Summary
Objective
The Action for Health in Diabetes (Look AHEAD) trial was a randomized controlled clinical trial to compare the effects of 10 years of intensive lifestyle intervention (ILI) with a control condition of diabetes support and education (DSE) on health outcomes in over 5,000 participants with type 2 diabetes. The ILI had significantly greater weight losses than DSE throughout the trial. The goal of this analysis is to describe the cost of delivering the intervention.
Methods
The ILI was designed to promote weight loss and increase physical activity. It involved a combination of group plus individual intervention sessions, with decreasing frequency of contact over the 10 years. The intervention incorporated a variety of strategies, including meal replacement products, to improve weight loss outcomes. The costs of intervention delivery were derived from staff surveys of effort and from records of intervention materials from the 16 US academic clinical trial sites. Costs were calculated from the payer perspective and presented in 2012 dollars.
Results
During the first year, when intervention delivery was most intensive, the annual cost of intervention delivery, averaged (standard deviation) across clinical sites, was $2,864.6 ($513.3) per ILI participant compared with $202.4 ($76.6) per DSE participant. As intervention intensity declined, costs decreased, such that from years 5 to 9 of the trial, the annual cost of intervention was $1,119.8 ($227.7) per ILI participant and $102.9 ($33.0) per DSE participant. Staffing accounted for the majority of costs throughout the trial, with meal replacements and materials to promote adherence accounting for smaller shares.
Conclusions
The sustained weight losses produced by the Look AHEAD intervention were supported by intervention costs that were within the range of other weight loss programmes. Future work will include an evaluation of the cost‐effectiveness of the ILI and will contain additional follow‐up data.
Keywords: Cost, diabetes, obesity, weight loss
Introduction
Recent clinical trials have shown that lifestyle interventions using behavioural counseling to induce weight loss and increase physical activity can have important health benefits 1, 2. The Diabetes Prevention Program (DPP), e.g. lifestyle intervention, reduced the risk of developing diabetes by 58% compared with a placebo intervention 3. In Look AHEAD, intensive lifestyle intervention (ILI) did not reduce cardiovascular morbidity and mortality in persons who were overweight or obese with type 2 diabetes 4, but it produced improvements in sleep apnea, incontinence and erectile dysfunction, reduced the incidence of high‐risk kidney disease and resulted in better long‐term diabetes control and some remission of diabetes, as well as fewer hospitalizations, compared with the control group 5, 6, 7, 8, 9, 10. An important concern often raised 11 is that these interventions are costly to provide: data are needed on the costs involved in offering different types of weight loss programmes. Such information will serve as the basis for cost‐effectiveness analyses that will inform decisions about the types of lifestyle interventions that should be offered in clinical and public health approaches to obesity.
The cost of offering weight loss programmes is highly variable. A recent review of commercial programmes provided information about monthly costs of programmes for participants 12. Whereas participation in self‐directed programmes may be free or cost less than $20 per month, weight loss programmes that include counseling, meal replacement products and medical monitoring cost $400–$700 per month. Similarly, although the lifestyle intervention provided in the DPP study was estimated (in year 2000 dollars) to cost $1,399 in year 1 ($162 per month) 13, the DPP has been translated into community‐based programmes (e.g. YMCA) that typically last 1 year and cost $400 to $600 per participant (or $33–$60 per month) 14. Clearly, the variability in these costs relates to the intensity of the programme (i.e. the number of sessions and whether they are offered in groups or individually), the type of staff used to deliver the programme (e.g. peers, nutritionists and physicians) and whether food products are provided. Costs also vary depending on what is included in the cost calculations. Some estimates focus on costs assessed from the perspective of the payer (which include labor costs and may or may not also include costs for renting space and for intervention materials). Other estimates target societal costs, which include both costs from the perspective of the payer and costs from the participant perspective (such as costs for the time spent in intervention sessions, time spent exercising and/or travel time). Costs of commercial programmes to the participant also include a profit margin to sustain the business model.
This paper presents an analysis of the costs involved in delivering the lifestyle intervention in Look AHEAD 15, a large multicentre clinical trial with an intensive weight loss and exercise intervention. The costs of delivering the ILI and its control conditions from the payer perspective are described. Costs associated with staffing group and individual sessions, providing intervention materials and supplying meal replacement products are differentiated. Methodological issues involved in estimating direct costs, especially costs related to space for conducting these interventions, are also discussed.
Research design and methods
Look AHEAD was a multicentre, randomized controlled trial in individuals who were overweight or obese with type 2 diabetes that evaluated the effect of an ILI focused on weight loss and physical activity relative to a control condition. The primary outcome was incidence of major cardiovascular events 4. Secondary outcomes included many other markers of health 15. To be eligible for enrollment, participants were aged 45–76 years, with a body mass index of at least 25 kg m−2 (27 kg m−2 if using insulin), HbA1c < 11%, systolic blood pressure < 160 mmHg, diastolic blood pressure < 100 mmHg and triglycerides < 600 mg dL−1 15. They underwent a maximal graded exercise test (to ensure that exercise could be safely prescribed) and completed 2 weeks of monitoring food intake and physical activity. They were then randomly assigned, with equal probability, to either the ILI or the control condition, referred to as diabetes support and education (DSE).
Participants were enrolled between 2001 and 2004. All informed consent procedures were approved by local Institutional Review Boards, and the consent forms were signed by the participants. The trial was registered at ClinicalTrials.gov Identifier: NCT00017953. The interventions continued through September 2012. Detailed descriptions of both the ILI and DSE interventions have been published elsewhere 16, 17. A brief synopsis follows.
Intensive lifestyle intervention
The ILI was designed to achieve and sustain an average loss of 7% or more of initial weight, primarily through an intensive regimen including diet modification and increased physical activity 16. During the first 6 months of ILI, participants attended three group meetings and one individual session per month. For the remainder of the first year, participants were provided two groups and one individual meeting per month. In months 13–48, participants attended monthly individual meetings that were followed approximately 14 days later with phone calls or e‐mails from interventionists. Optional monthly group meetings were also offered during these latter years.
The intervention sessions were typically led by registered dietitians (RDs) or exercise specialists. Individual sessions were planned to last about 30 min and usually were provided by a single interventionist. Group classes were often conducted by two or more staff members who might include a lifestyle interventionist and a research assistant or a combination of an RD and an exercise specialist. These sessions were scheduled for 60–90 min and were offered at several different times (day and evening sessions) to accommodate participants' schedules. Depending on the site, group classes varied from fewer than 10 participants to up to 20 members.
The ILI participants were asked to keep daily records of their food intake and physical activity. They were also instructed to attempt to reach behaviour and activity goals (described next) and turn in their diary records to the intervention staff at scheduled meetings. Intervention staff provided individualized feedback. The dietary goal of ILI participants included a calorie and fat gram prescription and use of meal replacement products to help participants adhere to their calorie goals. Goals were individualized on the basis of body weight. During the first 4 months, participants were provided servings of liquid meal replacement (e.g. Slim Fast, HMR, OPTIFAST and Ensure) to replace two meals and one snack per day. The physical activity component of the ILI consisted mostly of home‐based exercises with a goal of 175 min of moderate‐intensity physical activity per week. Lifestyle strategies were provided to facilitate adherence to diet and activity goals. Beginning in month 7, a ‘toolbox’ algorithm was implemented: participants who had not lost 5% of initial weight in the first 6 months were offered more advanced behavioural strategies or the optional use of a weight loss medication (orlistat) 16.
Diabetes support and education
The DSE intervention was designed to retain participants in the trial and consisted of educational sessions focused on diet, physical activity and social support 17. Four meetings were offered in year 1, three per year in years 2–4 and one meeting per year thereafter. Attendance at these meetings was optional. Each meeting lasted 90–120 min and was typically taught by a team that might include an RD, an exercise specialist, and a nurse educator or behaviour therapist. Different team members attended different sessions, on the basis of the topics covered. Each session was offered at several different times (daytime and evenings) to accommodate participants' schedules.
Assessment of costs involved in intensive lifestyle intervention and diabetes support and education delivery
The data used to estimate personnel costs came from two sources: (i) salary data from sites (year 2007) and (ii) periodic surveys of sites (see Appendix 1 for further details on timing of surveys and Appendix 2 for common staffing types). The year 2007 was used as a benchmark because it was the midpoint of intervention delivery from 2001 to 2012. The survey queried the number of specific types of staff members at the centre (e.g. the number of RDs), the percent of individual or group lifestyle sessions that were conducted by this type of staff member (by an RD) and the time spent in delivery. Although extensive training was done to ensure proper completion of these surveys, a review of the data suggested that sites interpreted the questions in different ways. For example, among sites that had three RDs and where individual sessions were always conducted by an RD, sites differed in reporting that each RD conducted 33% of the individual sessions or 100% of the individual visits. The latter led to the unlikely conclusion that three RDs were present at each individual session. To clarify the staffing patterns that were used, a biostatistician at the Coordinating Center called each site to verify the reported staffing pattern and correct errors. (See Appendix 4 for actual survey questions.)
Staffing models and time allocations were used to project personnel costs of delivering ILI and DSE. Research‐related staffing costs and facility (building/rent) costs were excluded, but both time spent preparing and delivering sessions were considered. Actual salaries and fringe benefits for each type of staff member (e.g. RD, exercise specialist and nurse) were obtained from each site and were used to calculate personnel costs. From a separate database, we also determined the number and type of visits attended by each participant and for those in ILI, their use of meal replacement products and orlistat. Although the study received meal replacement products and orlistat free of charge (from institutional donors), the costs that would have been associated with these products in typical programmes were used.
Differences in the costs (in 2012 dollars) of delivery between the ILI and DSE interventions over 9 years of follow‐up were examined. This cost estimate will be useful later in reporting the cost‐effectiveness of the Look AHEAD intervention.
Statistical methods
Staffing effort was collected beginning in 2001. Salary data (including fringe costs) for these efforts were obtained in 2007 dollars (to adopt a common benchmark within the time span of the intervention). For consistency and comparison to a previous Look AHEAD publication 7, salaries were inflation‐adjusted by 3% per year to be in 2012 dollars. Staff salaries associated with participant visits that occurred in 2001/2002 used this 2012 inflation‐adjusted salary, whereas those associated with participant visits in 2003 and beyond not only received the inflation adjustment to 2012 but also received a 3% salary increase for each year beyond 2002.
Annual staffing costs per participant in Table 4 were obtained by first summing costs within individual and year. Next, these sums were averaged within clinic, and finally, an across‐clinic average (and standard error) was obtained.
Meal replacement cost data were obtained at the clinic level by year. Costs were distributed equally among all ILI participants at the clinic during that year. Use of orlistat was collected on a per‐participant basis. The cost of orlistat was summed across all participants within site and year and was then distributed equally among all participants. Toolbox funds were dollars made available to clinics to assist participants struggling with weight loss (ILI) and were used to provide DSE participants with educational and other materials to aid in diabetes management.
Results
Participants' baseline characteristics and subsequent weight loss
Table 1 describes the demographic characteristics of participants. Nearly 60% of participants were women, and approximately one‐third were from racial and ethnic minorities. As reported previously, ILI and DSE participants lost an average of 8.6% and 0.7% of initial weight, respectively, at year 1. Mean losses from baseline were 4.7% and 1.1%, respectively, at year 4 and 6.0% and 3.5%, respectively, at an average of 9.6 years of follow‐up, when the intervention was terminated.
Table 1.
Baseline characteristics of Look AHEAD participants by intervention assignment
| Baseline characteristic | Diabetes support and education N = 2,575 | Intensive lifestyle intervention N = 2,570 |
|---|---|---|
| Mean (SD) or N (%) | Mean (SD) or N (%) | |
| Age | 58.8 (6.9) | 58.6 (6.8) |
| Sex | ||
| Female | 1,537 (59.7) | 1,526 (59.4) |
| Male | 1,038 (40.3) | 1,044 (40.6) |
| Race/Ethnicity | ||
| African‐American | 404 (15.7) | 400 (15.6) |
| Asian/Pacific Islander | 21 (0.8) | 29 (1.1) |
| Hispanic | 340 (13.2) | 340 (13.2) |
| Native American | 128 (5.0) | 130 (5.1) |
| Non‐Hispanic White | 1,631 (63.3) | 1,621 (63.1) |
| Other/Multiple | 51 (2.0) | 49 (1.9) |
| Body mass index (kg m−2) | ||
| <30 | 362 (14.1) | 403 (15.7) |
| 30 to <35 | 899 (34.9) | 918 (35.7) |
| 35 to <40 | 740 (28.7) | 672 (26.2) |
| >40 | 574 (22.3) | 577 (22.5) |
| Fitness (METS) | 7.18 (2.0) | 7.2 (1.9) |
| HbA1c (%) | ||
| <7.0 | 1,154 (44.8) | 1,197 (46.6) |
| 7.0 to 8.9 | 1,189 (46.2) | 1,185 (46.1) |
| 9.0 to 11.0 | 232 (9.0) | 188 (7.3) |
METS, metabolic equivalents; SD, standard deviation.
Staffing costs per session
Table 2 displays the protocol‐specified and actual average number of sessions attended per year per participant for DSE and ILI participants. The DSE participants attended 2.7 of the four sessions offered during year 1 and 2.0 to 2.3 of the three offered during years 2–4. After 4 years, one session per year was offered, but participants could attend the session again if desired; on average, participants attended 1.1 sessions per year. The time involved in offering these sessions, which included both preparation time and actual delivery time, averaged 4–6 h per session, and two or more providers were typically involved. (See Appendix 2 for typical staffing patterns.)
Table 2.
Staffing effort and cost per session by intervention group
| Year since randomization | DSE | ILI | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of sessions proposed | Number of sessions attended | Staff time per session (h) | Cost per participant per session ($) | Number of sessions proposed | Number of sessions attended | Staff time per session (h) | Cost per participant per session ($) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Year 1 sessions | ||||||||
| Group | up to 4 | 2.7 (1.1) | 4.5 (2.4) | 43.1 (33.4) | 30 | 25.6 (5.8) | 5.8 (2.8) | 33.0 (16.1) |
| Individual | 0 | 12 | 10.7 (2.8) | 1.5 (0.6) | 63.7 (28.6) | |||
| Phone | 0 | 1.2 (0.5) | 0.2 (0.1) | 12.3 (6.5) | ||||
| Year 2 sessions | ||||||||
| Group | up to 3 | 2.3 (1.0) | 4.8 (2.9) | 39.4 (24.4) | (>12 in person plus 12 other, group and individual combined) | 9.2 (6.4) | 4.6 (2.2) | 32.8 (12.4) |
| Individual | 0 | 9.4 (3.6) | 1.4 (0.4) | 61.3 (20.4) | ||||
| Phone | 0 | 3.9 (2.8) | 0.2 (0.1) | 11.7 (5.7) | ||||
| Year 3 sessions | ||||||||
| Group | up to 3 | 2.1 (0.9) | 5.2 (3.1) | 39.2 (18.5) | (>12 in person plus 12 other, group and individual combined) | 8.6 (6.7) | 4.1 (1.4) | 33.7 (11.8) |
| Individual | 0 | 8.4 (3.8) | 1.4 (0.4) | 57.1 (18.8) | ||||
| Phone | 0 | 4.8 (3.9) | 0.2 (0.1) | 11.3 (6.6) | ||||
| Year 4 sessions | ||||||||
| Group | up to 3 | 2.0 (0.9) | 5.2 (2.5) | 40.0 (19.3) | (>12 in person plus 12 other, group and individual combined) | 8.6 (6.8) | 4.4 (1.4) | 34.9 (11.4) |
| Individual | 0 | 8.2 (4.3) | 1.3 (0.4) | 55.4 (19.4) | ||||
| Phone | 0 | 4.1 (3.9) | 0.2 (0.1) | 10.3 (4.3) | ||||
| Years 5–9 sessions | ||||||||
| Group | 1 to 2 | 1.1 (0.1) | 6.1 (2.1) | 52.6 (27.6) | 12 offered | 6.6 (5.2) | 6.8 (5.1) | 51.8 (47.0) |
| Individual | 0 | 12 offered | 4.7 (3.2) | 1.1 (0.4) | 48.7 (23.0) | |||
| Phone | 0 | 2.2 (1.7) | 0.2 (0.1) | 9.1 (4.5) | ||||
For diabetes support and education (DSE), typical number of participants per group session ranged between 6 and 10 persons; for intensive lifestyle intervention (ILI), typical numbers ranged between 10 and 14 persons.
Standard deviations (SDs) represent variability among clinical sites.
Costs are expressed in 2012 dollars.
The ILI participants averaged 10.7 individual and 25.6 group sessions in their first year. This decreased to approximately nine individual and nine group sessions in year 2, eight group and eight individual sessions in years 3 and 4 and 6.5 contacts of each type thereafter. Phone sessions averaged one call per participant during the first year and four to five calls in subsequent years; each call lasted approximately 12 min. Preparation and delivery of the individual sessions were reported to take about 1.5 h per participant. Typically, one provider was involved. Group sessions, which typically involved two or more providers, required on average 5.8 h of effort (preparation and delivery) in year 1, 4.1 to 4.6 h in years 2–4 and 6.8 h in subsequent years. Although group sessions were longer than individual sessions, and involved more staff time, the costs involved in offering the group sessions were averaged across all participants who attended and were thus lower per participant than individual sessions.
Non‐staffing costs
Table 3 displays the annual costs of such items as meal replacements, orlistat, donations and toolbox items by treatment group. For DSE participants, non‐staffing costs were limited to donations and toolbox items alone (i.e. diabetes‐related educational materials) and averaged $86 per participant in year 1 and $45 per participant in subsequent years.
Table 3.
Annual non‐staffing costs, ILI and DSE
| DSE cost per participant per year | ILI cost per participant per year | |
|---|---|---|
| Mean | Mean (SD) | |
| Year 1 | ||
| Donations and toolbox | 86.0 | 479.7 |
| Orlistat | 0 | 44.7 (28.5) |
| Meal replacements | 0 | 798.0 (114.8) |
| Total | 86.0 | 1,322.4 (138.7) |
| Year 2 | ||
| Donations and toolbox | 45.0 | 309.1 |
| Orlistat | 0 | 59.3 (40.9) |
| Meal replacements | 0 | 651.1 (139.0) |
| Total | 45.0 | 1,019.5 (152.9) |
| Year 3 | ||
| Donations and toolbox | 45.0 | 309.1 |
| Orlistat | 0 | 52.3 (36.4) |
| Meal replacements | 0 | 512.7 (124.3) |
| Total | 45.0 | 874.1 (132.0) |
| Year 4 | ||
| Donations and toolbox | 45.0 | 309.1 |
| Orlistat | 0 | 28.0 (21.1) |
| Meal replacements | 0 | 366.0 (106.1) |
| Total | 45.0 | 703.1 (103.2) |
| Years 5–9 | ||
| Donations and toolbox | 45.0 | 309.1 |
| Orlistat | 0 | 12.7 (3.2) |
| Meal replacements | 0 | 207.2 (44.7) |
| Total | 45.0 | 529.0 (445.0) |
DSE, diabetes support and education; ILI, intensive lifestyle intervention; SD, standard deviation.
Year 1 meal replacements averaged 360.8 units per person 19.
For ILI participants, non‐staffing costs included meal replacements, orlistat, donations and toolbox items (including small exercise and diet equipment) (Appendix 3). Of these, meal replacements were the major contributor to cost. Non‐staffing costs were approximately $1,322 in year 1, declining to $529 in later years.
Total annual per participant costs
Table 4 displays the average annual cost per participant of intervention delivery, taking into account both staffing and non‐staffing costs. For DSE participants, the average per participant cost was approximately $202 during their first year, $124 to $136 during years 2–4 and thereafter, $103 per participant per year. For ILI participants, the average per participant cost for year 1 totaled $2,865. In later years, less attendance was required, and fewer meal replacements were used; thus, per participants costs declined. Per participant costs were $1,944 in year 2, $1,698 in year 3, $1,499 in year 4 and $1,120 in years 5–10.
Table 4.
Total annual cost of intervention delivery, staff and non‐staff costs combined
| Year since randomization | DSE total annual cost ($) per participant | ILI total annual cost ($) per participant |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| 1 | 202.4 (76.6) | 2,864.6 (513.3) |
| 2 | 135.6 (44.5) | 1,944.0 (315.3) |
| 3 | 127.3 (40.2) | 1,698.2 (317.2) |
| 4 | 125.0 (39.2) | 1,499.1 (300.2) |
| 5–9 | 102.9 (33.0) | 1,119.8 (227.7) |
DSE, diabetes support and education; ILI, intensive lifestyle intervention; SD, standard deviation.
SDs represent variability among clinical sites.
Costs are expressed in 2012 dollars.
Figure 1 shows the average per participant cost of the ILI and the DSE programmes during each year of the intervention. Costs were higher for ILI participants, especially during year 1. In year 1, the cost per participant per kg lost was $329.30.
Figure 1.
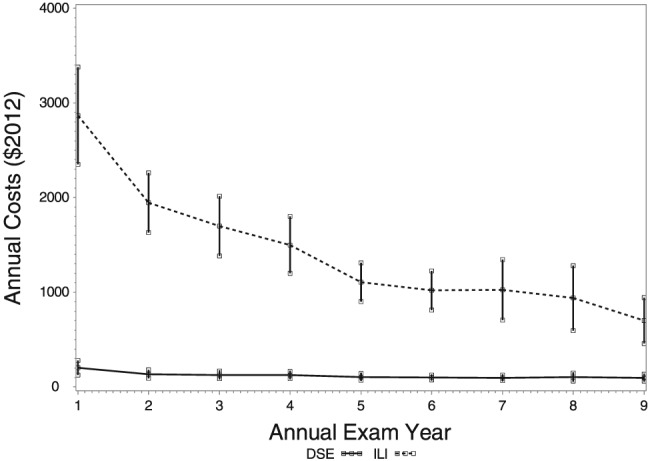
The average per participant cost of the ILI and the DSE programmes during each year of the intervention. DSE, diabetes support and education; ILI, intensive lifestyle intervention.
Conclusions
This paper provides detailed information about the cost of offering the ILI in Look AHEAD and important data about the treatment components that contributed to this cost. The cost of offering ILI (including staffing costs for individual session and group sessions and costs for meal replacement products and intervention supplies) was $2,865 per participant in year 1, $1,944 in year 2 and decreased gradually to $1,120 in years 5–9. In contrast, the DSE programme cost (including staffing and retention items) was only $202 per participant in year 1, $136 in year 2 and decreased to $103 in years 5 to 9. The major contributor to this difference was the intensity of the treatment contact: ILI participants were offered 12 individual and 30 group sessions in year 1, as compared with only four group sessions for the DSE group.
The primary component of the cost of the ILI was the staffing, which accounted for 60–70% of the per capita annual cost across years. The variability between clinics in the total annual cost of conducting the interventions likely reflects differences in the type and number of staff members that were used to conduct the sessions and differences in attendance at these sessions. The Look AHEAD lifestyle intervention included both group and individual sessions in an effort to capitalize on the strengths of these two different approaches. Group treatment programmes have produced better weight losses than individual programme 18, and thus, our programme relied primarily on group sessions, especially in year 1 of the programme when contacts were most frequent. Although group sessions were longer in duration and required more staff members than individual sessions, the fact that the costs are spread across many different individuals significantly reduces the per person cost. During year 1 of Look AHEAD, the per participant cost of an individual session was more than twice as much as a group class; however, by year 4, the study‐wide costs of group and individual sessions were similar because of the lower attendance at group sessions in later years. On the basis of the findings from the literature, use of meal replacement products was included in our lifestyle intervention to promote greater weight loss and maintenance 19. Although greater use of meal replacement products was associated with better outcomes 20, it is likely that those who use the meal replacement products were also adhering to other aspects of the intervention. Although meal replacement products were donated to the trial, if purchased, they would have cost $798 and $650, respectively, per participant in years 1 and 2, $513 in year 3, $366 in year 4 and $207 per participant in later years, because of the decreasing use of these items over time.
Costs of lifestyle intervention supplies, including items such as food scales and measuring cups, and exercise tools such as pedometers, fit stability balls and walking tapes, were estimated. These supplies cost approximately $300 per participant per year. It is unclear if these items were related to weight loss outcomes, but they are believed to contribute to retaining interest in the trial and motivating participants to continue to change eating and exercise behaviours.
Although cost of purchasing or renting space is often included in the direct cost calculations for a programme, this was not included in our calculation because of the difficulty estimating this parameter, both for group and individual sessions. In many programmes, space is rented for each intervention session, and a rental cost (e.g. $50 per room per hour) may be used to estimate this parameter. For example, Jakicic et al. conducted a group‐based lifestyle programme, with 42 group sessions plus individual make‐up meetings over 18 months. Their total per capita cost of space was estimated at $122 21. In other studies, a general estimate of overhead costs is provided by using a percentage of the personnel costs; using this approach, DPP estimated that the per capita cost of the lifestyle intervention was 69% of the cost of personnel or $519 during year 1 13. Whereas this latter calculation appears to include costs of the office space used by staff members, the approach taken by Jakicic et al. does not. Whether or not to include such space may depend on factors such as whether the staff member is a full‐time employee or comes to the site only to offer the treatment contacts. Other factors, such as where the space is located (within a hospital, in a commercial property or in space provided by a community partner; within or outside the city proper), will also influence the costs of space.
As previously reported 4, the ILI in Look AHEAD produced a mean weight loss of 8.6% at year 1 and 6.0% at the end of the intervention (median of 9.6 years). This weight loss was significantly greater than the 0.7% and 3.5% weight loss in DSE at year 1 and the end of intervention, respectively. As noted earlier, the lifestyle intervention had a large number of both short‐term and long‐term health benefits relative to DSE 4, 5, 6, 7, 8, 9, 10. In addition, the lifestyle intervention was associated with a reduction in medical care costs, with a 10% savings related to hospitalizations and a 7% savings in medication costs. Over 10 years, the ILI led to a mean relative per person cost saving of $5,280 7. Future work, which will incorporate additional follow‐up, will evaluate the overall cost‐effectiveness of the intervention. This evaluation will weigh the contributions of three components: healthcare cost savings, the costs involved in offering the lifestyle intervention and the health benefits that are associated with the intervention.
Our study has some limitations. Estimates of personnel time were based on cross‐sectional surveys. The rate that intervention tools (e.g. orlistat) were used by participants may have been increased because they were provided free of charge. Attendance rates in this clinical trial also may have been higher than those typically seen in clinical practice settings. Finally, we have used actual labor costs instead of Bureau of Labor Statistics national wage rates, which may limit generalization.
Recently, there have been a number of efforts to reduce the cost of lifestyle interventions, as provided in the DPP, by offering programmes at community centres, using lay community intervention staff 22, or by using digital media to deliver programmes 23. A meta‐analysis of this literature showed that there were no differences in weight loss achieved by trained professionals vs. lay educators 24. Similarly, these authors noted that there were no data on the cost‐effectiveness of 8, 12 or 16 treatment sessions, but that the more sessions participants attended, the greater their weight loss. Finally, the effectiveness, as well as the cost‐effectiveness, of lifestyle interventions used in combination with contemporary technology (e.g. activity monitors) remains unclear 25.
In conclusion, the cost of the ILI used in Look AHEAD was $2,864.60 per participant in the first year (or $329 per kg weight loss) but decreased over the subsequent 8 years of the intervention. These costs were driven primarily by personnel costs. Look AHEAD investigators currently are examining whether the costs of ILI in overweight and obese participants with type 2 diabetes are justified by a reduction in a variety of health problems and the associated medical care costs.
Central resources centres
DXA Reading Center, University of California at San Francisco Michael Nevitt, PhD (Principal Investigator); Ann Schwartz, PhD (Program Coordinator); John Shepherd, PhD (Co‐investigator); Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH.
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories Santica M. Marcovina, PhD, ScD (Principal Investigator); Jessica Chmielewski (Program Coordinator); Vinod Gaur, PhD.
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS (Principal Investigator); Ronald J. Prineas, MD, PhD (Principal Investigator); Charles Campbell (Program Coordinator); Zhu‐Ming Zhang, MD (Co‐investigator); Teresa Alexander; Lisa Keasler; Susan Hensley; Yabing Li, MD.
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities Robert Moran, PhD (Principal Investigator).
Hall‐Foushee Communications, Inc Richard Foushee, PhD; Nancy J. Hall, MA.
Federal sponsors
National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z.Yanovski, MD; Robert Kuczmarski, PhD.
National Heart, Lung and Blood Institute Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR.
Centers for Disease Control and Prevention Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD.
All other Look AHEAD staffs are listed alphabetically by site.
Funding
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135 and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women's Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service provided personnel, medical oversight and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical
Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc, a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc; Hoffmann‐La Roche Inc; Abbott Nutrition; and Slim‐Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Disclosure
Dr Goldman reports support from Precision Health Economics, outside the submitted work. Dr Cheskin reports grants from NIH during the conduct of the study and personal fees from Pressed Juicery, Inc, outside the submitted work. Dr Greenway reports grants from National Institutes of Health (NIDDK) during the conduct of the study. Dr Lewis reports grants from Novo Nordisk outside the submitted work.
Supporting information
Supporting info item
Rushing, J. , Wing, R. , Wadden, T. A. , Knowler, W. C. , Lawlor, M. , Evans, M. , Killean, T. , Montez, M. , Espeland, M. A. , Zhang, P. , and The Look AHEAD Research Group (2017) Cost of intervention delivery in a lifestyle weight loss trial in type 2 diabetes: results from the Look AHEAD clinical trial. Obesity Science & Practice, 3: 15–24. doi: 10.1002/osp4.92.
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
References
- 1. Batsis JA, Gill LE, Masutani RK, et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc 2016. DOI: 10.1111/jgs.14514.EPub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med 2013; 369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wing RR, Rosen RC, Fava JL, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD Trial. J Sex Med 2010; 7: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phelan S, Kanaya AM, Subak LL, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD Trial. J Urol 2012; 187: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espeland MA, Glick HA, Bertoni AL, Brancati FL, Bray GA, Clark JM, et al. Impact of an intensive lifestyle intervention on use and costs of medical services among overweight and obese adults with type 2 diabetes: the Action for Health in Diabetes. Diabetes Care, 2014;37:2548‐2556. PMCID: PMC4140155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gregg EW, Chen H, Wagenknecht LE, et al. Association of intensive lifestyle intervention with the remission of type 2 diabetes: the Look AHEAD study. JAMA 2012; 308: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuna ST, Reboussin DM, Borradaile KE, et al. Long‐term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013; 36: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Look AHEAD Research Group . Effect of a long‐term behavioral weight loss intervention nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomized clinical trial. Lancet Diabetes Endocrinol 2014; 10: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudzune KA, Bleich SN, Clark JM. Efficacy of commercial weight‐loss programs. Ann Intern Med 2015; 163: 399. [DOI] [PubMed] [Google Scholar]
- 12. The Diabetes Prevention Program Research Group . Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care 2003; 26: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Prevention Program Research Group . The 10‐year cost‐effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent‐to‐treat analysis of the DPP/DPPOS. Diabetes Care 2012; 35: 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The Look AHEAD . Research Group. Look AHEAD: Action for Health in Diabetes. Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003; 24: 610–628. [DOI] [PubMed] [Google Scholar]
- 15. Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006; 14: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Look AHEAD Research Group . The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials 2011; 8: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol 2001; 69: 717–721. [PubMed] [Google Scholar]
- 18. Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord 2003; 27: 537–549. [DOI] [PubMed] [Google Scholar]
- 19. Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped‐care intervention approach on weight loss in adults: a randomized clinical trial. JAMA 2012; 307: 2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wadden TA, West DS, Neiberg RH, et al. One‐year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: The DEPLOY Pilot Study. Am J Prev Med 2008; 35: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harvey‐Berino J, West D, Krukoski R, et al. Internet delivered behavioral obesity treatment. Prev Med 2010; 51: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali MK, Echouffo‐Tcheugui J, Williamson DF. How effective were lifestyle interventions in real‐world settings that were modeled on the Diabetes Prevention Program? Health Aff 2012; 31: 67–75. [DOI] [PubMed] [Google Scholar]
- 24. Jakicic JM, Davis KK, Roger RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long‐term weight loss: the IDEA randomized clinical trial. JAMA 2016; 316: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kozak AT, Buscemi J, Hawkins MA, et al. Technology‐based interventions for weight management: current randomized controlled trial evidence and future directions. J Behav Med 2016. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


