Abstract
Constipation is a common, often chronic, gastrointestinal disorder that can negatively impact the lives of those it affects and can be difficult to treat satisfactorily. The objective of this systematic review is to identify and analyze the available published literature on US Food and Drug Administration–approved prescription therapies for adults with constipation (episodic and chronic) and to assess their place in therapy, based on the methodologic strength and results of identified clinical trials. Ovid MEDLINE, PubMed, and EMBASE databases were used to search the published literature. Studies were included if they were randomized and prospective, conducted in adults (age ≥18), published as full-length manuscripts in English, and compared the test agent with placebo or a comparator(s). Studies were excluded if they involved patients with constipation attributed to secondary causes. Because fully published manuscripts from phase III efficacy trials involving the recently approved medication lubiprostone were not available, a manual search was performed of abstracts from the two annual major gastroenterology meetings (American College of Gastroenterology and Digestive Disease Week) from the past 4 years. Data on study design; number, age, and sex of patients; duration of treatment period; primary efficacy variable; secondary efficacy variables; adverse events; and discontinuations because of adverse events were abstracted from eligible articles. Eligible studies were assessed using well-established recommendations and a preformatted standardized form. A scoring system, with scores ranging from 1 to 15, was used to individually and separately assess the methodologic quality of the studies. Results of this analysis indicate a general lack of methodologically high-quality clinical trials supporting the use of lactulose and PEG 3350 to treat patients with chronic constipation, but data support their use in acute, episodic constipation. Conversely, high-quality evidence for tegaserod and lubiprostone in patients with chronic constipation does exist, though conclusions regarding the role in therapy for lubiprostone are still in development.
Keywords: Constipation, lactulose, lubiprostone, polyethylene glycol, PEG 3350, tegaserod
Constipation is a multisymptom gastrointestinal (GI) disorder that affects up to 27% of the North American population, with prevalence estimates between 12% and 19%.1 It affects twice as many women as men and, although reported among all age groups, tends to be associated with increasing age (although this finding has not been uniformly shown among studies).1 Available data indicate that patients with constipation have diminished quality of life compared with nonconstipated persons,2-4 and constipation has a substantial direct (healthcare utilization) and indirect (work absenteeism, decreased productivity) socioeconomic impact.4,5
Lifestyle measures (increased fluid, fiber, exercise) have been the cornerstone of a nonpharmacologic treatment approach to constipation and are generally regarded in clinical practice as good habits to encourage, particularly for patients with mild symptoms. However, convincing evidence demonstrating that lifestyle measures have a clinically relevant effect on bowel habits is scarce (particularly for patients with more severe symptoms).6 For patients who fail to achieve adequate relief from lifestyle interventions, numerous over-the-counter preparations typically represent the next step in the therapeutic algorithm. Although these agents provide satisfactory relief for many patients with mild symptoms, clinical evidence of their efficacy has been questioned for patients with more severe or chronic symptoms. Similar issues apply to the use of other nonprescription approaches, such as behavioral or complementary and alternative therapies.
Prescription medications are usually reserved for patients with severe constipation symptoms and those who experience symptoms on a chronic or recurrent basis. US Food and Drug Administration (FDA)-approved prescription therapies for constipation number only a few and have important differences related to their mechanisms of action and the specific patient populations for which they are intended. The aim of this systematic review is to identify and analyze the available published clinical trial evidence supporting FDA-approved prescription options for adults with constipation. Tegaserod (Zelnorm, Novartis) and lubiprostone (Amitiza, Sucampo Pharmaceuticals) are two medications with novel mechanisms of action that have recently been approved for use in patients with chronic constipation. Because these agents are less well known than polyethylene glycol (PEG) and lactulose, we sought to examine and highlight, in a comprehensive manner, the evidence that supports the use of all four of these therapies in specific patients and to review the clinical trial rates of their efficacy.
Methods
Literature Search
A search of the published literature using Ovid MEDLINE, PubMed, and EMBASE databases was performed. For Ovid MEDLINE (1966–December 2005) and PubMed (MEDLINE 1966–December 2005), constipation (English language) was combined with [drug (lactulose, polyethylene glycol, lubiprostone, or tegaserod)], and then the search was refined to include only adults and clinical trials. For PubMed (no time limit), search terms included constipation [title] OR constipated [title] AND clinical trial [publication type] NOT child* AND [drug (lactulose, polyethylene glycol, lubiprostone, or tegaserod)] [title] AND English [language]. For EMBASE (1980–December 2005), constipation (English language) was combined with [drug (lactulose, polyethylene glycol, lubiprostone, or tegaserod)]. Results were then limited to human trials, English language, and randomized trials or meta-analyses. Abstracts were screened, potentially relevant studies were reviewed, and selection criteria were applied. References within studies that met selection criteria were manually searched for other potentially relevant studies.
Our search for clinical trial data on the recently approved agent lubiprostone yielded little in full manuscript form. Therefore, we performed an extensive review of abstracts from the two annual major gastroenterology meetings (American College of Gastroenterology and Digestive Disease Week) from the past 4 years and included them in the analysis. This search yielded 13 published abstracts reporting on 11 trials.7-19 When the selection criteria were applied, four eligible abstracts met the inclusion criteria8-10,13 (two abstracts reported on the same trial).10,13 Personal communication with investigators and the maker of lubiprostone were also used to garner additional data related to the clinical trials of this agent.
Selection Criteria
Selection criteria are outlined in Table 1.20 For PEG, only trials involving the original PEG 3350 formulation were included because this is the only formulation that is FDA-approved for the treatment of patients with (occasional) constipation. No limits were placed on the number of patients enrolled in each study, and there was no upper age limit. With the exception of lubiprostone, abstracts were not included.
Table 1.
Study Selection and Methodological Criteria20
| Studies were included if they were: |
|
| Methodological criteria that were evaluated included: |
|
Other methodologic features examined included measurement of study protocol compliance and assessment of whether global outcome was included in the analysis.
Data Extraction and Analysis
Articles were reviewed and data abstracted to a standard form by each author independently. Data extracted from each review included study design; number, age, and sex of patients; duration of treatment period; primary and secondary efficacy variables; adverse events; and discontinuations because of adverse events. As much lubiprostone data as were available from the abstracts were retrieved, and investigators were contacted.
Qualitative Assessment of Study Methodology
Studies were assessed using well-established recommendations put forth by the Rome committee (Table 1). These guidelines detail the appropriate manner in which to design, conduct, analyze, and evaluate clinical trials involving functional GI disorders. A preformatted, standardized form and scoring system was used to individually and separately assess the methodologic quality of the selected studies. Disagreement was resolved through consensus. Studies received 1 point for the presence of each of the following 15 trial design characteristics: prospective, randomized, double-blinded, use of concealed allocation, placebo-controlled, sample size calculated a priori, prospectively defined primary efficacy variable, intention-to-treat (ITT) analysis, definition of constipation in keeping with the Rome criteria for constipation, parallel design, inclusion of a baseline observation period, follow-up at the end of therapy, treatment duration of at least 8 weeks, and use of a validated, patient-derived outcome measure. Selected studies were placed into 1 of 3 tertiles directly related to their methodologic quality scores. Studies scoring from 0–5 were considered low quality, those scoring from 6–10 were rated moderate quality, and studies scoring from 11–15 constituted the highest quality trials. Previous reports have demonstrated that limiting analysis to studies fulfilling a greater number of these methodologic benchmarks results in a more accurate assessment of treatment effect, likely because of minimization of multiple potential biases.21
Results
Lactulose
The literature search resulted in 15 articles; however, four were excluded because they did not study adult populations,22 studied only the effects on opioid-induced (secondary) constipation,23 or were not clinical trials involving actual patients.24,25 Two articles26,27 reported data from the same trial and were reviewed as one study. Ten different studies were therefore evaluated.26-36 The methodologic scores of these 10 trials involving lactulose ranged from 3–10, indicating low to moderate quality (Table 2).27-36 A review of the references within these articles failed to reveal additional trials that met selection criteria.
Table 2.
Lactulose Trials
| Study | Efficacy Measures | Safety |
|---|---|---|
| Dettmar et al28 Randomized, open-label, multicenter study of ispaghula husk (n=224) vs lactulose (n=91) or other (bisacodyl, docusate sodium, senna, magnesium sulfate, n=79) for simple constipation (≤7 d); 4 weeks of treatment; follow-up visits at 2 and 4 weeks TMS=5 (low quality) |
Data are based on ITT population
|
|
| Bouhnik et al29 Randomized, parallel-group, open-label, multicenter study of lactulose (n=33) vs PEG-4000 electrolyte solution (n=29) for constipation (Rome I criteria); 1-wk washout; 4 wks of treatment TMS=7 (moderate quality) |
Data based on ITT population Patient (based on daily diary card)
|
|
| Attar et al30 Randomized, open-label, parallel-group, multicenter study of PEG electrolyte solution (n=60) vs lactulose (n=55) in pts with chronic idiopathic constipation (≥3 mo with <3 stool/wk and/or straining at stool); 4 wks of treatment
|
Data based on PP population
|
|
| Kinnunen et al31 Open-label, randomized, controlled, crossover study of bulk laxative + senna vs lactulose in geriatric institutionalized patients (mean age 81.8 y; N=30) with 3-mo to 11-y history of constipation (<2 BM/wk); 1-wk run-in, 5-wk treatment period (P1). 1-wk washout, 5-wk treatment period (P2). TMS=5 (low quality) |
Data based on PP population
|
Greater frequency of loose stool with bulk laxative + senna, P<.05 Discontinuations due to AEs: none |
| Passmore et al27 Double-blind, randomized, multicenter crossover study of bulk laxative + senna vs lactulose syrup in elderly inpatients (mean age 82.9 y; N=85) with chronic constipation (<3 BMs/wk); 2-wk treatment, 3–5 d washout, 2-wk treatment. TMS=5 (low quality) |
Data based on PP population
|
No significant differences |
| Rouse et al32 Open-label, randomized, parallel group, 4-wk, multicenter study of lactulose vs ispaghula in patients (N=124) with ≥3-wk history of ≤3 BMs/wk TMS=3 (low quality) |
Data based on PP population (lactulose n=48; ispaghula n=45)
|
AEs not reported Discontinuations due to AEs: 2 in each group due to “concurrent effect/unpalatability” |
| Lederle et al33 Randomized, double-blind, crossover study of lactulose vs sorbitol in VA hospital or nursing home patients (age >65; N=31) with ≥1 y history of constipation; 2-wk lead-in with lactulose, randomization, 2-wk washout, 4-wk treatment, 2-wk washout, 4-wk treatment TMS=5 (low quality) |
Stool frequency, stool consistency, daily dose, use of other laxatives/enemas, treatment preference, occurrence and severity of bloating, cramping, flatulence, diarrhea, fecal incontinence, constipation, P=NS Nausea: lactulose > sorbitol, P<.05 | 1 patient in the lactulose group withdrew during 1st treatment period |
| Bass et al34 Double-blind, randomized, parallel-group study of lactulose syrup vs placebo in primary care patients (N=24) with chronic constipation (<3 BMs/wk for preceding 3 mo); 1-wk baseline (placebo), 1-wk treatment (double-blind). TMS=8 (moderate quality) |
Data based on PP population
†Improvement from baseline with placebo, P<.05 $ Lactulose > placebo, P≤.05 §Lactulose < placebo, P≤.05 |
|
| Sanders et al35 Double-blind, randomized study of 50% lactulose syrup (n=20) vs placebo (n=25) in elderly patients with constipation; 2-wk baseline, 12-wk treatment, 1-wk observation period. TMS=8 (moderate quality) |
Data based on PP population
|
Not reported |
| Wesselius-De Casparis et al36 Double-blind, randomized, multicenter study of 50% lactulose syrup (n=54) vs placebo (n=49) in elderly patients with chronic constipation; 2-wk baseline period, 3-wk treatment period, 2-wk observation period (no treatment) TMS=10 (moderate quality) |
Data based on ITT population Primary efficacy variable:
|
Not reported |
AEs = adverse events; BM = bowel movement; ITT = intention to treat; NS = not significantly different between treatment groups; PEG = polyethylene glycol; PP = per protocol; TMS = total methodology score.
Of the 10 different studies, five were double blinded,27,33-36 two were partially blinded,29,30 and three were open label.28,31,32 Three trials were of crossover design.27,31,33 All trials compared lactulose treatment with either placebo (three studies)34-36 or comparator treatments (seven studies).27-33 Treatment duration ranged from 1 to 12 weeks; only one study had a consecutive 12-week period with the same treatment.35 Most patients in these studies were female, and five studies involved only patients 60 and older.29,31,33,35,36 The definition of constipation used for patient eligibility for these studies varied, ranging from “simple constipation (≤7 days),” to fulfilling the Rome I criteria for functional constipation, to having variably defined “chronic constipation.”
Three of the 10 studies reported efficacy data from the ITT population,28,29,36 whereas seven reported only a per protocol (PP) analysis.27,30-35 None of the 10 studies delineated prospectively defined primary or secondary efficacy variables. Efficacy measures were wide ranging and included assessments of bowel function (stool frequency, stool consistency, straining at stool, stool passage); presence and severity of symptoms such as flatus, bloating, abdominal pain, and cramping; and overall effectiveness. Details concerning efficacy measures, along with statistically significant results, can be found in Table 2. In general, lactulose was more effective than placebo, but less effective than PEG or bulk laxative plus senna, at relieving symptoms of constipation. No statistically significant differences in adverse events associated with lactulose compared with placebo or comparator agents were noted. The most commonly reported adverse events for lactulose were abdominal pain/griping (defined as colicky or crampy abdominal pain), bloating, diarrhea, and gas. Discontinuations attributed to adverse events were not reported in 5 of the 10 studies28,31,34-36; reasons for discontinuation in studies that reported them included depression,30 concurrent effects (not further specified),32 and “intolerance of lactulose.”33
Polyethylene Glycol 3350
Of the 14 articles involving PEG identified in the literature search, 12 were excluded. Reasons for study exclusion included use of molecular weight formulations of PEG other than 3350 (seven studies),29,37-42 use of PEG plus electrolytes (three studies),43-45 analysis of the effects of PEG in patients with secondary (opioid-induced) constipation (one study),23 or trials not involving actual patients (one decision analysis).25 Careful review of the references within the two remaining articles revealed four additional trials for inclusion (one report46 included analysis of two different clinical trials of PEG; Table 3).46-49 Methodological scores for all PEG 3350 studies ranged from 7–10, indicating moderate quality (Table 3).
Table 3.
Polyethylene Glycol 3350 Trials
| Study | Efficacy Measures | Safety |
|---|---|---|
| Attar et al30 | See Table 2 | See Table 2 |
| Cleveland et al49 Randomized, double-blind, crossover study of PEG vs placebo in patients (N=23) with constipation; 1-wk placebo qualifying period, randomization if ≤3 BMs during prior wk, 2-wk treatment period, 2-wk treatment period with alternate treatment TMS=7 (moderate quality) |
Efficacy analysis based on PP population
|
Nausea
|
| DiPalma et al46 Randomized, double-blind, crossover study of PEG (17 g and 34 g) vs placebo in otherwise healthy ambulatory patients (N=50) with constipation; 7-d qualification period (placebo), randomization, three 10-d treatment periods TMS=7 (moderate quality) |
Efficacy analysis based on PP population
|
AEs:
|
| DiPalma et al46 Randomized, double-blind, placebo-controlled, crossover study of PEG vs placebo for constipation in long-term care patients with special needs from stroke or medical debility (N=35; mean age, 75.7); 7-d qualification period (placebo), randomization, three 10-d treatment periods TMS=7 (moderate quality) |
Efficacy analysis based on PP population
|
On 17 & 34 g doses, first 5 pts had diarrhea with very loose stool, leading to change to 6 and 12 g for remaining pts Discontinuations due to adverse events: “Several” |
| DiPalma et al47 Randomized, double-blind, placebo-controlled, multicenter, parallel-group study of PEG (n=80) vs placebo (n=71) in pts with history of constipation (≤2 BMs during qualification period); 1-wk qualification period, 14-d treatment TMS=8 (moderate quality) |
Efficacy analysis based on n=144
|
No differences between placebo and PEG groups Discontinuations due to AEs:
|
| DiPalma et al48 Randomized, double-blind, placebo-controlled, parallel-group study of PEG vs placebo in patients >19 y with history of constipation (Rome II criteria) (N=24); 72-hour treatment period TMS=10 (moderate quality) |
Efficacy analysis based on ITT population Primary efficacy variable
|
AEs:
|
AEs = adverse events; BM = bowel movement; ITT = intention to treat; NS = not significantly different between treatment groups; PEG = polyethylene glycol; PP = per protocol; TMS = total methodology score.
Of the six trials, five were double blinded46-49 and one was partially blinded30; three trials had a crossover design.46,49 All trials compared PEG 3350 treatment with either placebo (five studies)46-49 or lactulose (one study).30 Treatment duration ranged from 1 day to 30 days, with only a single study using a consecutive 4-week treatment period with the same agent.30 Most patients in these studies were female; one study involved elderly patients (mean age, 76 years).46 As in the lactulose trials, constipation was variably defined, generally as “3 or fewer bowel movements per week during a 1-week baseline period.” Only one study required that constipation be present for 3 months or longer.30
Two studies reported efficacy data from the ITT population,47,48 whereas four used PP analysis.30,46,49 Efficacy measures included assessments of bowel function (stool frequency, stool consistency, frequency of soiling, difficulty defecating, straining while defecating); presence and severity of symptoms such as flatus, bloating, abdominal pain, and cramping; and overall effectiveness. Only one study prospectively defined primary (stool frequency) and secondary efficacy endpoints.48 Details concerning efficacy measures, along with statistically significant results, can be found in Table 3. Overall, PEG 3350 was modestly more effective than lactulose in one study30 and significantly more effective than placebo in most parameters evaluated in four of the other five studies.46-49
Although diarrhea was the most commonly reported adverse event in these trials, PEG 3350 was generally well tolerated, and there were no statistically significant differences in adverse events reported with PEG 3350 compared with placebo or comparator agents. Discontinuations because of adverse events were not reported for four of the six studies, and in the two studies that did report discontinuations, rates were low. Specific reasons for discontinuation were not reported.30,46
Tegaserod
The literature search resulted in nine articles; however, seven involved patients with irritable bowel syndrome (IBS) and were excluded. The two articles50,51 that focused solely on patients with constipation had methodologic scores of 13 and 14 (Table 4).50,51 Review of the references within these articles revealed no additional trials that met selection criteria. Both studies were double-blind, parallel-group, placebo-controlled, 12-week trials. In both trials, most patients were female, and the mean age was 46 years. Inclusion criteria for these trials required that patients have at least a 6-month history of constipation, defined as an average of fewer than three complete spontaneous bowel movements (CSBMs) per week, together with one of the following 25% or more of the time: straining, incomplete evacuation, and very hard and/or hard stools. A bowel movement was defined as spontaneous if no laxative or enema was used in the 24 hours preceding the bowel movement and complete if the bowel movement resulted in a sensation of complete evacuation.
Table 4.
Tegaserod Trials
| Study | Efficacy Measures | Safety Measures |
|---|---|---|
| Kamm50 Double-blind, randomized, placebo-controlled, parallel-group, multicenter (123) trial of tegaserod (2 or 6 mg BID) vs placebo in 1,048 gastroenterology patients; Age: ≥18 y (mean, 46 y); Sex: 2 mg BID dose 359 F/58 M, 6 mg BID dose 369 F/62 M, placebo 363 F/53 M; symptoms: ≥6-month history of constipation (average of <3 CSBM per week, together with 1 of the following ≥25% of the time: straining, incomplete evacuation, very hard and/or hard stools) 2-wk baseline period (no treatment) 12-wk treatment period TMS=13 (high quality) |
Data based on ITT population Primary efficacy variable:
|
AEs = NS between groups Diarrhea:
|
| Johanson51 Double-blind, randomized, placebo-controlled trial tegaserod (2 or 6 mg bid vs placebo) in 1085 pts; age: 318 y (mean, 46 y); sex: 2 mg bid dose 400 F/50 M, 6 mg bid dose 406 F/45 M, placebo 407 F/40 M; symptoms: 36-month history of constipation (average of <3 CSBM per week, together with 1 of the following ≥25% of the time: straining, incomplete evacuation, very hard and/or hard stools) 2-week baseline period 12-wk treatment period TMS=14 (high quality) |
Data are based on ITT population Primary efficacy variable:
|
AEs = NS between groups Diarrhea: 2 mg BID dose, 4.5%; 6 mg BID dose, 7.3%; Placebo, 3.8% Discontinuations = NS btween groups 2 mg bid dose, 3.1%; 6 mg bid dose, 3.8%; placebo, 2.5% Due to diarrhea: 2 mg bid dose, 0.4%; 6 mg bid dose, 0.9%; placebo, 0% |
AEs = adverse events; CSBM = complete spontaneous bowel movement; ITT = intention to treat; NS = not significantly different between treatment groups; TMS = total methodology score.
Both studies reported efficacy data from the ITT population and used a prospectively defined primary efficacy variable. Both studies also evaluated a number of secondary efficacy variables, including an increase of one or more CSBMs per week over 12 weeks of treatment; stool frequency (CSBMs per week); straining; stool form; satisfaction with bowel habits; and bothersomeness of constipation, abdominal bloating/distension, and abdominal pain/discomfort. Details concerning efficacy measures, along with statistically significant results, can be found in Table 4. Overall, there was a statistically significant benefit of tegaserod compared with placebo in both trials (see Table 4 for details).
Tegaserod was associated with more diarrhea than placebo (Table 4). These episodes were generally reported during the first week of treatment, were transient, and did not result in hospitalization or electrolyte imbalances. Discontinuation attributed to diarrhea with tegaserod was less than 1%.
Lubiprostone
The literature search resulted in 13 published abstracts reporting data from 11 trials. No published manuscript reporting the results of a clinical trial with lubiprostone is yet available. Nine of the abstracts were excluded for the following reasons: two included patients with IBS14 or involved patients with drug-induced constipation17; two did not specify that the trial was conducted in patients ≥ 18 years of age and did not report a mean age for included patients11,12; four were retrospective, pooled analyses of previously reported results15,16,18,19; and one study was not randomized.7 The four eligible abstracts reported on 3 randomized, placebo-controlled trials in constipated patients8-10,13; two abstracts reported on the same trial.10,13 One trial was a dose-finding study that consisted of a 2-week baseline period and a 3-week active treatment period.8 The other two studies were identical phase III trials and consisted of a 2-week baseline period, a 4-week treatment period, and a 2-week drug-free follow-up period.9,10,13 Inclusion criteria for these trials required that patients have at least a 6-month history of constipation, which was defined as an average of fewer than three SBMs per week, together with one of the following 25% or more of the time: straining, incomplete evacuation, and/or hard stools. All trials reported results for the ITT population, prospectively used SBM frequency as a measure of efficacy, and reported 24-hour response using SBM. A number of secondary efficacy variables, including straining and stool consistency, were also measured. One study reported assessments of bloating and global assessment of constipation severity,8 and another reported results of subjects’ global assessments of treatment effectiveness.9 Details concerning efficacy measures, along with statistically significant results, can be found in Table 5.8-10,13 Overall, there was a statistically significant benefit of lubiprostone compared with placebo in all three trials.
Table 5.
Lubiprostone Trials
| Study | Efficacy Measures | Safety Measures |
|---|---|---|
| Johanson8 Randomized, placebo-controlled, dose-ranging study of lubiprostone (24 μg/d, 48 μg/d, or 72 μg/d vs daily placebo) in 127 constipated patients; Symptoms: <3 SBMs/wk and 6 months of at least one protocol-defined symptom (straining, hard stools, or sensation of incomplete evacuation) 25% of the time 3-wk treatment period TMS=8 (moderate quality) |
Data based on ITT population Average weekly number of SBMs: Significant increase during weeks 1 and 2 for 48 μg/d and 72 μg/d doses vs placebo. Percentage of patients with SBM within 24 hours: Significantly more patients in 48 μg/d and 72 μg/d groups vs placebo Stool consistency, bloating, and global assessment of constipation severity: statistically significant improvement in patients receiving lubiprostone versus placebo |
Most common AEs: nausea, headache, diarrhea, bloating Incidence of nausea was statistically significantly higher in patients receiving lubiprostone vs placebo. Discontinuations due to AEs: nausea (which increased in a dose-dependent fashion) resulted in four treatment discontinuations, equally distributed among the treatment groups Undisclosed number of patients in the placebo group (none in the lubiprostone group) withdrew due to lack of efficacy |
| Johanson9 Multicenter, randomized, placebo-controlled trial of lubiprostone 24 μg bid vs placebo bid in 242 constipated patients; mean age, 48.6 y; sex: 10% M/90% F; Symptoms: <3 SBMs/wk and 6 months of at least one Rome II criterion for functional constipation 2-wk drug-free baseline 4-wk treatment period 2-wk drug-free follow-up period TMS=13 (high quality) |
Data based on ITT population SBM frequency: lubiprostone > placebo at all weeks, P<.002 Time to first SBM/lubiprostone (57%) vs placebo (37%) within 24 hours of first dose, P=.0024 Improvement in straining and stool consistency: lubiprostone > placebo at all weeks, P<.001 Global assessment of treatment effectiveness: lubiprostone > placebo during the entire 4-wk treatment period, P<.0001 at all weeks |
Most common AEs: nausea, diarrhea, headache Discontinuations due to AEs: Lubiprostone = 9 patients Placebo: not reported |
| Johanson10,13 Multicenter, double-blind, randomized, placebo-controlled trial of lubiprostone (24 μg bid) vs placebo bid in 237 constipated patients; mean age, 45.8 y; sex: 12% M/88% F; Symptoms: <3 SBMs/wk and 6 months of at least one Rome II criterion for functional constipation 2-wk drug-free baseline 4-wk treatment period 2-wk drug-free follow-up period TMS=13 (high quality) |
Data based on ITT population SBM frequency: lubiprostone > placebo at all weeks, P<.002 Time to first SBM/lubiprostone (61%) vs placebo (31%) within 24 hours of first dose, P<.0001 Improvement in straining and stool consistency: lubiprostone > placebo at all weeks, P<.001 |
Most common AEs: nausea, diarrhea, headache Discontinuations due to AEs: Lubiprostone = 15 patients Placebo: not reported |
AEs = adverse events; SBM = spontaneous bowel movement; ITT = intention to treat; TMS = total methodology score.
In all randomized clinical trials of lubiprostone, the incidence of nausea in patients receiving lubiprostone was significantly higher than in those receiving placebo.8-10,13 In the dose-ranging study,8 no statistically significant differences were noted in the lubiprostone versus placebo groups with regard to the number of patients discontinuing treatment as a result of adverse events. A total of 24 (10%) patients were discontinued from the lubiprostone treatment groups because of adverse effects in the phase III trials of this agent, compared with two (0.8%) of those receiving placebo.9,10,13 Gastrointestinal adverse effects accounted for 75% of the adverse effects prompting withdrawal from these trials (Table 5).
Discussion
In years past, physicians largely relied on isolated, small research studies, anecdotal reports, and their own clinical experience to guide their practice. Evidence-based clinical practice provides physicians with the opportunity to evaluate therapeutic options using standardized assessment rules. Although some decry this approach because it appears to ignore the individual patient in favor of focusing on trends and effects involving large groups of patients, critical assessment of the medical literature using evidence-based rules (through comprehensive reviews, meta-analyses, and systematic reviews) will continue to have significant impact on the practice of medicine.
The treatment of constipation is an ideal topic for a systematic review because of the high prevalence of this disorder, its negative impact on quality of life, its high direct and indirect costs, and the multiple therapeutic agents available for its treatment. Although several medications are now FDA approved for the treatment of constipation, an objective review of the data supporting their use has been lacking. In this systematic review, we analyze the published data for the four medications—lactulose, PEG 3350, tegaserod, and lubiprostone—currently approved by the FDA for use in adults with constipation. The literature was carefully reviewed and analyzed to answer the following questions: (1) Are quality data available to support their efficacy and safety? (2) What types of patients (those with acute/occasional versus chronic constipation) were included in the clinical trials that demonstrated efficacy? (3) What is each drug’s mechanism of action?
Lactulose
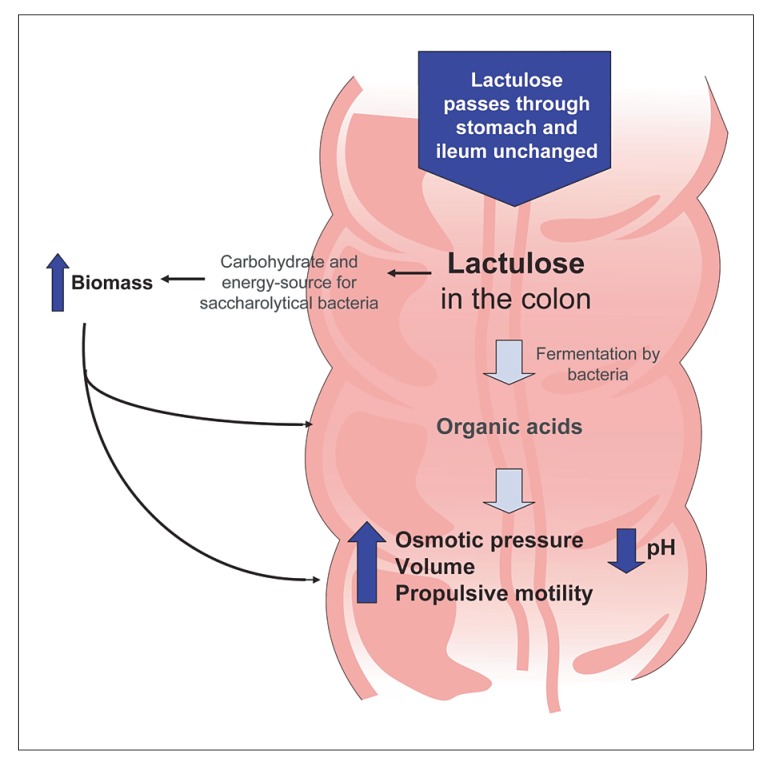
Lactulose is a nonabsorbable, synthetic disaccharide composed of the sugars D-galactose and D-fructose, which are fermented in the colon by bacteria. Products of this fermentation are primarily organic acids, such as lactic acid and small-chain fatty acids, that increase the osmotic load to the gut, thereby stimulating peristalsis (Figure 1).52 Our review reveals that few high-quality data support the use of lactulose in treating patients with constipation, either on an acute or a chronic basis. Methodology scores in evaluable studies ranged from 3–10, indicating weak to moderate evidence supporting its use in patients with constipation. Only three of the 10 published clinical trials of lactulose34-36 compared it with placebo, a critical comparison when determining a drug’s true efficacy. These three trials34-36 had small sample sizes (30–150 patients each), and two of them involved only elderly patients. Furthermore, constipation was variably defined in these studies—two required three or fewer bowel movements per week (one for 3 months or more), and the other required regular laxative use for chronic constipation. Two of the three studies were of short duration (1 and 3 weeks), did not prospectively define primary or secondary efficacy measures, and only reported efficacy results based on PP analysis. In the study that did use a prospectively defined primary endpoint, the endpoint was the need for additional laxatives during the treatment period and did not include bowel habit-specific parameters such as stool frequency or consistency.
Figure 1.
Mechanism of action of lactulose.
PEG 3350
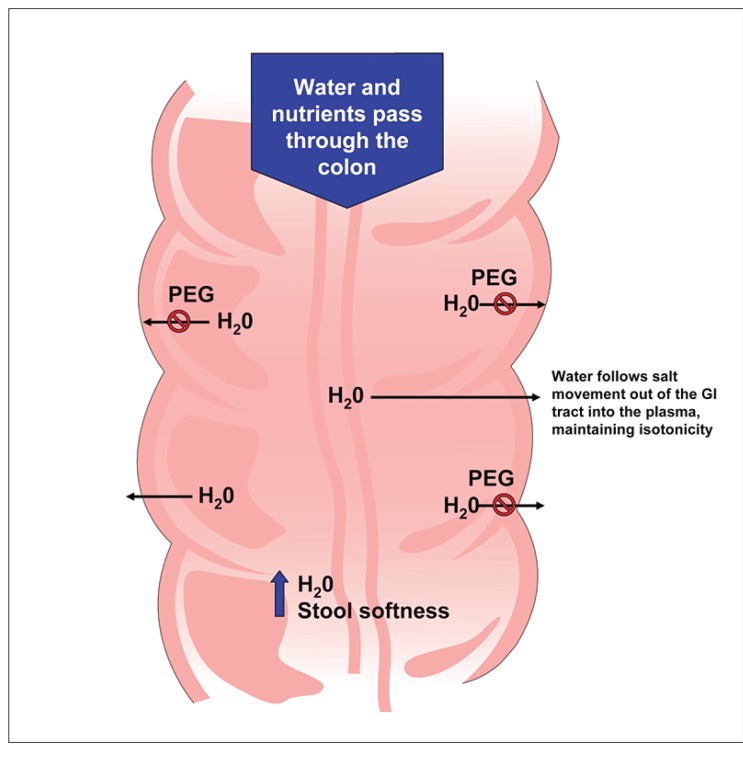
PEG is a nonabsorbable, nonmetabolized osmotic agent that retains water in the stool, softening the stool and increasing the number of bowel movements (Figure 2).52 PEG 3350 has gained popularity as a therapy for patients with constipation. Review of the published literature revealed five placebo-controlled trials and one trial comparing PEG 3350 with lactulose in adults with constipation; methodologic scores ranged from 7–10, indicating a moderate evidence base supporting its use in patients with constipation.30,46-49 Three of the five studies used a crossover design,46,49 which is not optimal for evaluating the therapeutic efficacy of a medication. This is especially true in the evaluation of medications for functional GI disorders, where the use of crossover design tends to accentuate the relatively high placebo response rate.53 Although constipation was more consistently defined in the studies of PEG 3350 relative to the lactulose studies, only one PEG 3350 study evaluated patients with functional or chronic constipation (as defined by the Rome I criteria).48 The major limitation of this study was its short treatment course—a single dose. All five placebo-controlled studies involved small sample sizes of fewer than 100 patients each, one involved elderly patients,46-49 four of the five did not use prospectively defined primary or secondary efficacy measures,46,47,49 and three of the five reported efficacy results based on PP analysis.46,49 Like lactulose, PEG 3350 has an onset of action between 24 and 48 hours. Although it is frequently used in clinical practice, high-quality evidence to support the use of PEG 3350 in patients with chronic constipation is lacking.
Figure 2.
Mechanism of action of polyethylene glycol.
GI = gastrointestinal; PEG = polyethylene glycol.
Tegaserod
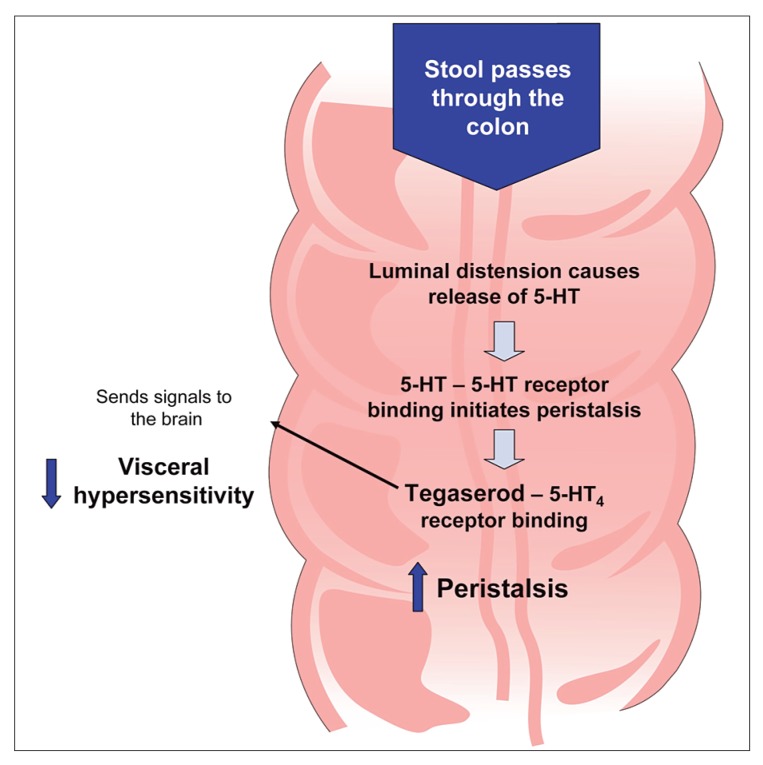
In contrast to lactulose and PEG 3350, which primarily affect stool form, tegaserod affects GI motility, secretion, and sensation. Tegaserod mimics serotonin by serving as a partial agonist at specific serotonin receptors (5-hydroxytryptamine type 4) distributed throughout the enteric nervous system. Stimulation of these receptors leads to the release of other neurotransmitters, ultimately resulting in enhanced peristaltic contractions, increased intestinal secretion, and reduced visceral hypersensitivity (Figure 3).54 The two clinical trials involving tegaserod for the treatment of patients with constipation were of high quality, with methodologic scores of 13 and 14.50,51 Each trial involved a large number of patients (> 1,500 in each study), was placebo-controlled and double-blinded, had consistently defined definitions of chronic constipation, and had prospectively defined primary and secondary efficacy measures. In addition, the studies were of acceptable duration (12 weeks). Patients can generally expect to have a spontaneous bowel movement within approximately 20 hours,51 and tegaserod has demonstrated benefits in relieving the multiple symptoms of chronic constipation, including straining and bloating.
Figure 3.
Mechanism of action of tegaserod.
5-HT = serotonin.
Lubiprostone
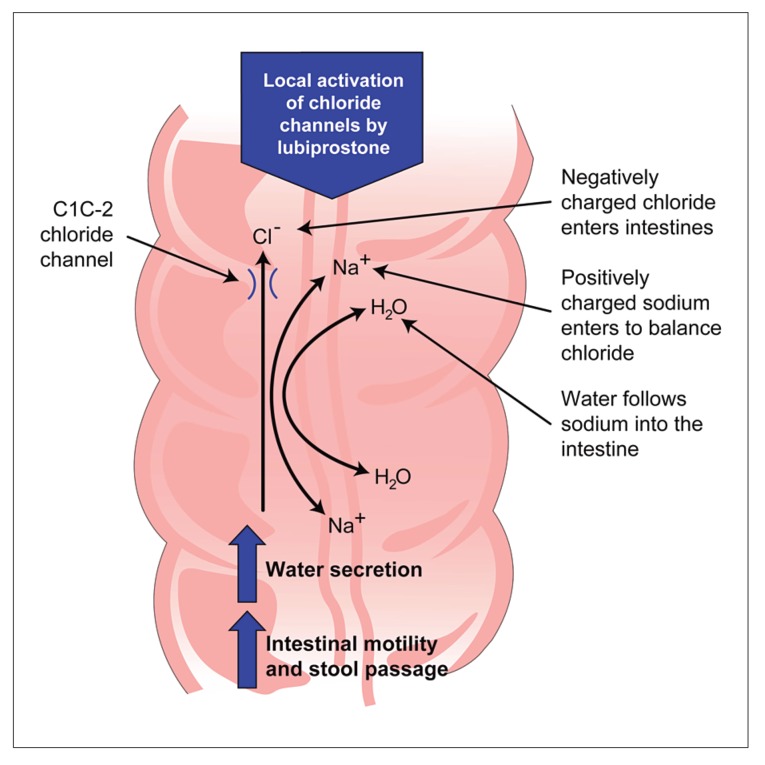
Lubiprostone is a bicyclic fatty acid.55 It works by locally activating the type 2 chloride channel (located in the apical intestinal membrane), thereby increasing intestinal fluid secretion without changing electrolyte concentrations in serum.55 Lubiprostone appears to enhance stool passage by increasing intestinal motility and by softening the stool (Figure 4).55,56 The manual search for lubiprostone abstracts produced three trials of moderate to high quality, with methodologic scores ranging from 8–13.8-10,13 Two of these trials were identically designed phase III trials, and one was a dose-ranging study. All trials were placebo controlled and had similar inclusion criteria. A statistically significant benefit of lubiprostone compared with placebo was demonstrated in all trials. All these trials, however, included relatively small populations and were of short treatment duration (3 and 4 weeks). Open-label data regarding the effects of lubiprostone over 24 weeks indicate that it is associated with a durable response for those patients who respond to and tolerate this medication.15 Because clinical data have only been published in abstract form and clinical use of lubiprostone has been extremely limited, firm conclusions regarding clinical applicability and treatment differences in patients with chronic constipation cannot be made at this time.
Figure 4.
Mechanism of action of lubiprostone.
Application of the findings from this review to clinical practice is highly dependent on the physician’s clinical judgment and experience. The approach to managing constipation should be individualized, keeping patient symptoms and treatment goals in mind. The evidence-based data presented here help put into perspective the amount and quality of clinical trial data for each of the currently available FDA-approved treatment options for adults with constipation. As demonstrated, high-quality evidence to support the use of lactulose in treating patients with constipation is lacking. Its mechanism of action and adverse effect profile support its use only in those with acute or occasional constipation. Similarly, evidence is lacking to support PEG 3350 for use in patients with chronic constipation. Its mechanism of action appears to be most suited to patients with intermittent, rather than chronic, symptoms. No published data have reported the effects of tegaserod or lubiprostone as therapies for occasional constipation; thus, among the FDA-approved medications, PEG 3350 has the highest quality of demonstrated efficacy for patients with nonchronic constipation.
Convincing evidence—in the form of high-quality, methodologically sound clinical trials—supports the use of tegaserod and lubiprostone in patients with chronic constipation. Data from the clinical trials of lubiprostone have only been reported in abstract form, and the drug has only recently been approved and marketed, two facts that necessarily limit conclusions about this drug’s future role in the treatment of constipation. Current evidence supports a role for lubiprostone in adults with chronic idiopathic constipation, but concerns regarding tolerability remain. For patients with chronic constipation, tegaserod has the soundest clinical evidence and experience basis to support its use.
A notable limitation of this review is the lack of comparable publications for all four agents evaluated. Because lubiprostone is a newly approved agent, we felt it was important to provide available data from trials that fit inclusion criteria (with the exception of publication status). We chose not to include a review of published abstracts for the other three agents because data from published manuscripts were viewed as a more complete, reliable data source.
Conclusion
Given the dearth of evidence-based efficacy data from high-quality clinical trials in patients with constipation, future research efforts should focus on designing studies in line with recognized and accepted methodologic parameters, including a clearly defined patient population (fulfillment of Rome criteria), adequate sample size and trial duration, and patient-derived outcomes. Important strides have been made in the past several decades in the design of novel molecular entities that target potential pathophysiologic factors underlying constipation (GI serotonergic agents, opioid receptor antagonists, chloride channel activators). Hopes are high that such efforts will continue and ultimately will lead to an enhanced understanding of constipation and a wider treatment armamentarium for patients with acute and chronic symptoms.
Acknowledgment
The authors thank Maribeth Bogush, PhD, for editorial assistance in preparing this manuscript.
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 3.Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 4.Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. PharmacoEconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 5.Harris LA. Prevalence and ramifications of chronic constipation. Manag Care Interface. 2005:23–30. [PubMed] [Google Scholar]
- 6.Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–242. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 7.Johanson JF, Gargano MA, Holland PC, et al. Multicenter open-label study of oral lubiprostone for the treatment of chronic constipation [abstract] Am J Gastroenterol. 2005;100 suppl:S331. [Google Scholar]
- 8.Johanson JF, Gargano MA, Patchen ML, Ueno R. Efficacy and safety of a novel compound, RU-0211, for the treatment of constipation [abstract] Gastroenterology. 2002;122:A-315. [Google Scholar]
- 9.Johanson JF, Gargano MA, Holland PC, et al. Phase III efficacy and safety of RU-0211, a novel chloride channel activator, for the treatment of constipation [abstract] Gastroenterology. 2003;124:A104. [Google Scholar]
- 10.Johanson JF, Gargano MA, Holland PC, et al. Initial and sustained effects of lubiprostone, a chloride channel-2 (CIC-2) activator for the treatment of constipation: data from a 4-week phase III study [abstract] Am J Gastroenterol. 2005;100 suppl:S324–S325. [Google Scholar]
- 11.Johanson JF, Gargano MA, Holland PC, et al. Phase III patient assessments of the effects of lubiprostone, a chloride channel-2 (CIC-2) activator, for the treatment of consitpation [abstract] Am J Gastroenterol. 2005;100 suppl:S329–S330. [Google Scholar]
- 12.Johanson JF, Gargano MA, Holland PC, et al. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation [abstract] Gastroenterology. 2004;126(2):A100. [Google Scholar]
- 13.Johanson JF, Gargano MA, Holland PC, et al. Phase III study of lubiprostone, a chloride channel-2 (CIC-2) activator for the treatment of constipation: safety and primary efficacy [abstract] Am J Gastroenterol. 2005;100 suppl:S328–S329. [Google Scholar]
- 14.Johanson JF, Panas R, Holland P, Ueno R. A dose-ranging, double-blind, placebo-controlled study of lubiprostone in subjects with irritable bowel syndrome and constipation (C-IBS) [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract 131. [Google Scholar]
- 15.Johanson JF, Panas R, Holland P, Ueno R. Long-term efficacy of lubiprostone for the treatment of chronic constipation [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract 1171. [Google Scholar]
- 16.Joswick TR, Ueno R, Wahle A, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in elderly vs non-elderly subjects [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract 51262. [Google Scholar]
- 17.Ueno R, Osama H, Engelke KJ. Effects of lubiprostone on morphine-induced constipation and analgesia [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract M1810. [Google Scholar]
- 18.Panas R, Ueno R, Wahle B, et al. Long-term safety and efficacy of lubiprostone for the treatment of chronic constipation in elderly subjects [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract S1260. [Google Scholar]
- 19.Joswick TR, Ueno R, Wahle, et al. Efficacy and safety of lubiprostone for the treatment of chronic constipation in male vs female subjects [abstract]; Abstract presented at: Digestive Disease Week; May 20-25, 2006; Los Angeles, Calif. Abstract M1195. [Google Scholar]
- 20.Veldhuyzen Van Zanten SJ, Talley NJ, Bytzer P, et al. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45(2):II69–II77. doi: 10.1136/gut.45.2008.ii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmers TC, Celano P, Sacks HS, Smith H., Jr Bias in treatment assignment in controlled clinical trials. N Engl J Med. 1983;309:1358–1361. doi: 10.1056/NEJM198312013092204. [DOI] [PubMed] [Google Scholar]
- 22.Urganci N, Akyildiz B, Polat TB. A comparative study: the efficacy of liquid paraffin and lactulose in management of chronic functional constipation. Pediatr Int. 2005;47:15–19. doi: 10.1111/j.1442-200x.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 23.Freedman MD, Schwartz HJ, Roby R, Fleisher S. Tolerance and efficacy of polyethylene glycol 3350/electrolyte solution versus lactulose in relieving opiate induced constipation: a double-blinded placebo-controlled trial. J Clin Pharmacol. 1997;37:904–907. doi: 10.1002/j.1552-4604.1997.tb04264.x. [DOI] [PubMed] [Google Scholar]
- 24.Lactulose (Chronulac) for constipation. Med Lett Drugs Ther. 1980;22:2–4. [PubMed] [Google Scholar]
- 25.Christie AH, Culbert P, Guest JF. Economic impact of low dose polyethylene glycol 3350 plus electrolytes compared with lactulose in the management of idiopathic constipation in the UK. Pharmaco Economics. 2002;20:49–60. doi: 10.2165/00019053-200220010-00005. [DOI] [PubMed] [Google Scholar]
- 26.Passmore AP, Davies KW, Flanagan PG, et al. A comparison of Agiolax and lactulose in elderly patients with chronic constipation. Pharmacology. 1993;47(1):249–252. doi: 10.1159/000139865. [DOI] [PubMed] [Google Scholar]
- 27.Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ. 1993;307:769–771. doi: 10.1136/bmj.307.6907.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dettmar PW, Sykes J. A multi-centre, general practice comparison of ispaghula husk with lactulose and other laxatives in the treatment of simple constipation. Curr Med Res Opin. 1998;14:227–233. doi: 10.1185/03007999809113363. [DOI] [PubMed] [Google Scholar]
- 29.Bouhnik Y, Neut C, Raskine L, et al. Prospective, randomized, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation. Aliment Pharmacol Ther. 2004;19:889–899. doi: 10.1111/j.1365-2036.2004.01918.x. [DOI] [PubMed] [Google Scholar]
- 30.Attar A, Lemann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut. 1999;44:226–230. doi: 10.1136/gut.44.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinnunen O, Winblad I, Koistinen P, Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47(1):253–255. doi: 10.1159/000139866. [DOI] [PubMed] [Google Scholar]
- 32.Rouse M, Chapman N, Mahapatra M, et al. An open, randomised, parallel group study of lactulose versus ispaghula in the treatment of chronic constipation in adults. Br J Clin Pract. 1991;45:28–30. [PubMed] [Google Scholar]
- 33.Lederle FA, Busch DL, Mattox KM, et al. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med. 1990;89:597–601. doi: 10.1016/0002-9343(90)90177-f. [DOI] [PubMed] [Google Scholar]
- 34.Bass P, Dennis S. The laxative effects of lactulose in normal and constipated subjects. J Clin Gastroenterol. 1981;3(1):23–28. doi: 10.1097/00004836-198100031-00005. [DOI] [PubMed] [Google Scholar]
- 35.Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc. 1978;26:236–239. doi: 10.1111/j.1532-5415.1978.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 36.Wesselius-De Casparis A, Braadbaart S, Bergh-Bohlken GE, Mimica M. Treatment of chronic constipation with lactulose syrup: results of a double-blind study. Gut. 1968;9:84–86. doi: 10.1136/gut.9.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaussade S, Minic M. Comparison of efficacy and safety of two doses of two different polyethylene glycol-based laxatives in the treatment of constipation. Aliment Pharmacol Ther. 2003;17:165–172. doi: 10.1046/j.1365-2036.2003.01390.x. [DOI] [PubMed] [Google Scholar]
- 38.Bazzocchi G. Polyethylene glycol solution in subgroups of chronic constipation patients: experience in obstructed defaecation. Ital J Gastroenterol Hepatol. 1999;31(3):S257–S259. [PubMed] [Google Scholar]
- 39.Lee DKC, Haggart K, Currie GP, et al. Effects of hydrofluoroalkane formulations of ciclesonide 400 micrograms once daily vs fluticasone 250 micrograms twice daily on methacholine hyper-responsiveness in mild-to-moderate persistent asthma. Br J Clin Pharmacol. 2004;58:26–33. doi: 10.1111/j.1365-2125.2004.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson A, Culbert P, Gillett H, Barras N. New polyethylene glycol electrolyte solution for the treatment of constipation and faecal impaction. Ital J Gastroenterol Hepatol. 1999;31(3):S249–S252. [PubMed] [Google Scholar]
- 41.Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerability of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46:522–526. doi: 10.1136/gut.46.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klauser AG, Muhldorfer BE, Voderholzer WA, et al. Polyethylene glycol 4000 for slow transit constipation. Z Gastroenterol. 1995;33:5–8. [PubMed] [Google Scholar]
- 43.Neri I, Blasi I, Castro P, et al. Polyethylene glycol electrolyte solution (Isocolan) for constipation during pregnancy: an observational open-label study. J Midwifery Women’s Health. 2004;49:355–358. doi: 10.1016/j.jmwh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Corazziari E, Badiali D, Habib FI, et al. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci. 1996;41:1636–1642. doi: 10.1007/BF02087913. [DOI] [PubMed] [Google Scholar]
- 45.Andorsky RI, Goldner F. Colonic lavage solution (polyethylene glycol electrolyte lavage solution) as a treatment for chronic constipation: a double-blind, placebo-controlled study. Am J Gastroenterol. 1990;85:261–265. [PubMed] [Google Scholar]
- 46.DiPalma JA, MacRae DH, Reichelderfer M, et al. Braintree polyethylene glycol (peg) laxative for ambulatory and long-term car facility constipation patients: report of randomized, cross-over trials. Online J Dig Health. 1999;1:1–7. [Google Scholar]
- 47.DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95:446–450. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]
- 48.DiPalma AM, DiPalma JA. Women’s colonic digestive health. Gastroenterol Nurs. 2002;25:3–8. doi: 10.1097/00001610-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Cleveland MV, Flavin DP, Ruben RA, et al. New polyethylene glycol laxative for treatment of constipation in adults: a randomized, double-blind, placebo-controlled study. South Med J. 2001;94:478–481. [PubMed] [Google Scholar]
- 50.Kamm MA, Muller-Lissner S, Talley NJ, et al. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [published correction appears in Am J Gastroenterol. 2005;100:735] [DOI] [PubMed] [Google Scholar]
- 51.Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796–805. doi: 10.1016/s1542-3565(04)00356-8. [DOI] [PubMed] [Google Scholar]
- 52.DiPalma JA. Current treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4(2):S34–S42. [PubMed] [Google Scholar]
- 53.Enck P, Klosterhalfen S. The placebo response in functional bowel disorders: perspectives and putative mechanisms. Neurogastroenterol Motil. 2005;17:325–331. doi: 10.1111/j.1365-2982.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 54.Lacy BE, Yu S. Tegaserod: a new 5-HT4 agonist. J Clin Gastroenterol. 2002;34:27–33. doi: 10.1097/00004836-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Orr KK. Lubiprostone: a novel chloride channel activator for the treatment of constipation. Formulary. 2006;41 [Google Scholar]
- 56.Lubiprostone: RU 0211, SPI 0211. Drugs Res Dev. 2005;6:245–248. doi: 10.2165/00126839-200506040-00009. [DOI] [PubMed] [Google Scholar]