Abstract
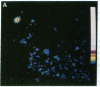
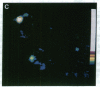
We have used intensified video fluorescence microscopy and digital image processing to observe and quantitate influenza virus (A/PR8/34/H1N1) fusion to human erythrocyte membranes. Viruses labeled with the lipid probe octadecylrhodamine B (R18) were seen to undergo fluorescence dequenching and eventual disappearance after exposure to pH levels known to induce virus-cell membrane fusion. Quantitative intensity measurements of single individual particles were possible. From these fluorescence data it has been possible to calculate the fraction of R18 dye molecules transferred from the virus to the cell. The redistribution of the lipid probe upon fusion at pH 5.0 had a t1/2 of 46 s, longer than expected for a free-diffusion model. The R18 loss was approximately twice as fast at pH 5.0 as at pH 5.1. No obvious delay until the start of fluorescence dequenching was observed after the pH changes, suggesting that activation processes are faster than the time resolution, 1-5 s, of the current method.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Robert-Nicoud M., Kaufman S. J., Jovin T. M. Fluorescence digital imaging microscopy in cell biology. Science. 1985 Oct 18;230(4723):247–256. doi: 10.1126/science.4048934. [DOI] [PubMed] [Google Scholar]
- Aroeti B., Henis Y. I. Fusion of native Sendai virions with human erythrocytes. Quantitation by fluorescence photobleaching recovery. Exp Cell Res. 1987 Jun;170(2):322–337. doi: 10.1016/0014-4827(87)90310-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal R., Bali-Puri A., Walter A., Covell D., Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987 Oct 5;262(28):13614–13619. [PubMed] [Google Scholar]
- Blumenthal R. Cooperativity in viral fusion. Cell Biophys. 1988 Jan-Jun;12:1–12. doi: 10.1007/BF02918347. [DOI] [PubMed] [Google Scholar]
- Boulay F., Doms R. W., Webster R. G., Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988 Mar;106(3):629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T. Direct observation of the budding and fusion of an enveloped virus by video microscopy of viable cells. J Cell Biol. 1988 Nov;107(5):1689–1695. doi: 10.1083/jcb.107.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. D., Blumenthal R. On the use of self-quenching fluorophores in the study of membrane fusion kinetics. The effect of slow probe redistribution. Biophys Chem. 1989 Nov;34(3):283–292. doi: 10.1016/0301-4622(89)80065-1. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Schoch C., Zech L., Blumenthal R. Gating kinetics of pH-activated membrane fusion of vesicular stomatitis virus with cells: stopped-flow measurements by dequenching of octadecylrhodamine fluorescence. Biochemistry. 1990 Feb 6;29(5):1303–1308. doi: 10.1021/bi00457a028. [DOI] [PubMed] [Google Scholar]
- Georgiou G. N., Morrison I. E., Cherry R. J. Digital fluorescence imaging of fusion of influenza virus with erythrocytes. FEBS Lett. 1989 Jul 3;250(2):487–492. doi: 10.1016/0014-5793(89)80782-3. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., de Boer T., Wilschut J. Characterization of the fusogenic properties of Sendai virus: kinetics of fusion with erythrocyte membranes. Biochemistry. 1985 Aug 27;24(18):4739–4745. doi: 10.1021/bi00339a005. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Loyter A., Citovsky V., Blumenthal R. The use of fluorescence dequenching measurements to follow viral membrane fusion events. Methods Biochem Anal. 1988;33:129–164. doi: 10.1002/9780470110546.ch4. [DOI] [PubMed] [Google Scholar]
- Maeda T., Ohnishi S. Activation of influenza virus by acidic media causes hemolysis and fusion of erythrocytes. FEBS Lett. 1980 Dec 29;122(2):283–287. doi: 10.1016/0014-5793(80)80457-1. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Sarkar D. P., White J. M., Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. Measurement by dequenching of fluorescence. J Biol Chem. 1989 Mar 5;264(7):3972–3978. [PubMed] [Google Scholar]
- Nussbaum O., Loyter A. Quantitative determination of virus-membrane fusion events. Fusion of influenza virions with plasma membranes and membranes of endocytic vesicles in living cultured cells. FEBS Lett. 1987 Aug 31;221(1):61–67. doi: 10.1016/0014-5793(87)80352-6. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring K. R., Smith P. D. Illumination and detection systems for quantitative fluorescence microscopy. J Microsc. 1987 Sep;147(Pt 3):265–278. doi: 10.1111/j.1365-2818.1987.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Fusion activity of influenza virus. A comparison between biological and artificial target membrane vesicles. J Biol Chem. 1986 Aug 25;261(24):10966–10969. [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]