Abstract
Pregnancy-related acute kidney injury (PRAKI) contributes to 3–7% of overall acute kidney injury (AKI) cases in Indian subcontinent. The aim of this study was to determine the outcomes of PRAKI and risk factors associated with renal injury and maternal mortality. One hundred and sixty-five patients with PRAKI, seen at M. S. Ramaiah Medical College between 2005 and 2014, were included in this, observational study. AKI was analyzed in terms of maximal stage of renal injury attained as per Risk, Injury, Failure, Loss of function, and End-stage renal disease (RIFLE) criteria. Outcomes included requirement for renal replacement therapy (RRT), maternal, and fetal mortality. Incidence of PRAKI was 1.56%, and the mean age of the study population was 25 years. Fifty percent of the patients were diagnosed with PRAKI during their first pregnancy. PRAKI was observed most commonly in the postpartum period (60%), followed by third trimester (32%); as per RIFLE criteria, failure was seen in 36% and injury in 34%. Thirty percent of cases required RRT. Sepsis (59%), pre-eclampsia, and eclampsia (56%) were the leading causes of PRAKI, while sepsis was the leading cause of maternal mortality. Maternal and fetal mortality were 20% and 22%, respectively. In univariate analysis, shock, hemorrhage requiring transfusion of >5 units packed red blood cells, oliguria, and “Loss” category of RIFLE were significantly associated with mortality. Majority of the patients (57%) required Intensive Care Unit care with a mean duration of admission at 7.3 days, and 75% was diagnosed with AKI at the time of admission. We report the lowest incidence of PRAKI in contemporary Indian literature. PRAKI was associated with high maternal and fetal mortality, with sepsis being the leading cause. No association was noted between mortality and initial stages of RIFLE criteria.
Key words: Obstetric renal failure, pregnancy-related acute kidney injury, RIFLE
Introduction
Pregnancy-related acute kidney injury (PRAKI) is a major cause of maternal and fetal morbidity and mortality in developing countries. With improvement in antenatal and postnatal care, the incidence of PRAKI in India has steadily declined from 22% in 1960s to 9% in 1980s,[1] and further down to 3–7% in 2000s;[2,3] however, the levels continue to remain higher than the levels seen in developed countries (1 in 20,000 pregnancies).[4] In developing countries, sepsis and hemorrhage account for >50% of cases of PRAKI,[5,6] in contrast to developed countries where chronic hypertension, renal disease and preeclampsia and eclampsia are important causes.[7,8] Cortical necrosis (CN) is an important cause of death and dialysis dependency in this population.[9]
The aim of our study was to study the clinical characteristics and outcomes of patients with PRAKI at our institution and review the recent literature on this topic.
Materials and Methods
In this prospective, observational study, 165 pregnant patients with a diagnosis of acute kidney injury (AKI) were admitted to our institution from 2005 to 2014. Records were analyzed for demographic characteristics, obstetric history, and clinical profile on admission. We excluded patients with preexisting diabetes mellitus, hypertension, contracted kidney, renal transplant recipients, or chronic kidney disease (CKD). CKD was defined as serum creatinine (S.cr) >1.5 mg/dl or presence of proteinuria >1+ on dipstick. Renal biopsy was done in patients with no renal recovery in terms of reduction in S.cr, urine output (u/o), or requirement of dialysis after 6 weeks from the time of diagnosis.
Definitions
AKI was defined on the basis of Risk, Injury, Failure, Loss of function, and End-stage renal disease (RIFLE) criteria.[10] PRAKI was defined as AKI diagnosed anytime during pregnancy or during postpartum phase (first 6 weeks postdelivery).
Pre-eclampsia was defined as blood pressure reading >140/90 mmHg diagnosed for the first time after 20 weeks of gestation with ≥2+ proteinuria on the dipstick.
Eclampsia was defined as the presence of new-onset grand mal seizures in a woman with pre-eclampsia.[11]
Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome was defined by the combination of thrombocytopenia (<100 G/L), elevated liver enzymes (aminotransferase >70 UI/L), and hemolysis.
Sepsis was defined as per the criteria laid down by the American College of Chest Physicians.[12] Sepsis is the clinical syndrome that results from a dysregulated inflammatory response to an infection that is nonresolving and deleterious, often leading to organ dysfunction. Sepsis is defined as the presence (probable or documented) of infection together with systemic manifestations of infection.
Outcomes
Evaluated outcomes included mortality, recovery of renal function or requirement of renal replacement therapy (RRT) and proteinuria. Renal recovery was defined as a decline in S.cr to ≤1.0 mg/dl and the presence of 24 h urine protein (UP) of <150 mg/day within 6 weeks of diagnosis of AKI. Patients who did not satisfy the criteria for renal recovery were subjected to a renal biopsy.
Statistical analysis
Data were analyzed using SPSS software version 18.0 by IBM. Chi-square test was used to find the factors associated with the outcome. Statistical significance was tested at P < 0.05. Risk estimate was calculated, and its 95% confidence interval was analyzed. Results are given as number, mean, median, and interquartile range for quantitative variables, and percentages for nominal variables.
Results
One hundred and sixty-five patients satisfied the criteria for PRAKI with an incidence of 1.56% in the context of all cases of AKI during the time period. Of these, five patients were lost to follow-up and have been excluded from the analysis related to renal and patient outcomes. The mean age of patients was 25 years. The majority of the patients were aged between 21 and 25 years (66%). Fifty percent of the patients were diagnosed with PRAKI during their first pregnancy. PRAKI was observed most commonly in the postpartum period (60%), followed by third trimester (32%). Ninety-three percent of women reported receiving antenatal care whereas 94% of patients delivered or aborted in an institutional setting. Ninety-four (57%) patients required Intensive Care Unit (ICU) level of care on admission. Average duration of ICU stay was 7.3 days (range 1–96 days).
The etiology of AKI was multifactorial in several patients [Figure 1]. Sixty patients (36%) were diagnosed with puerperal sepsis and 4% of cases were classified as septic abortion. Of patients with pre-eclampsia and eclampsia, 26% of patients had concomitant HELLP syndrome (n = 27) and 9.7% of cases were diagnosed with eclampsia (n = 9).
Figure 1.
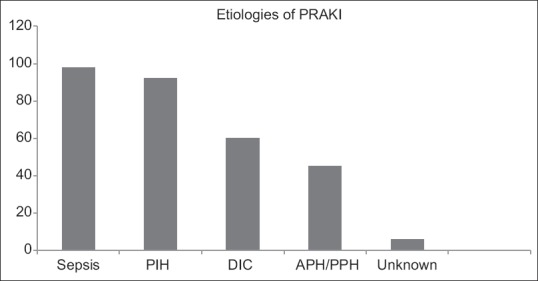
Etiologies of pregnancy-related acute kidney injury. Y axis: number (n) and X axis: etiologies. Sepsis: Majority cases of sepsis were proven or suspected bacterial sepsis. Incidence of septic abortion was 4%. Rare causes included systemic lupus erythematosus and hemolytic uremic syndrome. Acute fatty liver of pregnancy was seen in four cases
In terms of renal manifestations, 76% of the patients were diagnosed with AKI at the time of admission and the remaining patients developed AKI during the course of hospital stay. Oliguria, defined as u/o <0.5 ml/kg/h × 6 h), or anuria, was seen in 45% of cases. Mean S.cr on admission was 3.7 mg/dl. Distribution of PRAKI as per RIFLE criteria is described in Table 1. RRT (AKI-RRT group) was initiated in 49 (30%) patients in the form of intermittent hemodialysis or slow low-efficiency dialysis in 38 (77%) patients. The average duration of RRT was 11 days.
Table 1.
Division and outcomes of pregnancy-related acute kidney injury based on RIFLE criteria
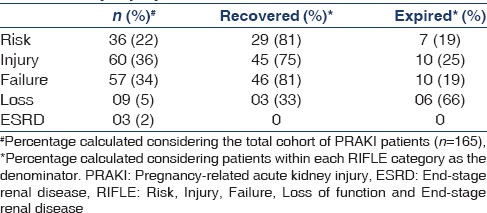
Outcomes of PRAKI in relationship to RIFLE criteria are outlined in Table 1. The incidences of biopsy-proven CN and long-term RRT were 4.8% and 1.8%, respectively. Of the patients with CN, partial CN was seen in five patients and complete CN was seen in three patients. Biopsy diagnosis of patients with persistent proteinuria (n = 6) included the following: membranoproliferative glomerulonephritis (n = 2), systemic lupus erythematosus, focal segmental glomerulosclerosis, membranous nephropathy, and mesangioproliferative glomerulonephritis (n = 1 each).
Maternal mortality was 20% (n = 33). Mortality with respect to RIFLE criteria is mentioned in Table 1, and its etiology is outlined in Table 2. With respect to pregnancy outcomes, 41 patients (24.8%) required lower section cesarean section. Fetal outcomes are outlined in Table 3.
Table 2.
Causes of death (n=33)

Table 3.
Fetal outcomes of pregnancy

In a univariate analysis, factors significantly associated with maternal mortality included hypotension and shock, hemorrhage requiring >5 units packed red blood cells, oliguria, sepsis, total bilirubin >5 mg/dl, requirement for RRT, and RIFLE “Loss” category.
Discussion
We report the lowest frequency of PRAKI of all the series from Indian subcontinent. Goplani et al. and Najar et al. reported 9% and 7% incidence of PRAKI, respectively, in their series.[3,5] Hassan et al.[13] from Pakistan reported a substantially higher incidence of PRAKI (30%) in their series. While there is no standardized definition for PRAKI, it is well established that an S.cr value >0.8 mg/dl or UP/cr ratio >300 mg in pregnancy is considered as renal dysfunction.[14] Other authors have utilized arbitrary S.cr values >1.5 mg/dl or 2 mg/dl, which can lead to under diagnosis of PRAKI, while early referrals to our institution and better provision of antenatal care may have contributed to lower incidence of PRAKI in our series.
RIFLE criteria have been utilized in contemporary PRAKI literature for classification of this entity and prognostication. While majority of our patients were classified into “Injury” and “Failure” categories, in another study of dialysis-dependent PRAKI patients, predictably a higher prevalence of “Loss” and “Failure” stages[15] was noted, whereas Bentata et al. noted the presence of “Risk” category in 50% patients with complete recovery of renal function in their cohort.[16] These studies as well as other studies indicate that the severity of renal failure in PRAKI correlates negatively with renal recovery.[17,18,19,20] More studies are required to explore the potential of RIFLE criteria to better define and classify this entity.
As noted in other series,[21,22,23] majority of PRAKI was observed in postpartum period or third trimester, and in primigravidas, a group known to be at high-risk for preeclampsia and eclampsia.[24,25] Similar to our cohort, sepsis contributed 30–60% cases of PRAKI in other series from Indian subcontinent, in contrast to 11% in Western literature.[7] We noted a low incidence of septic abortions in contrast to a higher incidence (12–50%) in some parts of India (12–50%) [Table 4].[3,21] A high incidence of puerperal sepsis highlights the need for improving the quality of antenatal and perinatal care. In literature, the contribution of pre-eclampsia and eclampsia to PRAKI is variable, (14–86% of PRAKI), and it is reported that the incidence of AKI in preeclampsia is 1–8.9%, while in HELLP syndrome, it is around 8–15% with varying severity of renal dysfunction.[26,27]
Table 4.
Cause of sepsis

The overall incidence of AKI-RRT in our study (30%) is lower than those reported in other Indian subcontinent series (60–94%).[3,18,21] The lower percentage of AKI-RRT in our series could be related to early detection of AKI either related to the criteria used for diagnosis or early referral to our center, leading to the earlier implementation of treatment. In contrast, Hildebrand et al.[22] report a much lower incidence of AKI-RRT of 1 in 10,000 deliveries from Canadian database explained by differences in socio-economic conditions. A large variability has been observed in the percentage of patients requiring long-term RRT, ranging from 0%[28] to 20–25%.[6,23] In our cohort, a very small percentage of patients required long-term RRT, attributable to a low incidence of CN and severity of AKI in comparison to other studies and early and aggressive management of PRAKI.
The mortality rate observed in our series (20%) is similar to those found in contemporary series from India and other developing countries;[3,7,21] however, it is higher than those noted in Western series.[7] Of note, none of the patients with purely pregnancy-induced hypertension (PIH) or HELLP died, similar to findings noted by several other authors.[27,28] It is evident that sepsis, severe hemorrhage, oliguria as well as AKI-RRT imply poor prognosis, findings which are consistent with reported determinants of maternal morbidity and mortality.[16] In terms of perinatal outcomes, our findings are in concordance with other authors noting 26–38% of perinatal mortality and 66% incidence of low birth weight in women with PIH and AKI.[25,27,28]
The strengths of our paper include a large sample size, availability of clinical data and reasonable length of follow-up with minimal patient attrition. Our paper highlights the vastly different characteristics of PRAKI in developing and developed nations, as well as regional disparities within India, which have a direct bearing on the long-term outcomes of this entity. Our limitations include lack of information regarding hemodynamic monitoring in our ICU and impact of conservative treatment measures on study outcomes as well as lack of a control group, which could have helped to define risk factors for the development of PRAKI.
Conclusion
PRAKI remains a challenging health issue in developing countries. Further improvements in antenatal and perinatal care have the potential to improve maternal and fetal outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful to Dr. David S. Goldfarb (New York University School of Medicine) for critically reviewing the manuscript and Dr. N. S. Shivaraj (Department of Biostatistics, M. S. Ramaiah Medical College) for assistance with statistical analysis.
References
- 1.Chugh KS, Sakhuja V, Malhotra HS, Pereira BJ. Changing trends in acute renal failure in third-world countries – Chandigarh study. Q J Med. 1989;73:1117–23. [PubMed] [Google Scholar]
- 2.Kumar KS, Krishna CR, Siva Kumar V. Pregnancy related acute renal failure. J Obstet Gynecol India. 2006;56:308–10. [Google Scholar]
- 3.Najar MS, Shah AR, Wani IA, Reshi AR, Banday KA, Bhat MA, et al. Pregnancy related acute kidney injury: A single center experience from the Kashmir Valley. Indian J Nephrol. 2008;18:159–61. doi: 10.4103/0971-4065.45291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gammill HS, Jeyabalan A. Acute renal failure in pregnancy. Crit Care Med. 2005;33(10 Suppl):S372–84. doi: 10.1097/01.ccm.0000183155.46886.c6. [DOI] [PubMed] [Google Scholar]
- 5.Goplani KR, Shah PR, Gera DN, Gumber M, Dabhi M, Feroz A, et al. Pregnancy-related acute renal failure: A single-center experience. Indian J Nephrol. 2008;18:17–21. doi: 10.4103/0971-4065.41283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahwa N, Bharani R, Kumar R. Post-partum acute kidney injury. Saudi J Kidney Dis Transpl. 2014;25:1244–7. doi: 10.4103/1319-2442.144259. [DOI] [PubMed] [Google Scholar]
- 7.Mehrabadi A, Liu S, Bartholomew S, Hutcheon JA, Magee LA, Kramer MS, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: Population based retrospective cohort study. BMJ. 2014;349:g4731. doi: 10.1136/bmj.g4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurrieri C, Garovic VD, Gullo A, Bojanic K, Sprung J, Narr BJ, et al. Kidney injury during pregnancy: Associated comorbid conditions and outcomes. Arch Gynecol Obstet. 2012;286:567–73. doi: 10.1007/s00404-012-2323-5. [DOI] [PubMed] [Google Scholar]
- 9.Prakash J, Vohra R, Wani IA, Murthy AS, Srivastva PK, Tripathi K, et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: A single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213–7. doi: 10.1093/ndt/gfl761. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure – Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ACOG Committee on Practice Bulletins – Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 12.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 13.Hassan I, Junejo AM, Dawani ML. Etiology and outcome of acute renal failure in pregnancy. J Coll Physicians Surg Pak. 2009;19:714–7. [PubMed] [Google Scholar]
- 14.Krane NK, Hamrahian M. Pregnancy: Kidney diseases and hypertension. Am J Kidney Dis. 2007;49:336–45. doi: 10.1053/j.ajkd.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Silva GB, Jr, Monteiro FA, Mota RM, Paiva JG, Correia JW, Bezerra Filho JG, et al. Acute kidney injury requiring dialysis in obstetric patients: A series of 55 cases in Brazil. Arch Gynecol Obstet. 2009;279:131–7. doi: 10.1007/s00404-008-0682-8. [DOI] [PubMed] [Google Scholar]
- 16.Bentata Y, Housni B, Mimouni A, Azzouzi A, Abouqal R. Acute kidney injury related to pregnancy in developing countries: Etiology and risk factors in an intensive care unit. J Nephrol. 2012;25:764–75. doi: 10.5301/jn.5000058. [DOI] [PubMed] [Google Scholar]
- 17.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siam S, Abd El Hameed AA, Matar H. Evaluation of acute kidney injury defined by RIFLE criteria and its association with mortality in critically Ill obstetric patients: A retrospective study. Med J Cairo Univ. 2011;79:589–93. [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonard M, Ducloy-Bouthors AS, Boyle E, Aucourt M, Gasan G, Jourdain M, et al. Postpartum acute renal failure: A multicenter study of risk factors in patients admitted to ICU. Ann Intensive Care. 2014;4:36. doi: 10.1186/s13613-014-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora N, Mahajan K, Jana N, Taraphder A. Pregnancy-related acute renal failure in eastern India. Int J Gynaecol Obstet. 2010;111:213–6. doi: 10.1016/j.ijgo.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand AM, Liu K, Shariff SZ, Ray JG, Sontrop JM, Clark WF, et al. Characteristics and outcomes of AKI treated with dialysis during pregnancy and the postpartum period. J Am Soc Nephrol. 2015;26:3085–91. doi: 10.1681/ASN.2014100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna A, Singh R, Prasad N, Gupta A, Bhadauria D, Kaul A, et al. Maternal, fetal and renal outcomes of pregnancy-associated acute kidney injury requiring dialysis. Indian J Nephrol. 2015;25:77–81. doi: 10.4103/0971-4065.136890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long PA, Abell DA, Beischer NA. Parity and pre-eclampsia. Aust N Z J Obstet Gynaecol. 1979;19:203–6. doi: 10.1111/j.1479-828x.1979.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 25.Prakash J, Pandey LK, Singh AK, Kar B. Hypertension in pregnancy: Hospital based study. J Assoc Physicians India. 2006;54:273–8. [PubMed] [Google Scholar]
- 26.Sibai BM, Villar MA, Mabie BC. Acute renal failure in hypertensive disorders of pregnancy. Pregnancy outcome and remote prognosis in thirty-one consecutive cases. Am J Obstet Gynecol. 1990;162:777–83. doi: 10.1016/0002-9378(90)91009-2. [DOI] [PubMed] [Google Scholar]
- 27.Gul A, Aslan H, Cebeci A, Polat I, Ulusoy S, Ceylan Y. Maternal and fetal outcomes in HELLP syndrome complicated with acute renal failure. Ren Fail. 2004;26:557–62. doi: 10.1081/jdi-200031750. [DOI] [PubMed] [Google Scholar]
- 28.Drakeley AJ, Le Roux PA, Anthony J, Penny J. Acute renal failure complicating severe preeclampsia requiring admission to an obstetric intensive care unit. Am J Obstet Gynecol. 2002;186:253–6. doi: 10.1067/mob.2002.120279. [DOI] [PubMed] [Google Scholar]


