Abstract
Hypertension is common in hemolytic uremic syndrome (HUS) and often difficult to control. Local renin-angiotensin activation is believed to be an important part of thrombotic microangiopathy, leading to a vicious cycle of progressive renal injury and intractable hypertension. This has been demonstrated in vitro via enhanced tissue factor expression on glomerular endothelial cells which is enhanced by angiotensin II. We report two pediatric cases of atypical HUS with severe refractory malignant hypertension, in which we targeted the renin-angiotensin system by using intravenous (IV) enalaprilat, oral aliskiren, and oral enalapril with quick and dramatic response of blood pressure. Both drugs, aliskiren and IV enalaprilat, were effective in controlling hypertension refractory to multiple antihypertensive medications. These appear to be promising alternatives in the treatment of severe atypical HUS-induced hypertension and hypertensive emergency.
Key words: Enalapril, enalaprilat, hemolytic uremic syndrome, malignant hypertension, renin
Introduction
Hypertension is common in hemolytic uremic syndrome (HUS), and often difficult to control in an acute stage. Malignant hypertension associated with HUS leads to reversible posterior encephalopathy syndrome, seizures, heart failure, and other adverse consequences which increase the morbidity and mortality of the disease. Renin-mediated mechanism is believed to be the main factor responsible for hypertension seen in these cases.[1,2,3] Drugs that act by blocking renin-angiotensin axis (RAS) are thus ideal for such cases, however, due to concern of progression of renal failure and lack of experience of these agents in children, these are not preferred or used commonly in acute stages. We hereby report two cases of HUS with severe refractory malignant hypertension in which we targeted RAS by using intravenous (IV) enalaprilat, oral aliskiren, and oral enalapril with quick and dramatic response of blood pressure (BP).
Case Reports
Case 1
A 6-year-old male was admitted with a history of vomiting, fever since 2 weeks, hematuria and decreased urine output since 1 week. On evaluation by his local practitioner, he was found to have anemia (hemoglobin [Hb] 6.3 g/dl), thrombocytopenia (platelet 72,000/mm3), active urine sediment (red blood cell [RBC] 40–60/hpf, albumin 3+), and azotemia (blood urea 200 mg/dl, creatinine 4.2 mg/dl). He had an episode of seizure (due to accelerated hypertension), hence was brought to our hospital for further management. On evaluation, he was hypertensive (BP 150/100 mmHg) with generalized edema, oliguria, and a normal systemic examination. Investigations were suggestive of HUS (Hb 4.8 g/dl, white blood cell [WBC] 11,190 cmm, platelet 1.84/mm3, peripheral smear: schistocytes positive, reticulocyte count 6.8%, lactate dehydrogenase [LDH] 4300 U/L, Direct Coombs test and Indirect Coombs tests were negative, urea 67 mg/dl, creatinine 2.6 mg/dl). Septic work up, dengue serology, and malarial antigen were negative, and he became afebrile on the 4th day of admission. His antinuclear antibodies (ANA) and antineutrophil cytoplasmic antibody (ANCA) were negative. He was started on empiric antibiotics (injection ceftriaxone) and daily plasmapheresis for HUS. Echocardiography and fundus were normal. Detailed complement regulator assay showed very high anti-Factor H antibody (41,000 IU). C3, C4, antigenic levels of Factor H, Factor I, Factor B, and CD46 were normal [Table 1]. He was given a blood transfusion and initiated on hemodialysis and daily plasma exchanges in view of oligo-anuric acute kidney injury (AKI).
Table 1.
Complement assay in cases*

For the child's height percentile, the BP percentiles were: 90th percentile: 113/72 mmHg and 95th percentile 117/76 mmHg (Blood pressure references used were as per the fourth report[4]). For arterial hypertension [Figure 1], he was started on sustained release nifedepine, clonidine, and metoprolol and subsequently prazosin with a gradual increase in dosage. However, arterial BP remained persistently high (>99th centile; up to 170/120 mmHg), and he developed blurring of vision, with abdominal pain and vomiting on the 3rd day of admission necessitating need for IV nitroglycerine (up to 5 mcg/kg/min) and subsequently labetolol infusion (up to 2 mg/kg/h) for refractory hypertension. Child had persistent arterial hypertension (>99th centile for his age), despite vigorous fluid removal in hemodialysis sessions.
Figure 1.
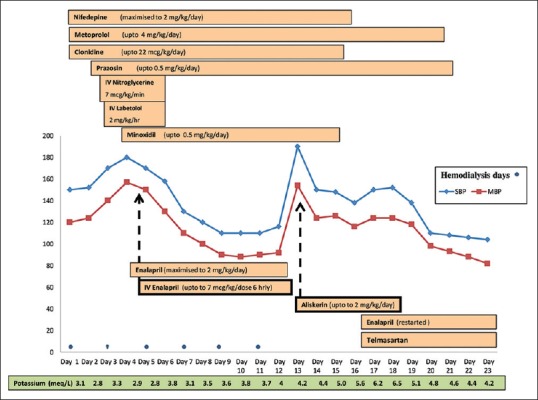
Response to antihypertensive medications in case 1
Oral enalapril and minoxidil were also added and dosage of other oral antihypertensives optimized to the maximal doses [Figure 1] but arterial BP remained high and was difficult to control. Oral enalapril was added on the same day of oral minoxidil. The starting dose was 0.2 mg/kg/day and was increased gradually. But since within 48 h of adding oral enalapril and oral minoxidil, child went into hypertensive emergency (BP 180/120 mmHg), with hallucinations and visual blurring, IV enalaprilat was added. Hypertension showed a significant improvement after addition of IV enalaprilat (10 µg/kg/dose q 8 hourly) on the 5th day of admission. There was a consistent fall of BP within hours of giving individual IV enalaprilat boluses (average fall in mean BP 9.5 mmHg). Arterial BP decreased to 110/78 mmHg; patient became asymptomatic, and nitroglycerine and labetolol infusions were tapered off successfully.
He developed neutropenia a week following enalaprilat therapy (WBC 1500 cmm, 50% neutrophils), which could not be attributed to any other cause; hence, it was stopped followed by a rebound in hypertension (arterial BP 190/136 mmHg). He was not dialysis dependent at this stage with a good urine output, and serum creatinine had fallen to 0.6 mg/dl, and his hemodialysis catheter was removed.
Aliskiren (2 mg/kg/dose) was then added with good response (arterial BP decreased to 150/110 mmHg over 24 h, and 138/110 mmHg over 48 h); however, it was withdrawn after 4 days due to hyperkalemia (serum potassium 6.5 mmol/L). After improvement of neutopenia, oral enalapril was reintroduced along with telmisartan. BP control improved within 48 h on this angiotensin-converting enzyme inhibitor-angiotensin receptor blockade (ACEI-ARB) combination (BP <90th percentile for age) along with other oral antihypertensive agents. Metoprolol and prazosin could be tapered off thereafter.
He received seven daily plasmapheresis sessions till active hemolysis subsided followed by alternate day sessions. He received prednisolone (1 mg/kg/day) and IV immunoglobulin 2 g/day (day 10 of admission), IV cyclophosphamide during his hospital stay. He was discharged after 3 weeks after achieving good BP control. He received total six doses of IV cyclophosphamide followed by maintenance azathioprine. At his 1 year of follow-up, he is doing well, normotensive (BP <90th percentile for age) and no proteinuria (urine protein/creatinine ratio <0.2) and a normal urine examination on angiotensin-converting enzyme (ACE) and ARB combination.
Case 2
A 7-month-old male was admitted with a history of vomiting, fever since 5 days and anuria since 2 days. There was no history of diarrhea or dysentery in the past. On admission, he was hypertensive (BP 130/60 mmHg), with pallor, facial puffiness, and normal systemic examination. Investigations were suggestive of atypical HUS-microangiopathic hemolytic anemia with AKI (Hb 7 g/dl, WBC 13,280/mm3, platelets 100,000/mm3, peripheral smear: schistocytes, elliptocytes, reticulocyte count 5.6%, LDH 4300 U/L) and active urine sediment (RBC 10–20/hpf, albumin 2+). The child had no evidence of pneumonia or sepsis, on clinical evaluation, and all cultures were sterile. The child had advanced azotemia (urea 216 mg/dl, creatinine 7.2 mg/dl) with severe hyperkalemia and metabolic acidosis. He was initiated on hemodialysis in view of anuric AKI. For HUS with evidence of ongoing hemolysis and dialysis dependence, he was started on daily plasmapheresis. A detailed complement assay (including C3, C4, antigenic levels of Factor H, Factor I, Factor B, CD46, and autoantibodies to Factor H) were normal [Table 1]. His ANA and ANCA were negative. BP was observed to be high since admission and increased up to 160/110 mmHg on serial monitoring, although patient remained asymptomatic. Echocardiography and fundus were normal.
For his arterial hypertension (>99th centile for age) [Figure 2], he was started on amlodipine and prazosin initially; dosage was increased to the maximal dose, and clonidine and oral enalapril were added on the 3rd and 4th day of admission, respectively. Despite the addition of multiple antihypertensive agents and dosage optimization and aggressive ultrafiltration in hemodialysis sessions, arterial BP showed only marginal decrease and mean arterial BP remained high (>99th centile for age; 100–110 mmHg). Intravenous enalaprilat (10 µg/kg/dose q 8 hourly) was added on day 6 of admission. The mean arterial BP improved (80 mmHg) within 12 h of addition of enalaprilat. Once arterial BP was controlled with no further increase, dose of oral enalapril was maximized, while tapering off IV enalaprilat and other antihypertensive medications were continued.
Figure 2.
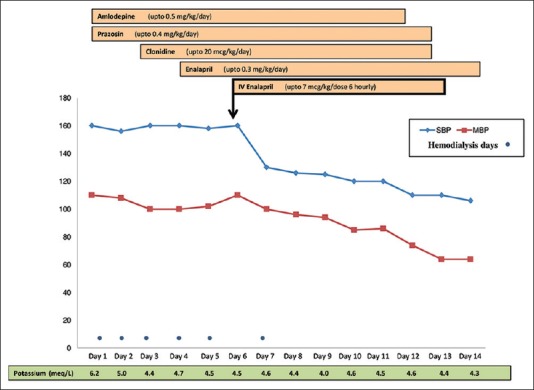
Response to antihypertensive medications in case 2
Daily hemolytic parameters and renal function were monitored. Both improved gradually on plasmapheresis. He received 5 daily and 3 alternate day plasmapheresis sessions. Hemodialysis was stopped by day 9 of admission. He was discharged in 2 weeks time in a stable condition, with adequate urine output and BP controlled on oral antihypertensive therapy. At a follow-up of 1 year, currently the infant is doing well, with serum creatinine 0.7 mg/dl, urine protein/creatinine ratio 1.5, and normal BP (BP <90th percentile for age) on oral enalapril 0.4 mg/kg/day.
Discussion
The extent of renal microangiopathic involvement appears to be responsible for the development of hypertension and renal failure in atypical HUS.[1] Renal ischemia is triggered which leads to maximal activation of RAS resulting in accelerated hypertension which is often very severe and resistant to antihypertensive therapy. Local RAS activation is believed to be an important key factor in the thrombotic microangiopathy in HUS leading to intractable hypertension. This has been demonstrated to occur via enhanced tissue factor expression on glomerular endothelial cells which is enhanced by angiotension II.[2] On the other hand, there are conflicting reports of plasma renin activity in HUS.[5,6,7] Two studies have shown elevated plasma renin levels in children with HUS, irrespective of systemic hypertension.[6,7]
Severe hypertension that ensues is a clinician's nightmare as it is hard to control despite use of multiple drug combinations and careful drug titration. In extreme cases, bilateral nephrectomy is ultimately required as a life-saving measure for achieving control of BP, thus again underlining the pivotal role of hyperreninemia in the development of hypertension in HUS.[1,3]
Although there is plenty of evidence in favor of renin-mediated mechanism in the pathogenesis of hypertension in HUS, in practice there is hesitation to use RAS inhibitors or their combinations in the acute stage for hypertension. This is due to fear of worsening of renal failure and lack of pediatric experience with newer RAS inhibitors. Oral ACEIs (enalapril, captopril) have been shown to be renoprotective and of benefit for long-term BP control and reduction in proteinuria in patients with persistent disease.[8] However, they are not preferred in the acute stage of disease, used with great caution and generally initiated after improvement of renal function. Moreover, ACEIs including enalaprilat are seldom used in hypertensive emergencies due to concerns regarding slow onset of action and variable effectiveness, especially in children.[9]
We used IV enalaprilat in both patients for acute hypertension who showed significant and consistent decline in BP. It helped in successful reversal of hypertensive urgency in the older child whose BP remained high and was extremely difficult to control despite multiple drugs including IV nitroglycerine and labetolol. Enalaprilat is the active metabolite of enalapril. The onset of action begins in 15 min, but the peak effect may take 1–4 h. The duration of action is usually 4–6 h. The half life of the drug is usually 11.1 h in infants and children.[10] There is one case series reporting the neonatal use of enalaprilat that reported that doses even at the lower end of what was used in this cohort may lead to significant, prolonged hypotension and oliguric acute renal failure.[11] If it is used in the newborn or children, it should be used with caution with ongoing monitoring of BP, serum potassium, and renal function. The adverse effects of enalaprilat are hyperkalemia, hypotension, cough, diarrhea, angioedema, and leucopenia (agranulocytosis). We encountered neutropenia in one of our patients a week after initiation of IV enalaprilat which reversed quickly on drug withdrawal. However, severe rebound hypertension also occurred on stopping enalaprilat, while other drugs were continued, which reiterates its effectiveness in the management of the hypertensive urgency. Hirschl et al. studied the BP response to IV enalaprilat in 35 patients with hypertensive crisis, and found that the extent of systolic and diastolic BP reduction correlated well with the pretreatment plasma renin and angiotensin II levels.[12] Thus, it appears that the status of RAS determines the efficacy of IV enalaprilat, and hence it was successful in both our patients with HUS-induced hypertension. Therapeutic enalaprilat levels can probably be achieved with 1/4th total cumulative dose of enalapril, administered as 6 hourly enalaprilat: recommended pediatric dosing is 5–10 mcg/kg/dose.[13] We used a dose of 10 mcg/kg/dose q 8 hourly in both of our cases based on the previous report.
We used aliskiren in the older child which too was very effective in lowering BP. Aliskiren is the first direct renin inhibitor available in an oral form approved for adults by the US Food and Drug Administration in 2007.[14,15] As renin is the first and rate-limiting step in angiotensin II synthesis in RAS, its direct inhibition is theoretically more advantageous as compared to ACE inhibition/ARB. As it is a nonpeptide molecule, it has better bioavailability and a long half-life and can, therefore, lower BP effectively. It is available in the form of 150 mg and 300 mg doses. Once administered orally, the effect of the drug peaks in 1–3 h, achieves its steady state in 5–7 days and has a half-life of 40 h.[15] Aliskiren produces dose-dependent BP reduction and its potency has been shown to be equivalent or better than ACEIs, ARBs in various trials.[15,16,17] Moreover, when administered as a combination with ACEI, it helps to block an increase in plasma renin activity induced by ACEI monotherapy. Combination therapy (aliskiren + ACEI) has been demonstrated to have greater BP-lowering potential as compared to either alone.[16] Dual RAS blockade however must be cautiously monitored as there is a higher chance of adverse effects. A case series of children with chronic kidney disease receiving combination aliskiren/ACEI showed >45% proteinuria reduction, however side effects in the form of hyperkalemia, worsening of renal function, and hypotension were seen.[17] Mild hyperkalemia was seen in our patient but was asymptomatic, and potassium normalized quickly on stopping the drug. Other adverse effects including nausea, angioedema, diarrhea, abdominal pain, and headache may be seen with aliskiren but are usually mild and not dose related. Adult studies have shown aliskiren to be a safe and effective antihypertensive drug; however, its use in children has been restricted so far due to the paucity of data. A recent prospective, randomized controlled in children between ages 6 and 17 years concluded that aliskiren in once daily doses of 2 mg/kg or 6 mg/kg was well tolerated and safe.[18] We used a dose of 2 mg/kg in case 1.
In the above two patients, we used two unconventional drugs: aliskiren and IV enalaprilat, both of which were very quick and effective in controlling high BP refractory to multiple antihypertensive medications and aggressive ultrafiltration during dialysis sessions. The limitation of the report is small number, multiple antihypertensives in these patients, and simultaneous use of plasma exchanges and immunosuppression in anti-Factor H antibody positive case 1 to help resolution of illness, which might make interpretation difficult. These appear to be promising alternatives in the treatment of severe atypical HUS-induced hypertension and hypertensive emergency, and there is a need to have more trials targeting renin in these cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ruggenenti P, Remuzzi G. Malignant vascular disease of the kidney: Nature of the lesions, mediators of disease progression, and the case for bilateral nephrectomy. Am J Kidney Dis. 1996;27:459–75. doi: 10.1016/s0272-6386(96)90155-9. [DOI] [PubMed] [Google Scholar]
- 2.Nestoridi E, Kushak RI, Tsukurov O, Grabowski EF, Ingelfinger JR. Role of the renin angiotensin system in TNF-alpha and Shiga-toxin-induced tissue factor expression. Pediatr Nephrol. 2008;23:221–31. doi: 10.1007/s00467-007-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habib R, Gagnadoux MF, Broyer M. Hemolytic-uremic syndrome in children and arterial hypertension. Arch Mal Coeur Vaiss. 1981;74:37–43. [PubMed] [Google Scholar]
- 4.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–76. [PubMed] [Google Scholar]
- 5.Proesmans W, VanCauter A, Thijs L, Lijnen P. Plasma renin activity in haemolytic uraemic syndrome. Pediatr Nephrol. 1994;8:444–6. doi: 10.1007/BF00856527. [DOI] [PubMed] [Google Scholar]
- 6.Grunfeld B, Gimenez M, Liapchuc S, Mendilaharzu J, Gianantonio C. Systemic hypertension and plasma renin activity in children with the hemolytic-uremic syndrome. Int J Pediatr Nephrol. 1982;3:211–4. [PubMed] [Google Scholar]
- 7.Powell HR, Rotenberg E, Williams AL, McCredie DA. Plasma renin activity in acute poststreptococcal glomerulonephritis and the haemolytic-uraemic syndrome. Arch Dis Child. 1974;49:802–7. doi: 10.1136/adc.49.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dyck M, Proesmans W. Renoprotection by ACE inhibitors after severe hemolytic uremic syndrome. Pediatr Nephrol. 2004;19:688–90. doi: 10.1007/s00467-004-1451-y. [DOI] [PubMed] [Google Scholar]
- 9.Temple ME, Nahata MC. Treatment of pediatric hypertension. Pharmacotherapy. 2000;20:140–50. doi: 10.1592/phco.20.3.140.34782. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Ishii M, Sugimura T, Chiba K, Kato H, Ishizaki T. The kinetic profiles of enalapril and enalaprilat and their possible developmental changes in pediatric patients with congestive heart failure. Clin Pharmacol Ther. 1994;56:160–8. doi: 10.1038/clpt.1994.119. [DOI] [PubMed] [Google Scholar]
- 11.Wells TG, Bunchman TE, Kearns GL. Treatment of neonatal hypertension with enalaprilat. J Pediatr. 1990;117:664–7. doi: 10.1016/s0022-3476(05)80711-5. [DOI] [PubMed] [Google Scholar]
- 12.Hirschl MM, Binder M, Bur A, Herkner H, Woisetschläger C, Bieglmayer C, et al. Impact of the renin-angiotensin-aldosterone system on blood pressure response to intravenous enalaprilat in patients with hypertensive crises. J Hum Hypertens. 1997;11:177–83. doi: 10.1038/sj.jhh.1000404. [DOI] [PubMed] [Google Scholar]
- 13.Reams GP, Lal SM, Whalen JJ, Bauer JH. Enalaprilat: An intravenous substitute for oral enalapril therapy. Humoral and pharmacokinetic effects. J Clin Hypertens. 1986;2:245–53. [PubMed] [Google Scholar]
- 14.Gradman AH, Kad R. Renin inhibition in hypertension. J Am Coll Cardiol. 2008;51:519–28. doi: 10.1016/j.jacc.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Bhatti AB, Gazali ZA. Can aliskiren be considered as a new novel drug for hypertension? Cureus. 2015;7:e375. doi: 10.7759/cureus.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uresin Y, Taylor AA, Kilo C, Tschöpe D, Santonastaso M, Ibram G, et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst. 2007;8:190–8. doi: 10.3317/jraas.2007.028. [DOI] [PubMed] [Google Scholar]
- 17.Stanton A, Jensen C, Nussberger J, O'Brien E. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42:1137–43. doi: 10.1161/01.HYP.0000101688.17370.87. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan JE, Keefe D, Zhou Y, Satlin L, Fang H, Yan JH. Pharmacokinetics, safety profile, and efficacy of aliskiren in pediatric patients with hypertension. Clin Pediatr (Phila) 2013;52:599–607. doi: 10.1177/0009922813483875. [DOI] [PubMed] [Google Scholar]


