 2 was used to determine whether the presence or absence of categorical variables differed by MSyn status. Pearson and partial correlation coefficients were calculated after log transformation of plasma TG, AT‐LPLA, FCW, insulin and other data as appropriate; regression coefficients were compared using Fisher's z transformation. HOMA‐IR was calculated as [(fasting insulin (μU/ml) × fasting glucose [mmol l−1])/22.5] 24. All tests were two‐tailed, and P‐values <0.05 were considered statistically significant.
Results
Subject characteristics (Table 1)
Table 1.
Subject characteristics
| Without MSyn overweight (N = 39) | MSyn overweight (N = 21) | Without MSyn obese (N = 40) | MSyn obese (N = 50) | |
|---|---|---|---|---|
| BMI (kg m−2) | 28 ± 1 | 29 ± 1‡ | 33 ± 3 | 34 ± 3 |
| Waist (cm) | 85 ± 1 | 92 ± 1*, † | 97 ± 1 | 101 ± 1* |
| Hip (cm) | 108 ± 1 | 109 ± 1**, ‡ | 119 ± 2 | 119 ± 1** |
| Body fat (%) | 43 ± 4 | 43 ± 3‡ | 49 ± 4 | 49 ± 4 |
| VAT area (cm2) | 124 ± 6 | 152 ± 7* | 143 ± 10 | 189 ± 8** |
| SAT area (cm2) | 372 ± 15 | 379 ± 19‡ | 479 ± 18 | 497 ± 13 |
| Systolic BP (mmHg) | 116 ± 2 | 123 ± 3* | 121 ± 15 | 131 ± 2** |
| Diastolic BP (mmHg) | 68 ± 1 | 69 ± 2 | 70 ± 1 | 75 ± 1* |
| Total Cholesterol (mg dL−1) | 209 ± 6 | 204 ± 10 | 193 ± 4 | 198 ± 5 |
| HDL‐C (mg dL−1) | 57 ± 14 | 47 ± 11**† | 55 ± 13 | 46 ± 11** |
| LDL‐C (mg dL−1) | 130 ± 5 | 123 ± 8 | 118 ± 4 | 121 ± 5 |
| TG (mg dL−1) | 118 ± 34 | 167 ± 56**, ‡ | 108 ± 25 | 160 ± 6** |
| Fasting Glucose (mg dL−1) | 93 ± 7 | 99 ± 7**, ‡ | 93 ± 6 | 102 ± 11** |
| HOMA‐IR | 2.2 ± 0.6 | 3.2 ± 1.1** | 2.7 ± 1.1 | 4.4 ± 2.0** |
| 2‐h Glucose (mg dL−1) | 129 ± 7 | 140 ± 8‡ | 119 ± 4 | 144 ± 7** |
BPI, body mass index; Hip, hip circumference; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SAT, subcutaneous abdominal adipose tissue; VAT, visceral abdominal adipose tissue; Waist, waist circumference. Significantly different from women without MSyn within the same BMI category:
P < 0.05,
P < 0.01. Significantly different from obese women without MSyn:
P < 0.05,
P < 0.01.
The 150 women were on average 60 ± 1‐year old and sedentary. As anticipated, the women with MSyn had higher waist circumference, TG, FPG, and BP and lower HDL‐C than women without MSyn (P's < 0.05, Table 1) despite similar BMI and body fat percentage. There was no effect of lipid lowering (N = 13) or anti‐hypertensive (N = 17) medications on waist circumferences (lipid lowering: 94 ± 2 vs. not: 99 ± 1 cm), (anti‐hypertensive: 97 ± 2 vs. not: 97 ± 1 cm.) or FPGs (lipid lowering: 101 ± 2 vs. not: 101 ± 1 mg dL−1), (anti‐hypertensive: 102 ± 3 vs. not: 109 ± 1 mg dL−1) in those with MSyn who were on vs. those not on these medications.
Body composition and metabolic characteristics of the subjects (Table 1)
The prevalence of MSyn was greater in those that were obese (50/90 or 56%) compared with those who were overweight (21/59 or 36%; P < 0.01). The women with MSyn had 31% higher VAT (MSyn: 176 ± 7 cm2 vs. without MSyn: 134 ± 6 cm2, P < 0.05), 11% higher intramuscular fat (19.4 ± 0.9 cm2 vs. 17.5 ± 0.6 cm2; P = 0.08), a higher prevalence of IGT (49% vs. 32%, P < 0.05) and were more insulin resistant by HOMA‐IR (P < 0.01) than women without MSyn. These differences are magnified when comparing the metabolic characteristics of the women who were overweight with MSyn to the women who were obese without MSyn. Despite weighing ~14 kg less and having 6% less body fat, the women who were overweight with MSyn had 21% lower SAT area, similar VAT area, higher TG, FPG and 2‐h glucose concentrations, and lower HDL‐C than the women who were obese without MSyn (Table 1).
Adipose tissue fat cell weight and lipoprotein lipase activity in metabolic syndrome
Despite comparable body fat percentage (47 ± 1 vs. 46 ± 1%), the women with MSyn had 15% larger ABD (mean: 0.76 ± 0.04 vs. 0.66 ± 0.02 µg TG/cell) and 11% GLT (mean: 0.78 ± 0.02 vs. 0.70 ± 0.02) FCWs (P's < 0.01). However, the difference in regional FCW reached significance in the obese group (P's < 0.05) but not in the overweight women (Figure 1). More women with MSyn had ABD FCWs above the overall 0.71‐µg TG/cell sample mean compared with women without MSyn (62% vs. 49%; P < 0.05), with similar results for GLT FCWs (58% vs. 38% above the 0.73‐µg TG/cell mean) (P < 0.05, Figure 1B,C). Despite larger adipocytes, the women with MSyn had 13% lower AT‐LPLA in the ABD (3.3 ± 0.3 vs. 3.8 ± 0.5 nmol FFA/min/106) and 13% lower in the GLT (4.5 ± 0.5 vs. 5.2 ± 0.7) (P's = NS) than those without MSyn (Figure 2).
Figure 1.
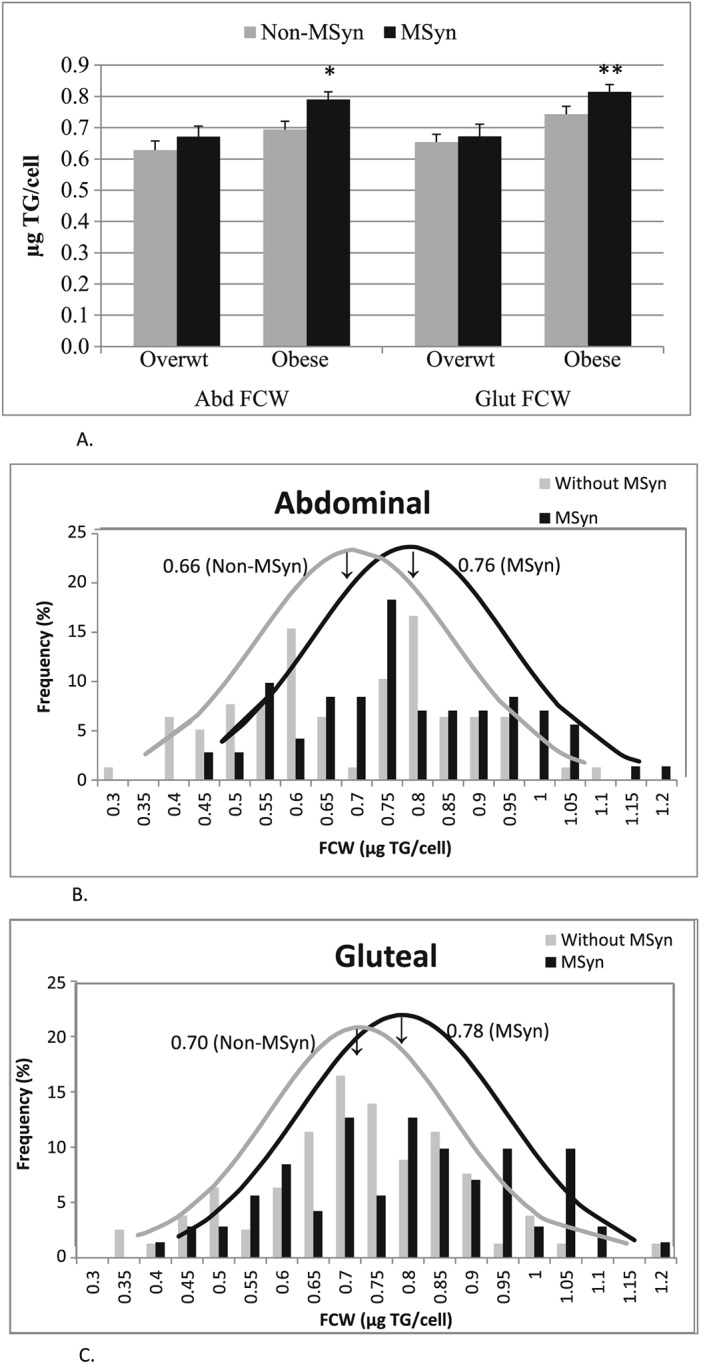
A Bar graph shows mean ± SEM fat cell weights within overweight (Overwt) and obese groups by metabolic syndrome status. *P < 0.05; **P < 0.01: significantly different than obese non‐MSyn. Figure 1B and C. The distribution of mean fat cell weights (FCWs) in women with and without MSyn shows that despite comparable obesity, FCWs of women with MSyn are shifted to larger cells and have a larger mean FCW in both the ABD (Figure 1B) and GLT (Figure 1C) sites than in women without MSyn.
Figure 2.
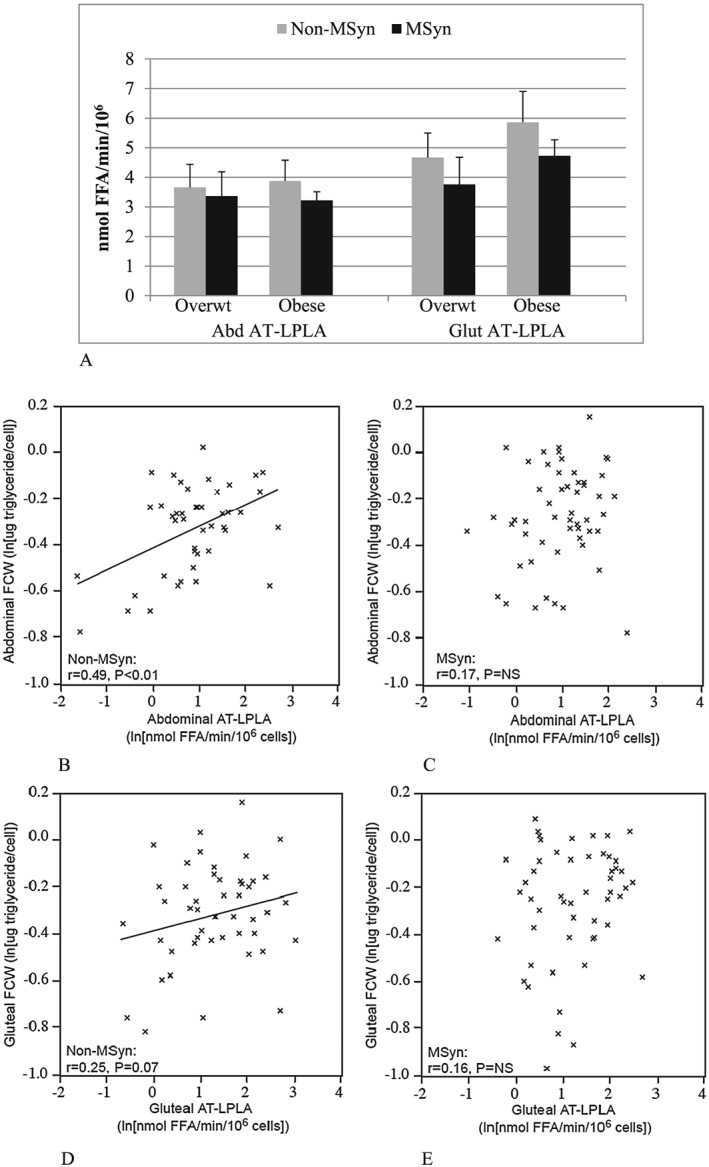
A Bar graph depicts mean ± SEM adipose tissue LPL activity within overweight (Overwt) and obese groups by MSyn status (P's = NS). Figure 2B–E. The scatter plots show a significant linear relationship between abdominal adipose tissue LPL activity (AT‐LPLA) and fat cell weight (FCW) in women without MSyn in the abdomen (r = 0.49, P < 0.01; Figure 2B) that approaches significance in the gluteal region (r = 0.25, P = 0.07; Figure 2D), but not in women with MSyn (abdominal: Figure 2C; gluteal: Figure 2E).
Relationships of adipocyte storage capacity and metabolic risk
There was a positive relationship in the women without MSyn between FCW and AT‐LPLA in the ABD (r = 0.49, P < 0.01) and a trend in the GLT (r = 0.25; P = 0.07) regions, but no relationship in either the ABD (r = 0.17) or GLT (r = 0.16) (P's = NS) in women with MSyn (Figure 2B–E). The regression coefficients for the relationship between ABD FCW and AT‐LPLA tended to differ between women with and without MSyn (P = 0.08). In the entire group, VAT was the strongest independent predictor of the MSyn components (Table 2) and also correlated significantly with ABD FCW (r = 0.29, P < 0.01) and approached significance with GLT (r = 0.17, P = 0.06) FCW; however, ABD FCW correlated less strongly than VAT with four components of the MSyn, and GLT FCW correlated with only two MSyn components after controlling for obesity and SAT (Table 2). SAT was directly related to both ABD FCW (r = 0.43, P < 0.01) and AT‐LPLA (r = 0.18, P < 0.05) independent of obesity, but neither SAT nor AT‐LPLA (Figure 2) correlated with the components of MSyn.
Table 2.
Metabolic syndrome is associated with VAT, fat cell hypertrophy and adipose tissue LPL activity
| VAT (cm2)† | Abdominal FCW (µg TG/cell)‡ | Gluteal FCW (µg TG/cell)‡ | Abdominal AT‐LPLA (ln[nmol FFA/min/106])§ | Gluteal AT‐LPLA (ln[nmol FFA/min/106])§ | |
|---|---|---|---|---|---|
| Waist (cm) | r = 0.58** | r = 0.22** | r = 0.12 | r = −0.06 | r = 0.04 |
| TG (ln[mg dL−1]) | r = 0.29** | r = 0.19* | r = 0.06 | r = −0.13 | r = −0.07 |
| HDL‐C (mg dL−1) | r = −0.20* | r = −0.27** | r = −0.24** | r = 0.01 | r = 0.01 |
| Systolic BP (mmHg) | r = 0.25** | r = 0.15 | r = 0.06 | r = 0.08 | r = −0.05 |
| Diastolic BP (mmHg) | r = 0.18* | r = 0.30** | r = 0.31** | r = 0.03 | r = −0.10 |
| Fasting Glucose (mg dL−1) | r = 0.30** | r = 0.11 | r = 0.13 | r = −0.02 | r = 0.02 |
| HOMA‐IR | r = 0.45** | r = 0.25** | r = 0.15* | r = 0.07 | r = 0.06 |
AT‐LPLA; adipose tissue lipoprotein lipase activity; BPI, body mass index; FCW, fat cell weight; Hip, hip circumference; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment‐estimated insulin resistance; LDL, low‐density lipoprotein; SAT, abdominal adipose tissue areas.
P < 0.05.
P < 0.01.
Controlled for body fat percentage, SAT and abdominal FCW.
Controlled for body fat percentage and SAT.
Controlled for body fat percentage and SAT.
Discussion
The results of this study provide insight into the role of increased abdominal fat distribution and reduced lipid storage capacity of subcutaneous adipocytes in the accumulation of visceral fat and the development of insulin resistance and MSyn in postmenopausal women who are overweight and obese. The greater metabolic dysfunction in women with MSyn correlated with VAT and was associated with lower AT‐LPLA and a limited capacity for continued expansion and lipid storage in subcutaneous abdominal adipose tissue, as supported by the absence of a positive correlation between ABD FCW and AT‐LPLA. In contrast, subcutaneous adipocyte lipid storage (FCW) correlated with increasing AT‐LPLA in women with the same degree of obesity without MSyn, likely increasing lipid storage, and preventing triacylglycerol spillover from subcutaneous into visceral sites and the development of insulin resistance and MSyn 7, 8. The larger adipocytes of women with MSyn seem to have reached their maximal capacity for lipid storage and expansion, attaining a ‘maximal rather than desirable’ size and plateauing. This may account for the preponderance of larger fat cells and mean FCWs compared with similarly obese women without MSyn. Despite a comparable degree of obesity, the women without MSyn have smaller adipocytes and a wider spectrum of adipocyte size that correlates directly with AT‐LPLA across a broader range of FCWs (Figure 2), supporting the notion of a greater reserve capacity for lipid storage.
Although fat cell number was not measured directly in this study, the smaller FCWs for the same degree of relative obesity and body fat in the women without MSyn suggest that they also may have more fat cells and greater adipogenesis than the women with MSyn of comparable obesity. In contrast, the smaller adipocytes of women without MSyn preserve their capacity for further adipocyte lipid storage with ensuing postmenopausal‐associated weight gain and thus avoid MSyn. This supports our hypothesis that the requisite subcutaneous adipocyte lipid storage capacity for normal metabolism is exceeded in women with MSyn leading to more lipid ‘spillover’, accumulation of VAT and insulin resistance than women of comparable obesity without MSyn 7, 8, 14.
The finding that women with higher subcutaneous FCW had higher risk of MSyn suggests that abnormal adipocyte biology is a critical regulator of whole body metabolism and risk for MSyn. Insulin resistance is associated with reduced adipogenic transcription factors and adipocyte differentiation in SAT 8, 9, 10, 15, 25, and if pro‐lipogenic and adipogenic adaptations could be stimulated similar to those observed with TZDs 11, other PPAR agonist‐therapy 12 and the overexpression of adiponectin in adipose tissue of transgenic mice with obesity 26, ectopic fat deposition might be prevented, improving insulin sensitivity and metabolic function. This theory is supported by many investigators' findings that a reduced capacity of subcutaneous adipocytes for lipid storage is associated with greater VAT and intramuscular fat, insulin resistance and metabolic dysfunction.
Acute (4 to 8 week) overfeeding studies show that young and middle‐aged adults with small adipocytes at baseline do not upregulate subcutaneous lipogenic and adipogenic genes, accumulate more ectopic fat and have greater metabolic dysfunction after weight gain than those who are able to expand SAT 25, 27, 28, 29, 30, supporting the detrimental metabolic effects of reduced subcutaneous adipogenic capacity. Further, in cross‐sectional studies, the reduced storage of fat in thigh SAT is independently associated with glucose intolerance and dyslipidemia 31. However, these subjects are younger, less obese, more active and more insulin sensitive than the postmenopausal women in the present study, whose weight gain is chronic because of longstanding caloric excess rather than acute exposure. It is likely that age, fitness, body composition and hormonal status affect the mechanisms regulating lipogenesis and adipogenesis, and their ability to adapt during acute overfeeding compared with longer term, chronic caloric excess 14.
The homogeneity of the study population of women who are overweight and obese with and without MSyn with respect to body composition and race, and the exclusion of older women with comorbidities such as T2DM, renal disease, hypertension and hyperlipidemia on multiple medications that could affect adipose tissue metabolism are strengths of this study. The well‐controlled diets and requirement for weight stability of the women on AHA diets before metabolic studies minimized known deficiencies in dietary recall to assure subject compliance. Further, the study methods for the assessment of VAT, SAT and intramuscular fat by CT scans and measurement of AT‐LPLA and FCW at both ABD and GLT sites contributed to the consistent findings across a large study population. However, we examined relatively healthy Caucasian postmenopausal women, thus limiting the generalizability of the results to other racial or ethnic groups or men where differences in VAT accumulation, hormones and metabolic risk exist 32, 33. Adipose aspirations were only collected for analyses from subcutaneous sites and not VAT, thus eliminating the direct comparison of lipid storage capacity among other sites and angiogenesis and genes related to adipogenesis, obesity and lipid storage in adipocytes (e.g. SREBP‐1, perilipin A and PPARγ) were not measured. The angiogenic capacity of SAT is higher than VAT and correlates negatively with insulin sensitivity 34, suggesting that there may be a threshold limiting the potential for angiogenesis and lipogenesis to promote SAT expandability in people with severe obesity. As the lipogenic capacity of adipose tissue increases in the transition from overweight to obese, subcutaneous angiogenesis and adipogenesis may become insufficient to sustain adipocyte expansion because of abnormal pro‐inflammatory adipokine secretion 35 and low LPL 36. Unfortunately, the current study is cross‐sectional with no feeding, weight loss or exercise interventions nor longitudinal follow‐up to examine these possibilities prospectively.
Conclusion
In conclusion, these findings support the hypothesis that lower AT‐LPLA will be associated with a limited capacity for subcutaneous adipocyte lipid storage and expansion, greater visceral fat accumulation and the development of MSyn in postmenopausal women who are overweight and obese. This could have clinical applicability, as it suggests that the development of novel therapies that might increase adipogenesis to enhance adipocyte expandability and lipid storage would limit regional lipid accumulation to SAT. This has the potential to prevent ectopic fat accumulation, one mechanism for the development of insulin resistance and MSyn in older, postmenopausal women with obesity.
Conflict of interest
The authors have no conflicts to disclose.
Funding
This work was supported by funds from National Institute on Aging (NIA) grants R01‐AG18408, R01‐AG20116, R01‐AG19310, and 5T32AG000219; Maryland Claude D. Pepper Older Americans Independence Center (P30 AG028747), NIDDK Mid‐Atlantic Nutrition Obesity Research Center (P30 DK072488); Department of Veterans Affairs and Baltimore VA Geriatric Research, Education and Clinical Center (GRECC); a VA Advanced Health Postdoctoral Fellowship; and VA Senior Research Career Scientist and Career Development Awards.
Acknowledgements
Our appreciation is extended to the women who participated in this study, John Sorkin, MD, PhD, for statistical consultation and the nurses, exercise physiologists, and registered dietitians for their assistance.
Serra, M. C. , Ryan, A. S. , and Goldberg, A. P. (2017) Reduced LPL and subcutaneous lipid storage capacity are associated with metabolic syndrome in postmenopausal women with obesity. Obesity Science & Practice, 3: 106–114. doi: 10.1002/osp4.86.
References
- 1. Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5‐year risk of death in older women. JAMA 1993; 269: 483–487. [PubMed] [Google Scholar]
- 2. Karelis AD, Brochu M, Rabasa‐Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab 2004; 30: 569–572. [DOI] [PubMed] [Google Scholar]
- 3. Ruderman NB, Schneider SH, Berchtold P. The “metabolically‐obese,” normal‐weight individual. Am J Clin Nutr 1981; 34: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 5. Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999‐2004). Arch Intern Med 2008; 168: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 6. Lin JW, Caffrey JL, Chang MH, Lin YS. Sex, menopause, metabolic syndrome, and all‐cause and cause‐specific mortality‐‐cohort analysis from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2010; 95: 4258–4267. [DOI] [PubMed] [Google Scholar]
- 7. Danforth E Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 2000; 26: 13. [DOI] [PubMed] [Google Scholar]
- 8. Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci 2002; 967: 363–378. [DOI] [PubMed] [Google Scholar]
- 9. Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 2010; 59: 2288–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clemente‐Postigo M, Queipo‐Ortuno MI, Fernandez‐Garcia D, Gomez‐Huelgas R, Tinahones FJ, Cardona F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One 2011; 6: e24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Souza CJ, Eckhardt M, Gagen K, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 2001; 50: 1863–1871. [DOI] [PubMed] [Google Scholar]
- 12. Barak Y, Nelson MC, Ong ES, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4: 585–595. [DOI] [PubMed] [Google Scholar]
- 13. McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014; 22: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan CY, Vidal‐Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans 2008; 36: 935–940. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Jansson PA, Nagaev I, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 2004; 317: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 16. Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and 'hyperleptinaemia'. Diabetologia 2007; 50: 625–633. [DOI] [PubMed] [Google Scholar]
- 17. Serra MC, Ryan AS, Sorkin JD, Favor KH, Goldberg AP. High adipose LPL activity and adipocyte hypertrophy reduce visceral fat and metabolic risk in obese, older women. Obesity (Silver Spring) 2015; 23: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African‐American and Caucasian women. J Gerontol A Biol Sci Med Sci 2003; 58: 181–189. [DOI] [PubMed] [Google Scholar]
- 19. Fried SK, Tittelbach T, Blumenthal J, et al. Resistance to the antilipolytic effect of insulin in adipocytes of African‐American compared to Caucasian postmenopausal women. J Lipid Res 2010; 51: 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab 2012; 302: E145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35(Suppl 1): S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol 1996; 270: E72–78. [DOI] [PubMed] [Google Scholar]
- 23. Berman DM, Nicklas BJ, Ryan AS, Rogus EM, Dennis KE, Goldberg AP. Regulation of lipolysis and lipoprotein lipase after weight loss in obese, postmenopausal women. Obes Res 2004; 12: 32–39. [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 25. McLaughlin T, Craig C, Liu LF, et al. Adipose Cell Size and Regional Fat Deposition as Predictors of Metabolic Response to Overfeeding in Insulin‐Resistant and Insulin‐Sensitive Humans. Diabetes 2016; 65: 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JY, van de Wall E, Laplante M, et al. Obesity‐associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007; 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alligier M, Gabert L, Meugnier E, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab 2013; 98: 802–810. [DOI] [PubMed] [Google Scholar]
- 28. Fabbrini E, Yoshino J, Yoshino M, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest 2015; 125: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A 2010; 107: 18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johannsen DL, Tchoukalova Y, Tam CS, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the "adipose tissue expandability" hypothesis. Diabetes Care 2014; 37: 2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48: 301–308. [DOI] [PubMed] [Google Scholar]
- 32. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009; 32: 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albu JB, Murphy L, Frager DH, Johnson JA, Pi‐Sunyer FX. Visceral fat and race‐dependent health risks in obese nondiabetic premenopausal women. Diabetes 1997; 46: 456–462. [DOI] [PubMed] [Google Scholar]
- 34. Gealekman O, Guseva N, Hartigan C, et al. Depot‐specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011; 123: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skurk T, Alberti‐Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007; 92: 1023–1033. [DOI] [PubMed] [Google Scholar]
- 36. Fried SK, Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J Lipid Res 1989; 30: 1917–1923. [PubMed] [Google Scholar]


