Summary
Background
Excess adipose tissue may lead to sequestrating of vitamin D, making it less available for use in the body.
Objective
This study determined if overweight or obese individuals (BMI > 25 kg m−2) had insufficient (<30 ng mL−1) levels of 25‐hydroxyvitamin D [25(OH)D] and, if so, would serum levels respond to exogenous supplementation.
Methods
Sixty‐three women who were overweight/obese (BMI = 31.07 ± 5.00 kg m−2) were randomly assigned in a double‐blind manner to receive 5,000 IU of vitamin D3 (D3) (n = 31) or a placebo (PL) (n = 32) daily. Serum 25(OH)D concentrations were measured by finger‐stick analyses at baseline and after 8 weeks of supplementation. Data were analyzed by using a 2 × 2 (group × time) repeated measure multivariate analysis of variance to determine group differences for pre‐values and post‐values (p < 0.05).
Results
On day one of the study, both D3 and PL groups had insufficient levels of vitamin D (mean ± SD) 24.03 ± 9.78 ng mL−1 and 23.62 ± 9.77 ng mL−1, respectively. After 8 weeks of supplementation, the D3 group 25(OH)D level rose to a mean of 43.57 ± 10.87 ng mL−1 (p < 0.001) versus the PL group whose 25(OH)D level remained statistically unchanged 24.31 ± 8.84 ng mL−1. Women who were overweight/obese had insufficient vitamin D levels prior to supplementation.
Conclusions
Following supplementation with 5,000 IU of vitamin D3, all subjects' 25(OH)D levels rose to a sufficient level (≥30 ng mL−1). The findings of this study concur with the Institute of Medicine and Endocrine Society recommendations in that two to three times the daily requirement of vitamin D is required to improve serum vitamin D levels in individuals who are overweight or obese.
Keywords: 25(OH)D levels, D3, obesity, overweight
Introduction
Vitamin D is a fat‐soluble vitamin that has many roles in the body. Perhaps, its most well‐known and important role is that it promotes the absorption of calcium from the small intestine and aids in retaining calcium that may otherwise be excreted. It also assists in maintaining phosphorus levels. Both calcium and phosphorus are crucial to healthy bone development and maintenance. Vitamin D deficiency in adults causes osteomalacia and an increased risk of falls 1. Receptors for vitamin D are found throughout the body, including genes and many types of cell membranes 2. Therefore, vitamin D is likely instrumental in many biological actions beyond bone health. Vitamin D deficiency [25(OH)D serum level <20 ng mL−1] and insufficiency [25(OH)D serum level 21–29 ng mL−1] 3, 4 have been associated with numerous health disorders including, but not limited to, hypertension; heart disease; certain cancers such as colon, breast and prostate; and type I diabetes 5, 6, 7. However, these roles are not as well understood at this time.
Unlike most other vitamins, which are generally obtained through dietary sources, vitamin D is not naturally present in most foods. Fatty fish and fish liver oils are among the few quality sources of vitamin D, and smaller amounts are found in beef liver and some mushrooms 8. Vitamin D can be produced by the body itself through exposure to sunlight. Skin exposed to ultraviolet‐B (UV‐B) radiation from sunlight converts cutaneous 7‐dehydrocholesterol into pre‐vitamin D3, which isomerizes to vitamin D3. The liver then converts vitamin D3 into 25‐hydroxyvitamin D [25(OH)D], which can then be converted into 1,25‐dihydroxyvitamin‐D [1,25(OH)2D] in the kidneys 2. Although 1,25(OH)2D is the active form of vitamin D, serum 25(OH)D is often used to assess vitamin D status in the body. This is due to its much longer half‐life (3–4 weeks vs. 3–4 h), as well as having a circulating level that is approximately 1,000‐fold greater 9.
Because vitamin D is produced by a healthy body under conditions of adequate sunlight, any factor that reduces the amount of UV‐B exposure may reduce the natural production of vitamin D and could put one at risk of deficiency or insufficiency. Living at latitudes greater than 37°N and below 37°S during the winter and early spring, the shorter days and greater cloud cover can all negatively affect 25(OH)D levels. Because of seasonal variation in UV‐B exposure, 25(OH)D levels are lowest in the winter and early spring 10. Physical barriers such as clothing, sunscreens and UV‐blocking glass can also negatively affect 25(OH)D levels 2, 11, 12.
However, insufficient vitamin D production is not limited to non‐biological factors. Skin pigmentation can play a significant role, as it can hinder UV‐B absorption, even under conditions of adequate exposure 2. People with lighter skin absorb more UV‐B rays than those with darker skin. The body also seems to lose its ability to produce vitamin D over time. Increasing age has been associated with lower levels of vitamin D, regardless of the season or location 13. These situations and conditions necessitate an increased focus on obtaining vitamin D from food and supplements.
Yet there are certain medical conditions that appear to inhibit the body's ability to adequately extract vitamin D from exogenous sources. These include digestive disorders such as celiac disease and Crohn's disease 14, 15. Also, there are medications taken for other adverse health conditions that can lead to malabsorption of vitamin D, such as corticosteroids, and certain cholesterol‐lowering drugs 16.
Interestingly, 25(OH)D levels have also been associated with body fat levels. There appears to be ample evidence that there is an inverse relationship between 25(OH)D and adiposity. Obese individuals are considered high risk for vitamin D deficiency. This may be partially due to a possible decreased exposure to UV‐B light due to limited mobility 17. However, there are also metabolic factors that may be considered.
One is that parathyroid hormone (PTH) can negatively affect 25(OH)D production. Increased levels of PTH, which are associated with obesity, can enhance the production of 1,25(OH)2D, creating negative feedback that reduces the synthesis of 25(OH)D in the liver 18. However, some research has indicated that 1,25(OH)2D may not always be elevated in the obese, despite high levels of PTH 19.
Additionally, because of vitamin D being fat soluble, it may be sequestered by excess adipose tissue, thus making it less available for use by the body 20. However, research by Drincic et al. challenged this claim when they demonstrated that once adjustments were made for body volume, 25(OH)D levels between obese and non‐obese subjects were the same 21.
Although the daily requirement for vitamin D in 19‐ to 70‐year‐old women with a body mass index (BMI) between 18 and 24.9 kg m−2 is 1,500–2,000 IU d−1, in women who are overweight or obese, the daily requirement is much higher 22. Both the Institute of Medicine (IOM) and the Endocrine Society have recommended that adults that are obese require 6,000–10,000 IU d−1 of vitamin D, in order to maintain a 25(OH)D level above 30 ng mL−1, followed by maintenance therapy of at least 3,000–6,000 IU d−1 1, 23. A recent study by Ekwaru et al. concurs with IOM and the Endocrine Society guidelines finding that vitamin D supplementation needs to be two to three times higher than the daily requirement in individuals who are obese and 1.5 times higher than that in individuals who are overweight in comparison with that in normal‐weight subjects 22. Maki et al. found that a 4‐month dose of 1,200 IU d−1 of vitamin D provided to men and women who were overweight with low 25(OH)D levels was unable to bring 25(OH)D up to adequate levels 24. Recently, Bhagatwala et al. demonstrated that monthly dosing with 60,000 (~2,000 IU d−1) and 120,000 (~4,000 IU d−1) increased 25(OH)D to adequate levels in vitamin D‐deficient young African–American men and women who were overweight and obese 25. Additional studies conducted on individuals who were overweight or obese all seem to indicate that a vitamin D dosage between 4,000–7,000 IU d−1 is required to increase vitamin D to adequate levels 26, 27, 28.
Evidence appears to indicate that in order for adults who are overweight and/or obese to acquire adequate levels of serum 25(OH)D, supplementation two to three times beyond the daily requirement of 1,200 IU d−1 may be required 1, 22, 23. The purpose of this study was to determine whether women who were overweight or obese with insufficient levels of 25(OH)D would respond favorably to daily supplementation of 5,000 IU of vitamin D3 over a period of 8 weeks. It was hypothesized that exogenous supplementation would be sufficient in increasing serum 25(OH)D to an adequate level of ≥30 ng mL−1.
Methods and procedures
Study design
This study was a randomized, double‐blind experimental trial and was approved by the Institution Review Board and Institutional Biosafety Committee at Northern Illinois University. Recruitment was carried out via flyers posted across the university campus and in various retail and/or service establishments within a 30‐mile radius of the campus. Interested participants were screened either via e‐mail or over the phone to determine if they qualified for the study. Provided they met inclusion and exclusion criteria, participants were sent a 3‐d food record to complete and were scheduled for their first visit. All participants voluntarily signed an Institutional Review Board‐approved informed consent prior to participation in the study.
Participant population
To be eligible to participate in this study, women must have met the following criteria: being healthy, having an age of 18–65 years, and having a BMI over 25 kg m−2. Those individuals who were pregnant, were hemophiliac and had hepatic or renal disease were taking more than 1,000 IU of supplemental vitamin D daily, and those using tanning beds were ineligible to participate.
Seventy‐one women who met the selection criteria participated in this study. Eight participants were excluded from the study (four participants were in the vitamin D group and four participants were in the placebo group) because of non‐compliance with the study protocol.
Anthropometric measurements
Anthropometric measurements were taken with subjects in lightweight clothing and bare feet. Height was measured by using a wall‐mounted stadiometer (Ayrton Stadiometer Model S100; Ayrton Corp., Prior Lake, MN, USA). Weight, fat mass, percent fat, fat‐free mass and BMI were assessed by using a bioelectrical impedance scale (Tanita Body Composition Analyzer TBF‐300A; Biospace Inc., Los Angeles, CA, USA). BMI was calculated by the Tanita analyzer by using the standard equation (kg m−2).
Vitamin D assessment
Serum 25(OH)D was assessed by the same two researchers throughout the study following the Institutional Biosafety Committee protocol for blood collection. A lancet was used to initiate a spot of blood from the finger, and then the finger was blotted on a special filter paper provided by ZRT laboratories (Beaverton, OR, USA). Samples were immediately labelled with the subject's name and stored at room temperature for 48 h until fully dry and then frozen at −10 °F until further analysis. Finger‐stick blood spots were analyzed by ZRT laboratories using liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). ZRT laboratories participates in the Vitamin D External Quality Assessment Scheme to ensure the analytical reliability of their 25(OH)D measurements, and it uses National Institute of Standards and Technology (NIST) Vitamin D standard reference material.
Dietary analyses
Finally, the participants were asked to complete a 3‐d food record (two weekdays and one weekend day) and bring it with them to the first visit. The primary investigator examined all the food records for completeness; missing portion sizes were clarified to eliminate missing data. This information was used to help assess the participants' dietary intake regarding total calories, carbohydrate, fat and protein as well as vitamin D and calcium. Food records were later analyzed by a graduate‐level nutrition student using Diet Analysis + for Windows Version 8 (2008; Stamford, CT, USA).
All of the aforementioned procedures were completed twice during the course of the study; once at the beginning of the study and, again, following the 8‐week supplementation period.
Dietary supplementation
Subjects were randomly assigned in a double‐blind manner to receive either 5,000 IU of vitamin D (D3 = 35) or maltodextrin as the placebo (PL = 36) (Shaklee Corporation, Pleasanton, CA, USA). Each subject was instructed to take five pills every day for the duration of the 8‐week study and to keep supplement bottles for recollection during the second visit. Supplement bottles were collected from each individual, and the number of pills remaining was recorded by investigators to assess compliance.
There were no differences between the groups at the beginning of the study on any of the measured dependent variables (p > 0.05). Baseline characteristics of each group can be found in Table 1.
Table 1.
Participant baseline characteristics ( )
| D3 supplement (n = 31) | Placebo (n = 32) | |
|---|---|---|
| Age (years) | 41.2 ± 14.6 | 45.3 ± 11.7 |
| Weight (kg) | 81.2 ± 16.1 | 86.4 ± 17.3 |
| BMI (kg m−2) | 31.3 ± 4.8 | 31.8 ± 5.1 |
| Body fat (%) | 37.8 ± 7.6 | 40.7 ± 6.7 |
| 25(OH)D (ng dL−1) | 24.0 ± 9.7 | 23.6 ± 10.4 |
Independent t‐tests indicated no significant differences between groups (p > 0.05).
BMI, body mass index.
Statistical analyses
Data were analyzed using a 2 × 2 (Group × Time) repeated measures multivariate analysis of variance using pasw statistics (version 18.0, 2009; SPSS Inc., Chicago, IL, USA). Separate univariate 1 × 2 anova tests were conducted for any significant main effects from the multivariate analysis of variance. Equivalency of groups at baseline was determined by independent t‐tests. The 3‐d food records were assessed for differences between and within groups by independent t‐tests and paired‐sample t‐tests. The significance of all tests was based on a p‐value <0.05.
Results
There was a significant main effect for Group (Wilk's Λ(6,56) = 0.727, p = 0.005, partial η 2 = 0.273) and a significant main effect for Time (Wilk's Λ(6,56) = 0.339, p < 0.000, partial η 2 = 0.601). Additionally, there was a significant Group × Time interaction (Wilk's Λ(6,56) = 0.441, p < 0.000, partial η 2 = 0.559).
Follow‐up univariate anovas on the interaction revealed that daily D3 supplementation significantly increased 25(OH)D levels in the experimental group compared with those in the placebo group (F (1,61) = 67.42, p < 0.000, partial η 2 = 0.525). This is displayed in Figure 1. There were no differences for any of the other dependent variables; all of which can be found in Table 2.
Figure 1.
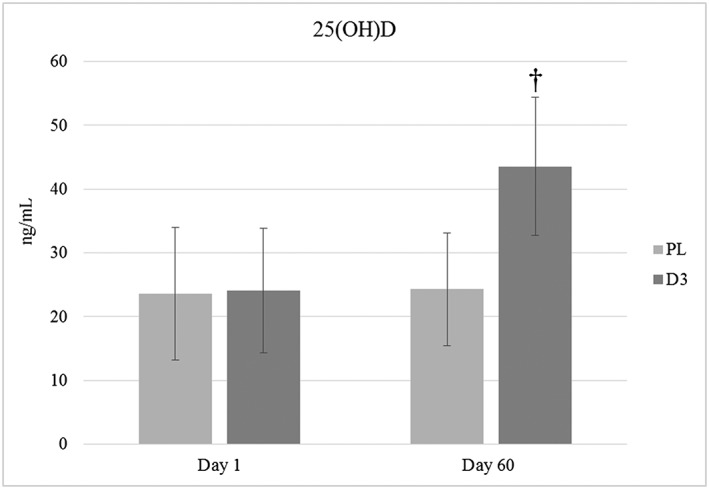
Blood serum 25‐hydroxyvitamin D levels before and after 8‐week supplementation. D3, supplement; PL, placebo. The superscript symbol (†) means different from day 1 (p < 0.05).
Table 2.
Dependent variables before and after 8‐week supplementation ( )
| Group | Day 1 | Day 60 | |
|---|---|---|---|
| Weight (kg) | D3 | 81.2 ± 16.1 | 81.4 ± 16.3 |
| PL | 86.4 ± 17.3 | 87.2 ± 16.5 | |
| BMI (kg m−2) | D3 | 30.3 ± 4.8 | 30.5 ± 5.0 |
| PL | 31.8 ± 5.1 | 32.1 ± 5.0 | |
| Body fat (%) | D3 | 37.8 ± 7.6 | 37.8 ± 7.3 |
| PL | 40.7 ± 6.7 | 41.5 ± 5.5 | |
| Fat mass (kg) | D3 | 31.5 ± 12.8 | 31.8 ± 12.6 |
| PL | 35.7 ± 12.4 | 36.9 ± 11.6 | |
| Fat‐free mass (kg) | D3 | 49.3 ± 6.0 | 49.7 ± 6.0 |
| PL | 50.7 ± 6.8 | 50.3 ± 5.7 | |
| 25(OH)D (ng mL−1) | D3 | 24.0 ± 9.7 | 43.6 ± 10.9† |
| PL | 23.6 ± 10.4 | 24.3 ± 8.8 |
Different from day 1 (p < 0.05).
D3, supplement; PL, placebo.
There were no differences in dietary intake between the groups at the beginning or upon conclusion of the study, nor were there any differences within the groups. Results of the 3‐d food diaries can be found in Table 3.
Table 3.
Three‐day food record results before and after 8‐week supplementation ( )
| Day 1 | Day 60 | |||
|---|---|---|---|---|
| D3 (n = 27) | PL (n = 27) | D3 (n = 27) | PL (n = 30) | |
| kcal d−1 | 1764.90 ± 399.51 | 1750.26 ± 475.05 | 1706.26 ± 349.18 | 1599.15 ± 351.71 |
| kcal kg−1 | 22.30 ± 6.04 | 21.27 ± 5.90 | 21.56 ± 5.29 | 19.00 ± 5.07 |
| Vitamin D per day (IU) | 109.67 ± 66.98 | 92.41 ± 62.99 | 107.21 ± 82.64 | 105.57 ± 80.68 |
| Calcium per day (mg) | 788.12 ± 324.09 | 745.06 ± 257.32 | 729.12 ± 272.47 | 730.35 ± 265.17 |
| % Protein per day | 17.78 ± 4.17 | 16.37 ± 3.38 | 17.48 ± 3.40 | 17.40 ± 2.99 |
| % Carbohydrate per day | 49.30 ± 9.61 | 51.74 ± 7.15 | 48.15 ± 9.16 | 49.20 ± 5.53 |
| % Fat per day | 33.89 ± 8.44 | 33.07 ± 6.25 | 34.78 ± 7.02 | 34.23 ± 6.83 |
Independent t‐tests and paired t‐tests indicated no significant difference between or within groups (p > 0.05).
Different from day 1 (p < 0.05).
D3, supplement; PL, placebo.
Supplement compliance
Supplement compliance was determined based on the number of pills left over after day 56, and the average level of compliance in taking either the vitamin D supplement or placebo was 94.9% ± 5.68% and 96.2% ± 4.09%, respectively. Two participants in the placebo group did not bring their remaining pills to be counted and therefore were not included in the aforementioned compliance rates.
Discussion
Daily D3 supplementation of 5,000 IU for 8 weeks is capable of increasing serum 25(OH)D to adequate levels in middle‐age women who were overweight or obese. To our knowledge, this is the first study using daily dosing of 5,000‐IU D3 supplementation exclusively in this population. The findings of this study concur with other studies 1, 22, 23.
There have been additional studies on individuals who are overweight or obese, indicating that higher amounts of supplemental vitamin D are necessary to achieve adequate vitamin D levels 26, 27, 28. Harris et al. supplemented pre‐diabetic African–American men and women who were overweight or obese with 4,000 IU d−1 of D3. At the conclusion of the 12‐week study, the treatment group had achieved adequate 25(OH)D serum levels (~32.5 ng mL−1) compared with the still‐deficient placebo control group (~15.0 ng mL−1) 26. Similar results were observed by Yiu et al. who administered 5,000 IU d−1 for 12 weeks in older, type II diabetic men and women with suboptimal serum 25(OH)D. While the placebo group experienced no change, the group receiving supplemental D3 had attained adequate 25(OH)D levels by the study's conclusion (58.6 ng mL−1) 27. Lastly, Wamberg et al. administered 7,000 IU to vitamin D‐deficient, obese, but otherwise apparently healthy middle‐age adult men and women for 26 weeks and were successful in increasing 25(OH)D to an adequate serum level of approximately 44 ng mL−1 28. Similar to Wamberg et al., the present investigation increased the 25(OH)D serum levels to an average of 43 ng mL−1 in apparently healthy, middle‐age women, although it was carried out with a lower supplementation amount of only 5,000 IU d−1. Collectively, these studies seem to indicate that in adults who are overweight and/or obese and who have suboptimal levels of 25(OH)D, daily supplementation of at least two to three times the daily requirement as recommended by IOM and the Endocrine Society appears to be necessary to achieve an adequate 25(OH)D status 1, 22, 23.
This study was conducted in Chicago, which is 41° north of the equator. At the initiation of this investigation, the mean vitamin D level for both groups was suboptimal, which is not uncommon in those living in colder climates during the winter months. This study was conducted intentionally in March through the first week in May to determine the effect of exogenous supplementation of vitamin D versus the effect of sun exposure. Supplementation brought most participants in the treatment group within an adequate range over a relatively short period of time. The 5,000‐IU d−1 dose was half the upper limit of 10,000 IU d−1 as determined by the IOM and Endocrine Society; thus, none of the subjects achieved a serum blood level close to what would be considered toxic. In fact, the highest achieved 25‐hydroxyvitamin D level was 60.20 ng mL−1, and vitamin D toxicity (hypercalcemia) is rarely seen before blood serum levels reach 150–200 ng mL−1 for a consistent period of time 29, 30. This is a notable finding and contributes to the body of research related to how much supplemental vitamin D is tolerated without observing negative effects. This also shows serum blood levels may also be increased to what is considered adequate by most researchers in a short period of time with 5,000 IU d−1 of vitamin D.
Adequate serum levels of vitamin D are important to maintain bone strength in children and adults. Severely deficient amounts of vitamin D cause osteomalacia (a softening of bones) particularly in the spine, pelvis and lower extremities in adults and increase fall risk 1, 31. Research also implicates vitamin D insufficiency as a risk factor for a variety of chronic diseases including type 1 and 2 diabetes, osteoporosis, cardiovascular disease, hypertension, metabolic syndrome and cancer 5, 31.
Information about the dietary intake of participants was collected through 3‐d food records, and the data were compared with current recommendations. Results from these data indicate that participants in both groups were not consuming an adequate amount of vitamin D from dietary sources. In fact, the mean average consumption was well below recommendations, therefore giving further support for use of vitamin D supplements in this population.
It is necessary to note the limitations and strengths of the design of this study. A limitation of this study was the relatively small size of the study sample. Future research should aim to recruit participants in higher numbers. Additionally, the 3‐d food diaries were dependent upon self‐reported information from the participants. This may have affected the accuracy of the dietary intake data.
A strength of the study was the time of year in which it was conducted – late winter into early spring. Exposure to sunlight is a possible confounding factor because this is difficult to control. The timing of this investigation was intended to minimize subjects' sun exposure, and investigators aimed to collect data during a time of year in Chicago, IL, USA, typically characterized by very little sun exposure and shorter periods of daylight, therefore decreasing the opportunity for subjects to alter their serum vitamin D levels through biological skin synthesis. The average temperature during the study period was 7.8 °C (46 °F), so it is likely that the weather conditions limited the amount of time that the participants spent outside.
The randomized, double‐blind design was also a strength of this study. This design limits bias on the part of the researchers and/or participants related to desired outcomes and expectations. Finally, the high rate of compliance (overall average of 95.6%) among the participants in taking their respective D3 supplement or the placebo strengthens the results of this investigation.
In conclusion, a promising finding was that there was a significant increase in serum vitamin D levels in women who were overweight or obese with daily supplementation of 5,000 IU of D3 after only 8 weeks. Reports by both IOM and the Endocrine Society recommend that 3,000–5,000 IU/d may be required in individuals who are overweight and obese for long‐term maintenance of adequate vitamin D levels.
Conflict of Interest Statement
No conflict of interest was declared.
Acknowledgements
The authors' responsibilities were as follows: J. M. L. was the principle investigator and was responsible for the general conception and study design, data collection and writing the initial draft of the manuscript. P. E. L. was responsible for the statistical analyses, writing the manuscript and extensive editing of the manuscript. Both authors have approved the final manuscript. This study was made possible through a grant provided by Shaklee Corporation (Pleasanton, CA, USA).
Lukaszuk, J. M. , and Luebbers, P. E. (2017) 25(OH)D status: Effect of D3 supplement. Obesity Science & Practice, 3: 99–105. doi: 10.1002/osp4.85.
References
- 1. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 2. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D‐lightful story. J Bone Miner Res 2007; 22(Suppl 2): V28–V33. [DOI] [PubMed] [Google Scholar]
- 3. Dawson‐Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16: 713–716. [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. Vitamin D: a D‐Lightful health perspective. Nutr Rev 2008; 66(10 Suppl 2): S182–S194. [DOI] [PubMed] [Google Scholar]
- 5. Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol 2008; 3: 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haines ST, Park SK. Vitamin D supplementation: what's known, what to do, and what's needed. Pharmacotherapy 2012; 32: 354–382. [DOI] [PubMed] [Google Scholar]
- 7. Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality‐a review of recent evidence. Autoimmun Rev 2013; 12: 976–989. [DOI] [PubMed] [Google Scholar]
- 8. Vieth R, Bischoff‐Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85: 649–650. [DOI] [PubMed] [Google Scholar]
- 9. Lips P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 2007; 22: 1668–1671. [DOI] [PubMed] [Google Scholar]
- 10. Bolland MJ, Grey AB, Ames RW, et al. The effects of seasonal variation of 25‐hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr 2007; 86: 959–964. [DOI] [PubMed] [Google Scholar]
- 11. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988; 67: 373–378. [DOI] [PubMed] [Google Scholar]
- 12. Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol 2013; 5: 51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am 2013; 42: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012; 142: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tavakkoli A, DiGiacomo D, Green PH, Lebwohl B. Vitamin D status and concomitant autoimmunity in celiac disease. J Clin Gastroenterol 2013; 47: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grober U, Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol 2012; 4: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr 1981; 34: 2359–2363. [DOI] [PubMed] [Google Scholar]
- 18. Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D‐endocrine system in blacks. J Clin Invest 1985; 76: 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25‐dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab 2004; 89: 1196–1199. [DOI] [PubMed] [Google Scholar]
- 20. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72: 690–693. [DOI] [PubMed] [Google Scholar]
- 21. Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012; 20: 1444–1448. [DOI] [PubMed] [Google Scholar]
- 22. Ekwaru JP, Zwicker JD, Holick MF, et al. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25‐hydroxyvitamin D in health volunteers. PLoS One 2014. DOI: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dietary Reference Intakes for Calcium and Vitamin D . Ross AC, Taylor CL, Yaktine AL, Del Valle HB. (eds). National Academy of Sciences: Washington DC, 2011. [PubMed] [Google Scholar]
- 24. Maki KC, Rubin MR, Wong LG, McManus JF, Jensen CD, Lawless A. Effects of vitamin D supplementation on 25‐hydroxyvitamin D, high‐density lipoprotein cholesterol, and other cardiovascular disease risk markers in subjects with elevated waist circumference. Int J Food Sci Nutr 2011; 62: 318–327. [DOI] [PubMed] [Google Scholar]
- 25. Bhagatwala J, Zhu H, Parikh SJ, et al. Dose and time responses of vitamin D biomarkers to monthly vitamin D3 supplementation in overweight/obese African Americans with suboptimal vitamin d status: a placebo controlled randomized clinical trial. BMC Obes 2015; 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris SS, Pittas AG, Palermo NJ. A randomized, placebo‐controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metab 2012; 14: 789–794. [DOI] [PubMed] [Google Scholar]
- 27. Yiu YF, Yiu KH, Siu CW, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013; 227: 140–146. [DOI] [PubMed] [Google Scholar]
- 28. Wamberg L, Kampmann U, Stodkilde‐Jorgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels – results from a randomized trial. Eur J Intern Med 2013; 24: 644–649. [DOI] [PubMed] [Google Scholar]
- 29. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88: 582S–586S. [DOI] [PubMed] [Google Scholar]
- 30. Holick MF. Vitamin D is not as toxic as was once thought: a historical and an up‐to‐date perspective. Mayo Clin Proc 2015; 90: 561–564. [DOI] [PubMed] [Google Scholar]
- 31. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81: 353–373. DOI: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]


