ABSTRACT
Cells have an intrinsic ability to self-assemble and self-organize into complex and functional tissues and organs. By taking advantage of this ability, embryoids, organoids and gastruloids have recently been generated in vitro, providing a unique opportunity to explore complex embryological events in a detailed and highly quantitative manner. Here, we examine how such approaches are being used to answer fundamental questions in embryology, such as how cells self-organize and assemble, how the embryo breaks symmetry, and what controls timing and size in development. We also highlight how further improvements to these exciting technologies, based on the development of quantitative platforms to precisely follow and measure subcellular and molecular events, are paving the way for a more complete understanding of the complex events that help build the human embryo.
KEY WORDS: Early development, Patterning, Self-assembly, Self-organization, Symmetry breaking, Tissue mechanics
Summary: This Review article discusses the basic physical and biological principles that underlie the self-organization of embryonic stem cells into organoids, and how this informs human development.
Introduction
Cells have a remarkable capacity to self-organize into complex and functional structures. Although many processes trigger cells to rearrange in adult organisms – healing upon injury, for example – the most dramatic assembly takes place in early development. During gastrulation (see Glossary, Box 1), which Lewis Wolpert anecdotally identified as the most important event in life (Wolpert and Vicente, 2015), cells undergo striking morphogenetic movements and changes in cell fate that transform a seemingly symmetric cluster of cells into an assembly of distinct and demarcated cell types. This process involves complicated, yet somehow perfectly orchestrated, interactions that ultimately lead to the generation of the three germ layers, which later contribute to organ formation. In mice, for instance, it takes only six and a half days to begin forming the body plan, starting from one cell (Wolpert et al., 2015). Leading up to this process, several major anatomical events occur, including morphogenetic movements, cavitation and polarization of tissues (Chazaud and Yamanaka, 2016). Each of these is influenced by various biochemical and physical cues, changes in gene expression, cell-cell interactions, and interactions between cells and the extracellular matrix (ECM) (Bedzhov et al., 2014a).
Box 1. Glossary.
Biomimetic. Imitating biological systems for the purpose of, for instance, modeling biological phenomena or materials science.
Cell/tissue tension. The total tension of a tissue or its interfacial tension is an interplay of single-cell surface tension, which tends to minimize the cell's area, and the tension from adhesive forces, which tends to maximize the contacts between cells. The single-cell surface tension is a combination of lateral membrane tension (coming from an osmotic difference between the solutions inside and outside the cell) and cortical tension.
Ectoderm. The germ layer that gives rise to the nervous system, the neural crest and the epidermis.
Embryoid. A more organized embryoid body, such as a cavitating or a multilayered cluster of differentiating ESCs that resembles an embryo at certain stages of early development. One example is a blastoid, a structure that contains the same cell types and tissue topology as a blastocyst.
Embryoid body. A 3D aggregate of differentiating ESCs.
Endoderm. The germ layer that gives rise to tissues of the digestive and respiratory systems.
Gastrulation. A process that transforms the early embryo into a multilayered structure with distinct germ layers.
Gastruloid. A multicellular in vitro model of a gastrulating embryo.
Mesoderm. The germ layer that gives rise to mesenchyme, connective tissues, mesothelium and various blood cells.
Organoid. A multicellular structure containing many of the cell types and tissue layers present in an adult organ, typically derived from stem cells in vitro.
Recently, many of these developmental events have been successfully recapitulated in vitro, allowing for the first time a highly quantitative approach to understanding these phenomena. These experiments have highlighted the remarkable ability of cells to organize into complicated structures through a process usually referred to as self-organization or, sometimes, as self-assembly (see Box 2 for the distinction between the two terms). Of note, the fact that the cells are a self-organizing system does not mean that they do not require appropriate culture conditions, such as the presence of signaling proteins and suitable mechanical properties of the surrounding medium.
Box 2. Self-organization and self-assembly – one and the same?
Self-organization and self-assembly are processes by which an ensemble of independent and isolated components spontaneously forms into a large-scale ordered structure (Whitesides and Boncheva, 2002). These processes are evident across all scales in biology and physics, from protein folding and the formation of membrane bilayers, to the flocking of birds. Although the two terms are most often used synonymously in biological systems, there is a distinction between them based on the processes by which the final structure is achieved. Namely, self-assembly is reserved for phenomena that lead to a thermodynamic equilibrium (and therefore are reversible and resistant to small perturbations), whereas self-organization is employed where the energy is continuously provided to the system (Jones, 2004). From a physical standpoint, the embryo is an open system far from equilibrium and its number of components increases with time. It is therefore a dynamically changing system where, at each step, the free-energy landscape is altered. Thus, most embryological processes should be viewed as self-organization. However, over longer time scales, some processes, such as sorting or epithelialization, can be viewed as quasi-static (i.e. in apparent equilibrium) and, under our definition, therefore as self-assembly. Of note, this distinction between the two processes is historically debated (Bensaude-Vincent, 2006).
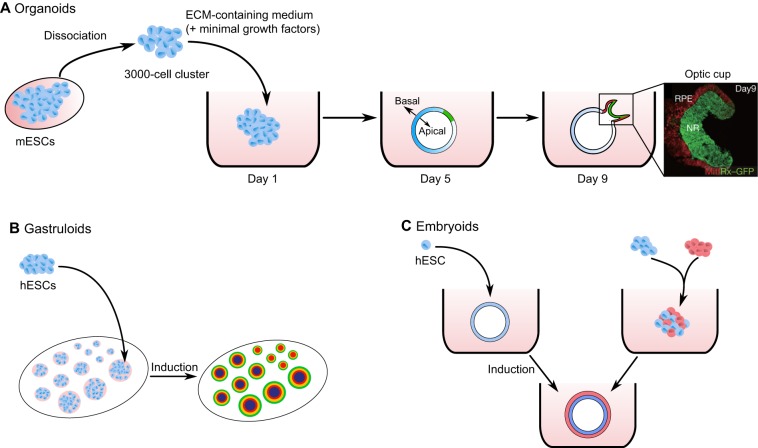
In a seminal example of self-organization, a cluster of dissociated mouse embryonic stem cells (ESCs) cultured in vitro was shown to spontaneously form an optic cup, exhibiting all layers of the neural retina, when presented with a medium containing low levels of growth factors and enriched with basal membrane proteins (Fig. 1A) (Eiraku et al., 2011). This structure, termed an organoid (see Glossary, Box 1), exhibits strong similarities to the in vivo tissue; it underwent changes in local tissue mechanics, invaginating to form the characteristic morphology of the optical cup (Fig. 1A) (Eiraku et al., 2011; Sasai et al., 2012). Importantly, this organoid formed without external scaffolding or mechanics, further demonstrating the innate ability of cells to self-generate functional multicellular structures. This approach was later adapted for making retinal tissue from human ESCs (hESCs) (Kuwahara et al., 2015). More recently, it was shown that human optic cup organoids engrafted onto injured eyes in primates continue to differentiate into a variety of retinal cell types, even creating synaptic contacts with the host (Shirai et al., 2016). Many other examples of organoids have also emerged (reviewed by McCauley and Wells, 2017), including gut (Sato et al., 2009; Spence et al., 2011), kidney (Takasato et al., 2015), pancreas (Greggio et al., 2013) and even brain (Eiraku et al., 2008; Lancaster et al., 2013) organoids. Their generation often involves modifications to 3D culture conditions and mimicking the assumed in vivo signaling events with extrinsic factors. Although making organoids has obvious potential health benefits, significant refinements and developments will be needed before they may become routinely applied in medicine. Importantly, however, these various types of organoids constitute promising new model systems that can be used for understanding fundamental aspects of human development that cannot otherwise be studied. They facilitate the exploration of signaling pathways in cell specification and organogenesis and also, from a more quantitative, mechanical standpoint, they delineate the physical basis of tissue and organ shaping (Lancaster and Knoblich, 2014).
Fig. 1.
Self-organization into organoids, gastruloids and embryoids. (A) A cluster of dissociated mouse embryonic stem cells (mESCs) cultured in a medium containing extracellular matrix (ECM) proteins and minimal growth factors spontaneously self-organizes, first into a polarized quasi-spherical epithelial tissue, then later giving rise to a structure resembling an optic cup. Rx+ cells (green) mark the retinal anlage; Mitf+ cells (red) mark the epithelial shell of the optic cup. NR, neural retina; RPE, retinal pigment epithelium. Microscopy image adapted with permission (Eiraku et al., 2011). (B) Dissociated human embryonic stem cells (hESCs) are seeded on a surface patterned with polymerized ECM proteins, creating demarcated cell colonies of defined size and shape. Subsequent addition of morphogen may give rise to the patterned differentiation of cells. In the case of BMP4 induction, patterned cells form gastruloids with all germ layers (Deglincerti et al., 2016b; Warmflash et al., 2014). Colors in patterned cell colonies represent different germ layers. (C) Some routes by which cells can be induced to form a multilayered embryoid. Left pathway: hESCs form an organized 3D structure, and subsequent induction leads to pattern formation. Right pathway: the mixing of multiple cell types gives rise to sorting and differentiation into an organized embryoid.
In general, an organoid is defined as a structure in which pluripotent or progenitor stem cells are differentiated into multiple cell populations that self-organize/assemble into a tissue that resembles an organ in vivo (Clevers, 2016; Fatehullah et al., 2016; Kicheva and Briscoe, 2015; Lancaster and Knoblich, 2014; Sasai et al., 2012; Turner et al., 2016). In a similar vein, researchers have also generated structures referred to as embryoid bodies, embryoids and gastruloids (see Glossary, Box 1; Fig. 1B,C). Embryoid bodies have been widely used for some time as models of early development, and typically are disorganized 3D clusters of pluripotent or differentiated cells. We consider the term embryoid to represent a more organized embryoid body that arises as a consequence, for instance, of cell polarization induced by the ECM in the surrounding medium or due to the correct topology of multiple cell types representing an embryo at a certain time of development. Gastruloids, by contrast, are models of a gastrulating embryo, either in 2D (Etoc et al., 2016; Warmflash et al., 2014) or in 3D (Turner et al., 2016; van den Brink et al., 2014). Self-assembly/organization has also been observed directly in attached mammalian blastocysts in vitro. These studies established that human (Deglincerti et al., 2016a; Shahbazi et al., 2016) and mouse (Bedzhov et al., 2014b; Morris et al., 2012) embryos can be cultured ex vivo in the absence of maternal tissues, showing important self-organizing events. Although these assays are currently ethically limited to studying human embryos for only up to 14 days of development, they provide a complementary approach to embryoid and gastruloid experiments for quantitatively studying early human development and are likely to become increasingly popular in the coming years.
In light of these recent advances in generating organoids, embryoids and gastruloids, understanding how cells self-organize and self-assemble to generate discrete tissues and organs is essential. It is equally crucial that quantitative in vitro assays are developed to assure reproducibility of the organoid-generation process, as one of main issues in the field is that methods for the spontaneous formation of organoids usually result in heterogeneous and poorly reproducible structures. In addition to the vital roles played by gene regulation, signaling pathways and cell differentiation, many classical questions concerning tissue mechanics are now resurfacing. For example, what are the driving forces of self-organization/assembly in early development? How is it so well controlled to create biomimetic structures (see Glossary, Box 1) on a dish with very little external intervention? How are mechanical and biochemical signals coupled? The aim of this Review is to explore these and similar questions, highlighting examples in which quantitative approaches have contributed significantly to our understanding of early development. We will not focus on detailed molecular aspects of signaling and patterning nor on methods to produce organoids, as these topics have been thoroughly reviewed elsewhere (e.g. Clevers, 2016; Fatehullah et al., 2016; Kicheva and Briscoe, 2015; Lancaster and Knoblich, 2014; McCauley and Wells, 2017; Turner et al., 2016). Instead, we focus on the three ingredients that we believe are key for creating a functional organoid: (1) self-organization/assembly, which involves the correct positioning of cells with respect to one another; (2) breaking of symmetry, whereby, for instance, a seemingly homogenous cluster of cells gives rise to the body axis; and (3) the control of developmental timing and size.
Self-assembly and self-organization
Pattern formation during embryogenesis is based on the physical and morphological properties of cells, the signals they receive, and the mechanical properties of tissues. As development progresses these properties undergo changes, generating dynamic juxtapositions of different cell types. For example, placing dissociated hESCs on a dish coated with polymerized ECM proteins, such as laminin, and culturing in medium that maintains pluripotency leads to gradual epithelialization in compact colonies (Fig. 2A). This formation of an epithelium from a non-epithelial state is an example of self-assembly. It is driven by effective, strong cell-cell interactions involving the cell adhesion molecule E-cadherin, actin polymerization, and other cell-linking proteins (Adams et al., 1998; Alberts et al., 2014; Vasioukhin et al., 2000). These adhesive factors can also regulate cell movements and cell sorting, and hence can influence tissue shape and structure. Finally, mechanical cues from the external environment, as well as external growth factors and signals, also play a key role in determining cell fate and thus self-organization.
Fig. 2.
Features of ESC self-organization and patterning. (A) A simple example of polarity-based self-organization. Dissociated ESCs on a surface coated with polymerized ECM proteins form a polarized epithelium, with cells exhibiting apical (green) and basolateral (orange) surfaces. (B) Sorting driven by the minimization of tissue surface energy. Cells with the strongest cell-cell interactions (based on adhesive energy or tissue tension) migrate toward the interior of a cell cluster, while those with the weakest cell-cell interactions migrate toward the exterior. (C) Lumen formation in ESCs. Cells embedded in a gel of polymerized ECM proteins polarize and form a spherical embryoid with a lumen at its center. The proteins actin and ezrin are enriched at the apical part of polarized cells. Microscopy image reproduced with permission (Taniguchi et al., 2015). (D) Mechanical properties affect cell differentiation and patterning. Shown is an example demonstrating that cells cultured on a softer surface have a higher propensity for mesodermal differentiation than those cultured on a stiff surface. (E) Geometric confinement may give rise to a signaling gradient. Shown is an example of the BMP4-induced differentiation of hESCs grown in colonies of different sizes. The cells sense BMP4 only at the edge of the colony (i.e. only the cells in between the two dotted circles are competent to receive the BMP4 signal), inducing the secretion of an inhibitor which, together with the BMP4, establishes a signaling gradient. The result is a radially symmetric pattern of gene expression resembling that of germ layer formation in gastrulation. As the signaling gradient is constant, the inner cell fates do not arise in small colonies. TE, trophectoderm. Microscopy images adapted with permission (Deglincerti et al., 2016b).
The role of adhesive forces
Adhesive forces have been suggested to play a prominent role in orchestrating the self-organization/assembly of cells within the embryo. Interestingly, the proteins that mediate these interactions, such as cadherins and their partner proteins the catenins, sense not only mechanical but also biochemical signals (Shawky and Davidson, 2015). Given the role of cadherins in epithelialization, can modifying cell-cell interactions trigger rearrangements in vitro? This idea emerged from early cell-mixing experiments. Mixing cells from different tissues of a vertebrate embryo leads to sorting, such that cells regroup according to their relative positions within the embryo (Holtfreter, 1944; Steinberg, 1970; Townes and Holtfreter, 1955). For instance, when dissociated cells taken from the retina, liver, heart or limb bud of a five-day chick embryo were reaggregated in vitro in different binary combinations, the cells always de-mixed, irrespective of the combination, and reassembled to join with the same cell type (Steinberg, 1963). Eventually, one cell type ended up surrounding the other, where the relative positions of tissues matched those in the embryo (Steinberg, 1963). This sorting might be driven by the minimization of total tissue surface energy, which places the cells with the strongest interactions and/or highest tissue tension (see Glossary, Box 1) at the interior, surrounded by layers of other tissues of decreasing strength of intercellular interactions (Fig. 2B). In support of this hypothesis, germ layer aggregates from the leopard frog Rana pipiens spontaneously stratify in vitro according to tissue tension, with tension decreasing from inside to outside (Davis et al., 1997). At the molecular level, differences in cadherin expression (both the amount and type) give rise to cell sorting in a tension-dependent manner in vitro and in vivo (Foty and Steinberg, 2005; Friedlander et al., 1989; Godt and Tepass, 1998; González-Reyes and St Johnston, 1998; Katsamba et al., 2009; Schotz et al., 2008; Steinberg and Takeichi, 1994). More recent efforts, however, have challenged this notion. Indeed, in some cases, the differential expression of cadherins does not lead to cell sorting in vivo or in embryoid bodies (Moore et al., 2009, 2014; Ninomiya et al., 2012). Taking into account that cells undergo active processes such as polarization, the in vivo and in vitro results can be reconciled. Namely, by modeling the dynamic interactions involving cadherins, ECM and the cytoskeleton, it was shown that cell sorting no longer necessarily scales with the absolute surface density of cadherin, as it depends on a variety of factors (Amack and Manning, 2012).
While it is understood that physical forces within a tissue facilitate the massive cell movements that occur during the self-organization/assembly of organoids, how the adhesive energy couples with cortical tension is not fully understood. Moreover, the extent to which sorting takes part in embryogenesis, not only to actuate cell motion but also to potentially aid differentiation and cell fate specification, is largely unknown (Fagotto, 2014).
Cadherin levels also play a role in the process of epithelial-to-mesenchymal transition (EMT) – the reverse of epithelialization. It is this process that frees tightly packed epithelial cells by altering cell-cell contacts and by activating processes that promote cell migration. In particular, E-cadherin is downregulated during EMT, during which time cell-cell interactions are decreased, and is replaced by N-cadherin, which exhibits weaker inter-protein interactions (Moore et al., 2014). At the same time, cells alter their signaling profile and reshape their cytoskeleton, which powers cell movement (Lamouille et al., 2014). Importantly, EMT plays a role in germ layer formation as epithelial epiblast cells ingress at the primitive streak (Stern, 2004).
Factors that regulate cavitation and lumenization
Recent advances in 3D tissue culture approaches, including those involving organoid, gastruloid and embryoid assays, have also provided the means to study the formation of cavities and lumina. For example, by embedding dissociated hESCs into a gel of ECM proteins it was shown that hESCs spontaneously form a lumen (Fig. 2C) due to interactions with the ECM (Bedzhov and Zernicka-Goetz, 2014; Kadoshima et al., 2013; Taniguchi et al., 2015), in the same way as shown previously for Madin-Darby canine kidney (MDCK) and human colorectal cancer cells, both of which are common models of epithelia (Martin-Belmonte and Mostov, 2008; Rodriguez-Fraticelli and Martin-Belmonte, 2013). In the case of developing MDCK cysts, actin-associating proteins such as ezrin are trafficked to the cytokinetic plane as single cells first divide (Bryant et al., 2014), forming an actin-rich site and marking the initiation region for the apical membrane (Apodaca et al., 2012). Cells then self-organize into an epithelium, forming a lumen at their apical sides. Apparently, in the case of dissociated human pluripotent stem cells, the lumen can already be seen after the first division between two cells in 3D culture (Taniguchi et al., 2015). By modifying the 3D culture medium, it has also been shown that human pluripotent stem cells can differentiate into amnion-like cells surrounding a lumen at the center of the colony (Shao et al., 2016). These approaches for studying cavity formation open up exciting routes for studying developmental events involving epithelial transitions and highlight an important morphological element in the bioengineering of organoids.
Input from external mechanical cues
Mechanical cues provided by the surrounding environment can also affect the self-organization of cells. In an organism as simple as Volvox (a type of green algae), gastrulation brings about a dramatic reorganization, which is believed to be a consequence of cell shape changes alone. The Volvox embryo is a spherical monolayer that turns itself inside-out, exposing its flagella to the exterior (Kirk, 2005). Time-lapse live imaging and mathematical modeling have demonstrated that a few cells reshape into wedges, inducing local tissue curvature which, together with successive tissue extension and contraction, provide the mechanical force for complete embryo inversion (Hohn et al., 2015). The transfer of mechanical force across the embryo is achieved by a kinesin motor that is localized at the cytosolic bridges connecting all the cells (Nishii et al., 2003). Another recent study implied that, although the cellular and signaling events in gastrulation can be vastly different among species, it is the mechanics of gastrulation that might be evolutionarily conserved (Brunet et al., 2013). This study used a myosin inhibitor to prevent cell movements during gastrulation in zebrafish. Although this treatment did not affect the initial specification of germ layers, an early mesodermal marker, ntl (also known as ta; a brachyury ortholog), was not expressed in the embryo. However, by injecting these cells with magnetic beads, gastrulation could be mimicked by an external magnetic field used to pull the cells. This process rescued ntl expression and the associated gastrulation movements in the embryo (Brunet et al., 2013). According to the authors, a possible explanation for this finding is that mechanical forces trigger mechanosensory nuclear translocation of β-catenin, an evolutionarily conserved pathway, to initiate the expression of downstream mesodermal genes.
Mesoderm formation is also highly sensitive to the mechanical properties of the substrate (Fig. 2D). By differentiating hESCs grown on hydrogel substrates of different stiffness, it was found that a soft substrate (with a stiffness of 400 Pa) promotes more abundant expression of mesodermal markers than does a stiff (60 kPa) substrate (Przybyla et al., 2016). Of note, the stiffness of a typical plastic or glass tissue culture dish is >1 GPa. This study also suggested that stiff substrates promote the degradation of β-catenin in an integrin-dependent manner, thereby inhibiting mesodermal differentiation (Przybyla et al., 2016).
Generating micropatterns: the role of confining external signals
The embryo is a dynamically changing system far from equilibrium and it is not clear if, at any stage of development, it reaches an interfacial tissue tension equilibrium. Nevertheless, some equilibrium thermodynamic phenomena described in this section, such as adhesion-driven or tension-driven sorting, seems to explain some aspects of self-organization behavior in organoid formation almost surprisingly well. It also demonstrates the importance of physical properties and tissue mechanics in helping the relevant signaling pathways to differentiate cells and direct them to their destined tissue. Importantly, the fate of a cell is under the control of a complex signaling network, which ultimately leads to the formation of germ layers and the correct spatial organization of patterning in an embryo. Understanding how many signaling pathways function and integrate to pattern cells in an embryo is a very challenging task. One major caveat of using embryoid bodies and organoids to understand the underlying mechanisms of particular signaling pathways is that these systems are often not reproducible in terms of size, proportion of cells of a certain fate, and the shape of fate boundaries. Recently, advances in biophysical methodologies and high-resolution live-cell imaging have enabled important progress in this direction by modeling self-assembly/organization processes in highly quantitative platforms. By spatially confining hESCs onto circles of submillimeter size, for example, it has been shown that, upon application of bone morphogenetic protein 4 (BMP4), colonies differentiate into a radially symmetric pattern (Fig. 1B, Fig. 2E). The resultant pattern essentially mimics that seen in a gastrulating embryo, with each ring expressing genes associated with different tissues (from the edges inward): presumptive extraembryonic tissue, endoderm, mesoderm, and ectoderm (see Glossary, Box 1) (Deglincerti et al., 2016b; Warmflash et al., 2014). Interestingly, this system has uncovered that receptors of the transforming growth factor β (TGFβ) superfamily of growth factors (including that of BMP4) are localized apically at the colony edge but laterally at the colony center. As BMP4 produces its own inhibitor, noggin, a morphogen gradient is formed from the edges inward, causing radial patterning (Fig. 2E) (Etoc et al., 2016).
It has also been shown that manipulating colony size by micropatterning can direct the cells into mesoderm versus endoderm upon differentiation (Lee et al., 2009), which is likely to be a consequence of edge sensing and a reaction-diffusion mechanism. Micropatterning has also been used to study developmental processes beyond gastrulation, namely during the formation and subsequent characterization of cardiomyocytes (Ma et al., 2015).
Breaking symmetry
Breaking symmetry is necessary for successful establishment of the body axis and can occur at multiple scales, from intracellular to embryological (Stern, 2004). One classic example of breaking symmetry is the fertilization of an amphibian egg. In this context, sperm entry breaks the maternal cylindrical symmetry imposed in the unfertilized egg and the centriole of the sperm organizes the egg's microtubules such that the pole opposite to the entry point becomes the dorsal side of the embryo (Wolpert et al., 2015). However, reproducibly recapitulating symmetry breaking in vitro has proven to be challenging. Indeed, in making the optic cup in vitro, despite exhibiting striking similarities to in vivo cell organization, the organoid still lacked some asymmetric aspects, such as the gapped structure at the ventralmost region of the optic cup seen in the embryo (Sasai et al., 2012). In addition, the spontaneous reaggregation of chick embryo cells in sorting experiments, although resulting in the correct placement of cell types relative to one another, resulted in a radially symmetric pattern, despite these cells not having a radially symmetric pattern in the embryo (Steinberg, 1963). Similarly, the BMP4-induced differentiation of micropatterned hESCs, although recapitulating germ layer formation, did not break radial symmetry (Warmflash et al., 2014).
How is symmetry broken in vivo? In the case of a single cell, cell polarization may be seen as a cellular symmetry-breaking event, marked by a redistribution of cytoskeletal and membrane-associated proteins (Fig. 3A, left). This process is essential in early development and occurs during a process called compaction, whereby the cells in an embryo form a tightly packed cluster and increase the contacts among them (Fig. 3A, right); in the mouse embryo, this phenomenon typically takes place at the 8- to 16-cell transition (White et al., 2016a; Ziomek and Johnson, 1980). During organoid formation, ESCs embedded in a gel of polymerized ECM proteins can also form a cavity due to cell polarization (as depicted in Fig. 2C). But what can break symmetry at an embryological scale? In classical developmental biology experiments, Spemann and Mangold demonstrated that transplanting a cluster of cells, termed the organizer, from one newt gastrula into the ventral side of another gives rise to a second body axis, ultimately creating a two-headed tadpole (Spemann and Mangold, 1924). The grafted cells contributed to axial mesodermal derivatives (such as the notochord), whereas the nervous system (with the exception of the floor plate) was derived from the host. Importantly, this experiment established that symmetry breaking is caused by localized inductive signals (Ozair et al., 2013; Spemann and Mangold, 1924). In asking how early these symmetry-breaking signals are established, it was shown that two to three blastomeres of an eight-cell stage Xenopus embryo are sufficient to give rise to a second body axis when transplanted into a 64-cell embryo (Gimlich and Gerhart, 1984). This region of the embryo, termed the Nieuwkoop center, gives rise to the Spemann-Mangold organizer. Depending on the stage of the embryo at transplantation, the transplanted organizer can act as an inducer of symmetry breaking to the surrounding cells or directly contribute its cells to the progeny. Therefore, a local source of signaling and competency of surrounding cells to respond to these signals are the minimal requirements of symmetry breaking. In the case of dorsoventral axis formation in the frog, this process involves a localized BMP4 concentration to specify dorsal fate, while the BMP4 inhibitors Chordin, Follistatin and Noggin are secreted by the Spemann organizer and together form a ventralizing BMP4 gradient (Harland, 1994; Hemmati-Brivanlou et al., 1994; Hemmati-Brivanlou and Melton, 1992, 1994; Sasai et al., 1994; Smith and Harland, 1992).
Fig. 3.
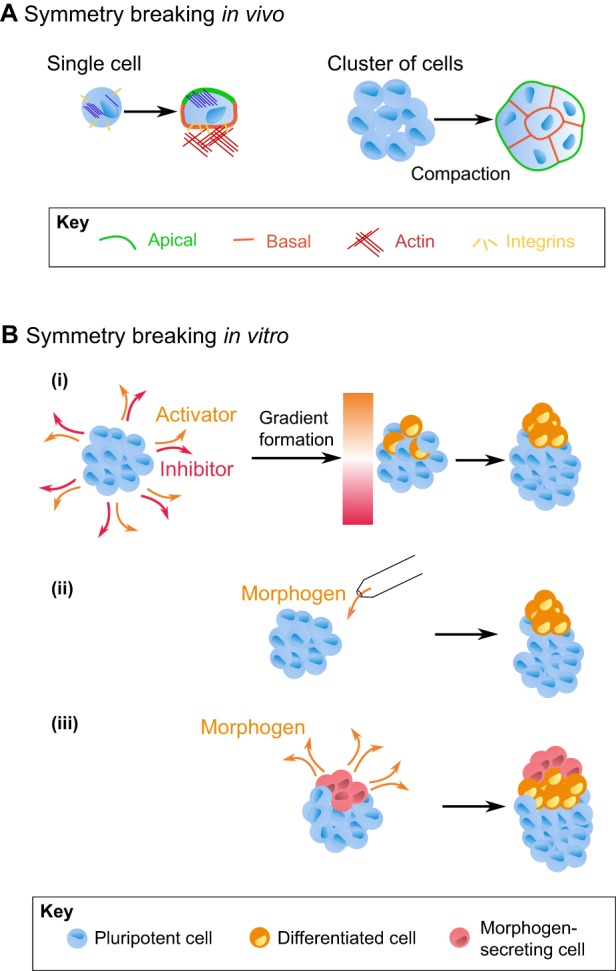
Breaking symmetry in cells and organoids. (A) (Left) Symmetry breaking at the level of a single cell. In this example, cells reorganize their cytoskeleton and membrane-anchored proteins to form apical-basal polarity, with, among many other proteins, integrins on the basal side and actin on the apical. (Right) Symmetry breaking at the multicellular level. In the case of the early mouse embryo, cell polarization underlies the process of compaction, whereby a cluster of eight loosely connected, non-polarized cells becomes tightly packed, significantly increasing cell-cell contacts and leading to the polarization of cells. (B) Examples of potential approaches to induce symmetry breaking in organoids. (i) Breaking symmetry with a diffusion-reaction mechanism. Adding morphogens to embryoid bodies can induce the secretion of inductive and inhibitory molecules from cells. Via a reaction-diffusion (Turing) process, an initially homogenously distributed signal can then, after reaching steady state, give rise to a stable signaling gradient, which in turn can trigger asymmetric changes in cell fate within the organoid. (ii) Symmetry breaking can also be induced by locally delivering a morphogen with a micropipette; in this case, the cells exposed to the highest level of morphogen will be induced to change fate. (iii) Symmetry breaking via the local secretion of morphogen from engrafted cells (red) in an organoid made up of another cell type (blue).
Local signals are also important for establishing the anterior-posterior body axis. In the mouse, for example, signals from the extraembryonic ectoderm and the cells of the distal visceral endoderm (DVE), which migrate to the anterior part of the embryo, restrict the signals coming from the primitive streak to within the proximal posterior regions of the epiblast. This process breaks symmetry and helps form the anterior-posterior axis (Fig. 3A) (Stower and Srinivas, 2014). Left-right asymmetry, on the other hand, in various species is induced by a concentration gradient of Nodal and its inhibitor Lefty. In vertebrates, one model hypothesizes that the rotary motion of cilia causes a leftward flow of extracellular fluid, in turn leading to asymmetric activation of the Nodal cascade (Blum et al., 2014).
Tissue mechanics is another potential ingredient contributing to symmetry breaking. By culturing post-implantation mouse embryos in a confined space, it was demonstrated that external mechanical forces, which are likely to arise from maternal tissues, are key for the formation of an ectopic thickened cell layer resembling the DVE, which is in turn responsible for setting up the anterior-posterior axis (Hiramatsu et al., 2013). Interestingly, a more recent study has suggested that the anterior-posterior axis of a mouse embryo can form in the absence of any maternal cues (Bedzhov et al., 2015). The full extent to which tissue mechanics can influence axis formation in the gastrulating embryo is, therefore, yet to be determined.
Despite this growing knowledge of the signaling pathways and cellular events that lead to symmetry breaking, the precise molecular and physical underpinnings that cause asymmetry, as well as the precise timing of symmetry-breaking events, remain elusive. Moreover, it remains unclear whether one can differentiate hESCs into true extraembryonic cell types, which could be an important technical limitation to achieving spontaneous symmetry breaking in vitro. Nonetheless, some recent work has shown that embryoid bodies or smaller aggregates can, to an extent, break symmetry, as evidenced by the localized expression of primitive streak genes, either in response to exogenous Wnt stimulation (ten Berge et al., 2008) or upon stimulation with activin A or the Wnt agonist CHIR99021 (van den Brink et al., 2014). The latter demonstration seems robust and even shows resemblance to axis formation and elongation as seen in the mouse embryo. Importantly, symmetry breaking as it is observed in these experiments does not require an initial local morphogen source and is highly sensitive to initial aggregate size (van den Brink et al., 2014). This observation is, of course, not at odds with the physical basis of patterning; Turing famously theorized that an interplay of activators and their inhibitors in a reaction-diffusion model can give rise to patterning from an initially uniform morphogen field (Turing, 1952). Subsequent models deployed minimal ingredients that lead to symmetry breaking in the morphogen concentration landscape (Gierer and Meinhardt, 1972). Mathematical modeling (Corson and Siggia, 2012; Gierer and Meinhardt, 1972; Turing, 1952), together with the highlighted experimental examples, can thus provide important insights into the variables (such as dimensionality and cell density) that need to be considered when creating organoids in a dish.
It is also becoming increasingly clear that symmetry breaking may occur much earlier in development than previously thought, prior to body axis formation or even lineage separation. Indeed, it appears that seemingly morphologically equivalent cells in embryos are actually quite different in terms of their molecular profiles. For example, recent experiments have shown that there is variable intercellular expression of proteins as early as the four-cell stage of a mouse embryo, even though the separation of fates into primitive endoderm, epiblast and trophectoderm takes place at the 16- to 32-cell transition (Goolam et al., 2016; White et al., 2016b). Furthermore, by measuring the binding dynamics of transcription factors to DNA, it was shown that Sox2, a transcription factor controlling pluripotency, exhibits much longer binding to DNA in specific blastomeres of the four-cell embryo; these blastomeres with longer-lived Sox2-DNA complexes later contributed to the pluripotent cells of the inner cell mass. According to this study, the first symmetry-breaking event may be caused by cleavage at the two- to four-cell transition (Goolam et al., 2016; Torres-Padilla et al., 2007). In agreement, single-cell transcriptomic analyses of mouse embryos show that at least one blastomere in a four- to eight-cell stage embryo exhibits low levels of expression of a gene downstream of Sox2 (Goolam et al., 2016), suggesting that cell lineage determination occurs much earlier than morphologically observed. These observations also reinforce the notion that cell division partitioning errors (Shi et al., 2015) or random transcriptional noise (Eldar and Elowitz, 2010; Elowitz et al., 2002) could be the mechanism that drives symmetry breaking in the embryo.
In summary, several common mechanisms emerge from the analysis of symmetry breaking both in vivo and from reconstituted systems. Some symmetry-breaking events seem cell-autonomous, being determined parentally or encoded in asymmetric gene expression among the cells of a very early embryo. Other symmetry-breaking events are caused by a morphogen gradient set by a local source. Finally, reaction-diffusion mechanisms may give rise to symmetry breaking despite an initially homogenous distribution of the morphogen. With the advancement of 3D culture techniques, future efforts (Fig. 3B) will hopefully provide a better understanding of these symmetry-breaking events, especially at the single-cell and molecular levels. For example, as previously demonstrated (van den Brink et al., 2014), the simple addition of morphogen to a medium surrounding 3D cultures may give rise to symmetry breaking due to a diffusion-reaction mechanism (Fig. 3B). A better understanding of the inhibitors that cells secrete upon stimulation, combined with the ability to create different tissue topologies, might provide insights into many developmental processes. Moreover, it would be very interesting to determine the extent to which creating a local morphogen gradient breaks symmetry. Such experiments could be performed, for instance, using micropipette injection of the morphogen, microfluidics techniques, or perhaps by grafting different cell types (Fig. 3B). Importantly, these experiments will provide unique insights into the molecular underpinnings of symmetry breaking and might also help in devising methods to make organoids that more closely resemble their in vivo counterparts.
The control of size and timing during development
Even after cells correctly self-organize and symmetry is broken, the proper development of an organism requires the precise control of timing and size. Our understanding of the regulation and integration of these two variables during development remains cursory. In particular, understanding how patterning scales with organism size, and what determines the absolute size of an organism, remain key questions in the field. The latter is intricately linked to timing control, as there is an evident correlation between organism size and developmental timing, but also since each step in development occurs at consistent time intervals in the same species.
Size control and scaling
Pattern formation is known to scale with embryo size; for this reason, pattern positions are relative in the embryo, not absolute. Much of this topic has been studied in the fly, with various models proposed (Kicheva and Briscoe, 2015). For example, wing imaginal disc development along the anterior-posterior axis is thought to be under the control of a gradient of Decapentaplegic (Dpp, a BMP analog). Interestingly, the gradient adjusts itself to compartment size, with Dpp levels maximal at the center and minimal at the edges, and with the pattern scaled accordingly (Hufnagel et al., 2007; Nellen et al., 1996; Teleman and Cohen, 2000). Theoretical explanations for other notable examples of scaling in the fly have also been proposed, such as the scaling of the maternal Bicoid (Bcd) gradient that sets the anterior-posterior axis early in development (He et al., 2015).
Important lessons in developmental scaling have also come from studies of the frog. After cutting the frog embryo in half, the dorsal side, containing the organizer, develops into a proportionally patterned, albeit smaller, embryo (Reversade and De Robertis, 2005). Moreover, despite size differences among amphibian species and the variability in egg and gastrula size of the same species, the animals always pattern in the same way. It has also been shown that the BMP inhibitor Chordin forms a dynamic feedback loop with its stabilizing protein Sizzled: at the ventral side of the amphibian embryo, Sizzled controls Chordin levels and thus the BMP gradient, which in turn sets the pattern (Inomata et al., 2013). Very different from these examples, it has been shown that the scaling of neural tube pattern in two birds of different size is due to a difference in the threshold at which the cells of the two species respond to signals (Uygur et al., 2016). Other examples and theoretical models exist that further explain the scaling of pattern formation in vivo (Kicheva and Briscoe, 2015; Umulis and Othmer, 2013).
But what is known about scaling and pattern formation in vitro? For example, 2D gastruloids formed on confined colonies do not scale their patterning with tissue size (Warmflash et al., 2014). Instead, with the decreased tissue size they lose the fates that are normally present at the colony interior, as would be expected for a signaling mechanism that is restricted to the edge (Fig. 2E) (Etoc et al., 2016). It is currently unclear whether organoids and embryoids exhibit any growth or pattern scaling control. It will thus be interesting to test if the size control mechanisms seen in embryos can be recapitulated in vitro.
The control of developmental timing in vivo and in vitro
Although the above experiments provide some insights into the existence of a robust size-scaling mechanism, much remains to be discovered. In addition, little is known about the regulation of timing in development. The onset of some events in the mouse preimplantation embryo seems to be affected by a complex combination of factors that could provide some ʻmeasure' of time, including embryo size, total cell numbers, cell division count, nuclear-cytoplasmic ratio, DNA replication, and specific effectors in the cytoplasm (Kojima et al., 2014). For example, it has been proposed that there is an inhibitory cytoplasmic factor present in the one-cell embryo that could govern embryo size early in development; the dilution of this factor during cleavage below some threshold could trigger the onset of compaction (Lee et al., 2001). Similarly, a threshold in nuclear-cytoplasmic ratio, which exponentially increases prior to compaction, could be another timing factor (Kojima et al., 2014). A particularly intriguing experiment showed that after obliterating even as much as 90% of the mouse embryo at around gastrulation, the embryo in most cases recovers by the end of organogenesis, although with a reduced total mass, implicating a coordinated action between timing and embryo size (Snow and Tam, 1979). A similar effect was seen after removing blastomeres from a four-cell mouse embryo using micromanipulation; proliferation is accelerated until cell number catches up and development progresses normally (Power and Tam, 1993). In both cases, the developmental timing of particular organs was not coordinated with that of others, as compared with that seen in the healthy control, although in most cases development recovered by E12.5 (Kojima et al., 2014).
These experiments suggest that there are underlying mechanisms that can accelerate and thus control the dynamics of tissue proliferation and growth up to and during organogenesis, and possibly later as well. However, what these mechanisms are is still a mystery. The timing of formation of some organoids, such as brain organoids, more or less follows developmental timing in vivo, depending on the assay used (Gaspard et al., 2008; Turner et al., 2016). Furthermore, the timing of neural differentiation seems to be species-specific, as seen in vivo, as illustrated by the slower neural maturation of hESCs compared with primate ESCs (Otani et al., 2016) and by the faster in vitro differentiation of mouse cells into neurons compared with human cells (Wichterle et al., 2002). In the case of the optic cup discussed earlier, it took much longer to form organoids from human cells than from mouse cells (Kuwahara et al., 2015), again reflecting the relative developmental timing of these species. One is tempted to speculate that timing is independent from one tissue to another, supported by the observation that developmental compensation time in the mouse is not the same for each organ, as discussed above (Kojima et al., 2014). Other explanations are possible: self-organization itself, for example, could be the limiting factor. Future studies are clearly needed to obtain a better understanding of the spatiotemporal control of embryonic development in mammals. How does size control couple with timing? What are the roles of signaling molecules, compared with the role of mechanical influences? Are differentiation and development as a whole linked with size and timing regulation at the level of a single cell? Organoids, embryoids and gastruloids provide a new platform for the study of mechanisms that underlie size and timing control in development, and the rapid advances in generating, characterizing and quantitatively analyzing these structures are likely to lead to many discoveries in the near future.
Future perspectives
Various self-assembly and self-organizing events take place during the development of an embryo, organoid or gastruloid. As we have highlighted in this Review, it is becoming clear that multiple complex factors influence how cells form patterned tissues, both in vivo and in vitro. It is possible that for these reasons some organoids, such as brain organoids, seem to yield more reproducible morphologies than other organoids (van de Wetering et al., 2015). Possible explanations for this observation are that brain organoids typically contain many more cell types than other organoids and so the landscape of interactions is much more complex. Furthermore, neural induction takes longer than the differentiation of other tissues, so any errors introduced at the start might be compounded as time progresses. Finally, the technical limitations of tissue culture are likely to affect the way organoids develop. Although organoids in vitro are typically made by culturing stem cells under conditions that mimic, as closely as possible, the in vivo situation, we are still a long way from having a full understanding of all of the complex cell-cell and cell-ECM interactions, as well as the signaling network, that take place during development. Therefore, work in the near future requires knowledge obtained from developmental biology and its integration with physics and bioengineering to generate more robust and reproducible organoid cultures.
We predict several aspects that will see significant development in the near future. (1) Micropatterning. Many key questions have been answered using embryoid bodies; however, these systems typically yield disorganized structures and are therefore difficult to use to address quantitative questions. The development of micropattern-based assays offers reproducible ways of making organoids that are much more amenable for quantitative studies, opening an important chapter in studying human development. (2) 3D cultures. Recent efforts in creating more organized 3D structures from hESCs – embryoids – are likely to progress even further by the development of sophisticated hydrogels, which will allow cells to organize into more complex structures in a reproducible way (Gjorevski et al., 2016). These systems might also provide a means to study symmetry-breaking events by locally delivering morphogens, either by microinjection techniques or using co-cultures (Fig. 3B). (3) Single-cell sequencing. The impressive advancement of single-cell sequencing techniques in recent years will greatly improve organoid development by allowing researchers to compare the molecular signatures of cells in these structures with their presumptive counterparts in the embryo. These methods will also help us to gain insights into the molecular underpinnings of self-organization processes in organoids and embryoids, in both time and space. (4) Live-cell microscopy. We predict that rapid developments in light sheet microscopy coupled with gene editing technologies will provide important insights into the molecular basis of lineage specification and the morphogenetic changes that occur in early development at resolutions that have never been possible before.
Each of these techniques has its own limitations. For example, while 2D patterned cultures lack the 3D architecture of an embryo, 3D embryoids do not faithfully represent the shape of a human embryo, and 3D gastruloids do not generate the same topological organization of different tissues as seen in the embryo. Therefore, no one technique alone is sufficient for answering all of the key questions in the field of early development, and their integration is thus crucial. Altogether, the development of these methods paves the way toward regenerative medicine and possibly even the bioengineering of human organs. Perhaps more importantly than the utilitarian aspects, the study of ʻoids' marks a major step toward the long overdue understanding of our own origin.
Acknowledgements
We thank Eric Siggia, Gist Croft and Zeeshan Ozair for constructive criticism of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a National Institutes of Health/Department of Health and Human Services grant (#5RO1Hd080699) to A.B. M.S. is a Junior Fellow of the Simons Foundation (#429966). Deposited in PMC for release after 12 months.
References
- Adams C. L., Chen Y. T., Smith S. J. and Nelson W. J. (1998). Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 142, 1105-1119. 10.1083/jcb.142.4.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K. and Walter P. (2014). Molecular Biology of the Cell. New York: Garland Science. [Google Scholar]
- Amack J. D. and Manning M. L. (2012). Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212-215. 10.1126/science.1223953 [DOI] [PubMed] [Google Scholar]
- Apodaca G., Gallo L. I. and Bryant D. M. (2012). Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 14, 1235-1243. 10.1038/ncb2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzhov I. and Zernicka-Goetz M. (2014). Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032-1044. 10.1016/j.cell.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzhov I., Graham S. J., Leung C. Y. and Zernicka-Goetz M. (2014a). Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzhov I., Leung C. Y., Bialecka M. and Zernicka-Goetz M. (2014b). In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 9, 2732-2739. 10.1038/nprot.2014.186 [DOI] [PubMed] [Google Scholar]
- Bedzhov I., Bialecka M., Zielinska A., Kosalka J., Antonica F., Thompson A. J., Franze K. and Zernicka-Goetz M. (2015). Development of the anterior-posterior axis is a self-organizing process in the absence of maternal cues in the mouse embryo. Cell Res. 25, 1368-1371. 10.1038/cr.2015.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude-Vincent B. (2006). Self-assembly, self-organization: a philosophical perspective on a major challenge of nanotechnology. Archive ouverte en Sciences de l'Homme et de la Société, halshs-00350831 https://halshs.archives-ouvertes.fr/halshs-00350831. [Google Scholar]
- Blum M., Feistel K., Thumberger T. and Schweickert A. (2014). The evolution and conservation of left-right patterning mechanisms. Development 141, 1603-1613. 10.1242/dev.100560 [DOI] [PubMed] [Google Scholar]
- Brunet T., Bouclet A., Ahmadi P., Mitrossilis D., Driquez B., Brunet A.-C., Henry L., Serman F., Béalle G., Ménager C. et al. (2013). Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. 4, 2821 10.1038/ncomms3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Roignot J., Datta A., Overeem A. W., Kim M., Yu W., Peng X., Eastburn D. J., Ewald A. J., Werb Z. et al. (2014). A molecular switch for the orientation of epithelial cell polarization. Dev. Cell 31, 171-187. 10.1016/j.devcel.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud C. and Yamanaka Y. (2016). Lineage specification in the mouse preimplantation embryo. Development 143, 1063-1074. 10.1242/dev.128314 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2016). Modeling development and disease with organoids. Cell 165, 1586-1597. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- Corson F. and Siggia E. D. (2012). Geometry, epistasis, and developmental patterning. Proc. Natl. Acad. Sci. USA 109, 5568-5575. 10.1073/pnas.1201505109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. S., Phillips H. M. and Steinberg M. S. (1997). Germ-layer surface tensions and “tissue affinities” in Rana pipiens gastrulae: quantitative measurements. Dev. Biol. 192, 630-644. 10.1006/dbio.1997.8741 [DOI] [PubMed] [Google Scholar]
- Deglincerti A., Croft G. F., Pietila L. N., Zernicka-Goetz M., Siggia E. D. and Brivanlou A. H. (2016a). Self-organization of the in vitro attached human embryo. Nature 533, 251-254. 10.1038/nature17948 [DOI] [PubMed] [Google Scholar]
- Deglincerti A., Etoc F., Guerra M. C., Martyn I., Metzger J., Ruzo A., Simunovic M., Yoney A., Brivanlou A. H., Siggia E. et al. (2016b). Self-organization of human embryonic stem cells on micropatterns. Nat. Protoc. 11, 2223-2232. 10.1038/nprot.2016.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K. and Sasai Y. (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519-532. 10.1016/j.stem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T. and Sasai Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Eldar A. and Elowitz M. B. (2010). Functional roles for noise in genetic circuits. Nature 467, 167-173. 10.1038/nature09326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz M. B., Levine A. J., Siggia E. D. and Swain P. S. (2002). Stochastic gene expression in a single cell. Science 297, 1183-1186. 10.1126/science.1070919 [DOI] [PubMed] [Google Scholar]
- Etoc F., Metzger J., Ruzo A., Kirst C., Yoney A., Ozair M. Z., Brivanlou A. H. and Siggia E. D. (2016). A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell 39, 302-315. 10.1016/j.devcel.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F. (2014). The cellular basis of tissue separation. Development 141, 3303-3318. 10.1242/dev.090332 [DOI] [PubMed] [Google Scholar]
- Fatehullah A., Tan S. H. and Barker N. (2016). Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246-254. 10.1038/ncb3312 [DOI] [PubMed] [Google Scholar]
- Foty R. A. and Steinberg M. S. (2005). The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255-263. 10.1016/j.ydbio.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Friedlander D. R., Mège R. M., Cunningham B. A. and Edelman G. M. (1989). Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc. Natl. Acad. Sci. USA 86, 7043-7047. 10.1073/pnas.86.18.7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N., Bouschet T., Hourez R., Dimidschstein J., Naeije G., van den Ameele J., Espuny-Camacho I., Herpoel A., Passante L., Schiffmann S. N. et al. (2008). An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351-357. 10.1038/nature07287 [DOI] [PubMed] [Google Scholar]
- Gierer A. and Meinhardt H. (1972). A theory of biological pattern formation. Kybernetik 12, 30-39. 10.1007/BF00289234 [DOI] [PubMed] [Google Scholar]
- Gimlich R. L. and Gerhart J. C. (1984). Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev. Biol. 104, 117-130. 10.1016/0012-1606(84)90042-3 [DOI] [PubMed] [Google Scholar]
- Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M. E., Ordóñez-Morán P., Clevers H. and Lutolf M. P. (2016). Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560-564. 10.1038/nature20168 [DOI] [PubMed] [Google Scholar]
- Godt D. and Tepass U. (1998). Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature 395, 387-391. 10.1038/26493 [DOI] [PubMed] [Google Scholar]
- González-Reyes A. and St Johnston D. (1998). The Drosophila AP axis is polarised by the cadherin-mediated positioning of the oocyte. Development 125, 3635-3644. [DOI] [PubMed] [Google Scholar]
- Goolam M., Scialdone A., Graham S. J. L., Macaulay I. C., Jedrusik A., Hupalowska A., Voet T., Marioni J. C. and Zernicka-Goetz M. (2016). Heterogeneity in Oct4 and Sox2 targets biases cell fate in 4-cell mouse embryos. Cell 165, 61-74. 10.1016/j.cell.2016.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio C., De Franceschi F., Figueiredo-Larsen M., Gobaa S., Ranga A., Semb H., Lutolf M. and Grapin-Botton A. (2013). Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452-4462. 10.1242/dev.096628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. M. (1994). Neural induction in Xenopus. Curr. Opin. Genet. Dev. 4, 543-549. 10.1016/0959-437X(94)90070-J [DOI] [PubMed] [Google Scholar]
- He F., Wei C., Wu H., Cheung D., Jiao R. and Ma J. (2015). Fundamental origins and limits for scaling a maternal morphogen gradient. Nat. Commun. 6, 6679 10.1038/ncomms7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A. and Melton D. A. (1992). A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature 359, 609-614. 10.1038/359609a0 [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A. and Melton D. A. (1994). Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77, 273-281. 10.1016/0092-8674(94)90319-0 [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Kelly O. G. and Melton D. A. (1994). Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77, 283-295. 10.1016/0092-8674(94)90320-4 [DOI] [PubMed] [Google Scholar]
- Hiramatsu R., Matsuoka T., Kimura-Yoshida C., Han S.-W., Mochida K., Adachi T., Takayama S. and Matsuo I. (2013). External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos. Dev. Cell 27, 131-144. 10.1016/j.devcel.2013.09.026 [DOI] [PubMed] [Google Scholar]
- Höhn S., Honerkamp-Smith A. R., Haas P. A., Trong P. K. and Goldstein R. E. (2015). Dynamics of a volvox embryo turning itself inside out. Phys. Rev. Lett. 114, 178101 10.1103/PhysRevLett.114.178101 [DOI] [PubMed] [Google Scholar]
- Holtfreter J. (1944). A study of the mechanics of gastrulation. J. Exp. Zool. 95, 171-212. 10.1002/jez.1400950203 [DOI] [Google Scholar]
- Hufnagel L., Teleman A. A., Rouault H., Cohen S. M. and Shraiman B. I. (2007). On the mechanism of wing size determination in fly development. Proc. Natl. Acad. Sci. USA 104, 3835-3840. 10.1073/pnas.0607134104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H., Shibata T., Haraguchi T. and Sasai Y. (2013). Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann's organizer signals. Cell 153, 1296-1311. 10.1016/j.cell.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Jones R. A. L. (2004). Soft Machines: Nanotechnology and Life. Oxford: Oxford University Press. [Google Scholar]
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M. and Sasai Y. (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284-20289. 10.1073/pnas.1315710110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsamba P., Carroll K., Ahlsen G., Bahna F., Vendome J., Posy S., Rajebhosale M., Price S., Jessell T. M., Ben-Shaul A. et al. (2009). Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc. Natl. Acad. Sci. USA 106, 11594-11599. 10.1073/pnas.0905349106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva A. and Briscoe J. (2015). Developmental pattern formation in phases. Trends Cell Biol. 25, 579-591. 10.1016/j.tcb.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Kirk D. L. (2005). Volvox: a Search for the Molecular and Genetic Origins of Multicellularity and Cellular Differentiation. Cambridge: Cambridge University Press. [Google Scholar]
- Kojima Y., Tam O. H. and Tam P. P. L. (2014). Timing of developmental events in the early mouse embryo. Semin. Cell Dev. Biol. 34, 65-75. 10.1016/j.semcdb.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Ozone C., Nakano T., Saito K., Eiraku M. and Sasai Y. (2015). Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun. 6, 6286 10.1038/ncomms7286 [DOI] [PubMed] [Google Scholar]
- Lamouille S., Xu J. and Derynck R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178-196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A. and Knoblich J. A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P. and Knoblich J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373-379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. R., Lee J. E., Yoon H. S., Roh S. I. and Kim M. K. (2001). Compaction in preimplantation mouse embryos is regulated by a cytoplasmic regulatory factor that alters between 1- and 2-cell stages in a concentration-dependent manner. J. Exp. Zool. 290, 61-71. 10.1002/jez.1036 [DOI] [PubMed] [Google Scholar]
- Lee L. H., Peerani R., Ungrin M., Joshi C., Kumacheva E. and Zandstra P. W. (2009). Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Res. 2, 155-162. 10.1016/j.scr.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Ma Z., Wang J., Loskill P., Huebsch N., Koo S., Svedlund F. L., Marks N. C., Hua E. W., Grigoropoulos C. P., Conklin B. R. et al. (2015). Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 6, 7413 10.1038/ncomms8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F. and Mostov K. (2008). Regulation of cell polarity during epithelial morphogenesis. Curr. Opin. Cell Biol. 20, 227-234. 10.1016/j.ceb.2008.01.001 [DOI] [PubMed] [Google Scholar]
- McCauley H. A. and Wells J. M. (2017). Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development 144, 958-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Cai K. Q., Escudero D. O. and Xu X.-X. (2009). Cell adhesive affinity does not dictate primitive endoderm segregation and positioning during murine embryoid body formation. Genesis 47, 579-589. 10.1002/dvg.20536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Tao W., Meng Y., Smith E. R. and Xu X.-X. (2014). Cell adhesion and sorting in embryoid bodies derived from N- or E-cadherin deficient murine embryonic stem cells. Biol. Open 3, 121-128. 10.1242/bio.20146254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. A., Grewal S., Barrios F., Patankar S. N., Strauss B., Buttery L., Alexander M., Shakesheff K. M. and Zernicka-Goetz M. (2012). Dynamics of anterior-posterior axis formation in the developing mouse embryo. Nat. Commun. 3, 673 10.1038/ncomms1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen D., Burke R., Struhl G. and Basler K. (1996). Direct and long-range action of a DPP morphogen gradient. Cell 85, 357-368. 10.1016/S0092-8674(00)81114-9 [DOI] [PubMed] [Google Scholar]
- Ninomiya H., David R., Damm E. W., Fagotto F., Niessen C. M. and Winklbauer R. (2012). Cadherin-dependent differential cell adhesion in Xenopus causes cell sorting in vitro but not in the embryo. J. Cell Sci. 125, 1877-1883. 10.1242/jcs.095315 [DOI] [PubMed] [Google Scholar]
- Nishii I., Ogihara S. and Kirk D. L. (2003). A kinesin, invA, plays an essential role in volvox morphogenesis. Cell 113, 743-753. 10.1016/S0092-8674(03)00431-8 [DOI] [PubMed] [Google Scholar]
- Otani T., Marchetto M. C., Gage F. H., Simons B. D. and Livesey F. J. (2016). 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467-480. 10.1016/j.stem.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozair M. Z., Kintner C. and Brivanlou A. H. (2013). Neural induction and early patterning in vertebrates. Wiley Interdiscip. Rev. Dev. Biol. 2, 479-498. 10.1002/wdev.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M.-A. and Tam P. P. L. (1993). Onset of gastrulation, morphogenesis and somitogenesis in mouse embryos displaying compensatory growth. Anat. Embryol. 187, 493-504. 10.1007/BF00174425 [DOI] [PubMed] [Google Scholar]
- Przybyla L., Lakins J. N. and Weaver V. M. (2016). Tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell 19, 462-475. 10.1016/j.stem.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B. and De Robertis E. M. (2005). Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123, 1147-1160. 10.1016/j.cell.2005.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli A. E. and Martin-Belmonte F. (2013). Mechanical control of epithelial lumen formation. Small GTPases 4, 136-140. 10.4161/sgtp.24303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Eiraku M. and Suga H. (2012). In vitro organogenesis in three dimensions: self-organising stem cells. Development 139, 4111-4121. 10.1242/dev.079590 [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K. and De Robertis E. M. (1994). Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779-790. 10.1016/0092-8674(94)90068-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J. et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, U262-U147 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Schötz E.-M., Burdine R. D., Jülicher F., Steinberg M. S., Heisenberg C. P. and Foty R. A. (2008). Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J. 2, 42-56. 10.2976/1.2834817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M. N., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty N. M., Campbell A., Devito L. G., Ilic D. et al. (2016). Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700-708. 10.1038/ncb3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Taniguchi K., Gurdziel K., Townshend R. F., Xue X., Yong K. M., Sang J., Spence J. R., Gumucio D. L. and Fu J. (2016). Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. (in press), 10.1038/nmat4829. 10.1038/nmat4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawky J. H. and Davidson L. A. (2015). Tissue mechanics and adhesion during embryo development. Dev. Biol. 401, 152-164. 10.1016/j.ydbio.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Chen Q., Li X., Zheng X., Zhang Y., Qiao J., Tang F., Tao Y., Zhou Q. and Duan E. (2015). Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development 142, 3468-3477. 10.1242/dev.123950 [DOI] [PubMed] [Google Scholar]
- Shirai H., Mandai M., Matsushita K., Kuwahara A., Yonemura S., Nakano T., Assawachananont J., Kimura T., Saito K., Terasaki H. et al. (2016). Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc. Natl. Acad. Sci. USA 113, E81-E90. 10.1073/pnas.1512590113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C. and Harland R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829-840. 10.1016/0092-8674(92)90316-5 [DOI] [PubMed] [Google Scholar]
- Snow M. H. L. and Tam P. P. L. (1979). Is compensatory growth a complicating factor in mouse teratology? Nature 279, 555-557. 10.1038/279555a0 [DOI] [PubMed] [Google Scholar]
- Spemann H. and Mangold H. (1924). Über induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Dev. Genes Evol. 100, 599-638. [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M. et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105-109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. (1963). Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401-408. 10.1126/science.141.3579.401 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. (1970). Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 173, 395-433. 10.1002/jez.1401730406 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. and Takeichi M. (1994). Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA 91, 206-209. 10.1073/pnas.91.1.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. (2004). Gastrulation: from Cells to Embryo: Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Stower M. J. and Srinivas S. (2014). Heading forwards: anterior visceral endoderm migration in patterning the mouse embryo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130546 10.1098/rstb.2013.0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M., Er P. X., Chiu H. S., Maier B., Baillie G. J., Ferguson C., Parton R. G., Wolvetang E. J., Roost M. S., Lopes S. M. et al. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, U564-U238 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Shao Y., Townshend R. F., Tsai Y.-H., DeLong C. J., Lopez S. A., Gayen S., Freddo A. M., Chue D. J., Thomas D. J. et al. (2015). Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Reports 5, 954-962. 10.1016/j.stemcr.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman A. A. and Cohen S. M. (2000). Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103, 971-980. 10.1016/S0092-8674(00)00199-9 [DOI] [PubMed] [Google Scholar]
- ten Berge D., Koole W., Fuerer C., Fish M., Eroglu E. and Nusse R. (2008). Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508-518. 10.1016/j.stem.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Padilla M.-E., Parfitt D.-E., Kouzarides T. and Zernicka-Goetz M. (2007). Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445, 214-218. 10.1038/nature05458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes P. L. and Holtfreter J. (1955). Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 128, 53-120. 10.1002/jez.1401280105 [DOI] [PubMed] [Google Scholar]
- Turing A. M. (1952). The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. Biol. Sci. 237, 37-72. 10.1098/rstb.1952.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. A., Baillie-Johnson P. and Martinez Arias A. (2016). Organoids and the genetically encoded self-assembly of embryonic stem cells. BioEssays 38, 181-191. 10.1002/bies.201500111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umulis D. M. and Othmer H. G. (2013). Mechanisms of scaling in pattern formation. Development 140, 4830-4843. 10.1242/dev.100511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygur A., Young J., Huycke T. R., Koska M., Briscoe J. and Tabin C. J. (2016). Scaling pattern to variations in size during development of the vertebrate neural tube. Dev. Cell 37, 127-135. 10.1016/j.devcel.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Francies H. E., Francis J. M., Bounova G., Iorio F., Pronk A., van Houdt W., van Gorp J., Taylor-Weiner A., Kester L. et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933-945. 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink S. C., Baillie-Johnson P., Balayo T., Hadjantonakis A.-K., Nowotschin S., Turner D. A. and Martinez Arias A. (2014). Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development 141, 4231-4242. 10.1242/dev.113001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Yin M. and Fuchs E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209-219. 10.1016/S0092-8674(00)81559-7 [DOI] [PubMed] [Google Scholar]
- Warmflash A., Sorre B., Etoc F., Siggia E. D. and Brivanlou A. H. (2014). A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847-854. 10.1038/nmeth.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Bissiere S., Alvarez Y. and Plachta N. (2016a). Mouse embryo compaction. Curr. Top. Dev. Biol. 120, 235-258. 10.1016/bs.ctdb.2016.04.005 [DOI] [PubMed] [Google Scholar]
- White M. D., Angiolini J. F., Alvarez Y. D., Kaur G., Zhao Z. W., Mocskos E., Bruno L., Bissiere S., Levi V. and Plachta N. (2016b). Long-lived binding of Sox2 to DNA predicts cell fate in the four-cell mouse embryo. Cell 165, 75-87. 10.1016/j.cell.2016.02.032 [DOI] [PubMed] [Google Scholar]
- Whitesides G. M. and Boncheva M. (2002). Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 99, 4769-4774. 10.1073/pnas.082065899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H., Lieberam I., Porter J. A. and Jessell T. M. (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385-397. 10.1016/S0092-8674(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Wolpert L. and Vicente C. (2015). An interview with Lewis Wolpert. Development 142, 2547-2548. 10.1242/dev.127373 [DOI] [PubMed] [Google Scholar]
- Wolpert L., Tickle C. and Arias A. M. (2015). Principles of Development: USA: Oxford University Press. [Google Scholar]
- Ziomek C. A. and Johnson M. H. (1980). Cell surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell 21, 935-942. 10.1016/0092-8674(80)90457-2 [DOI] [PubMed] [Google Scholar]