Abstract
Schwann cells (SCs) are known to play an important role for the regeneration of mammalian peripheral nerves. Their effect is likely due to the production of neuronotrophic and/or supportive factors. Here we study the effect of intraocular transplant of SCs on the survival of rat retinal ganglion cells (RGCs) after the intracranial section of the optic nerve. SCs were injected intraocularly in adult hooded rats. Surviving RGCs were retrogradely labeled with horseradish peroxidase applied to the proximal stump of the optic nerve. Results show that intraocular transplants of SCs promote the survival of a large number of RGCs for periods as long as 9 and 14 weeks after optic nerve section. In experimental retinae, surviving RGCs were 2- to 8-fold more numerous than in controls. This finding suggests that SCs are the source of factors that promote the survival of RGCs. Nerve growth factor is produced by SCs, and the intraocular injection of nerve growth factor has been previously shown to promote RGC survival. The rescuing effect of SCs on RGCs is greater than that obtained by intraocular injection of nerve growth factor. This greater effect may be due to the action of other neurotrophic factors produced by SCs or by transplanted SCs producing NGF in a sustained fashion.
Full text
PDF

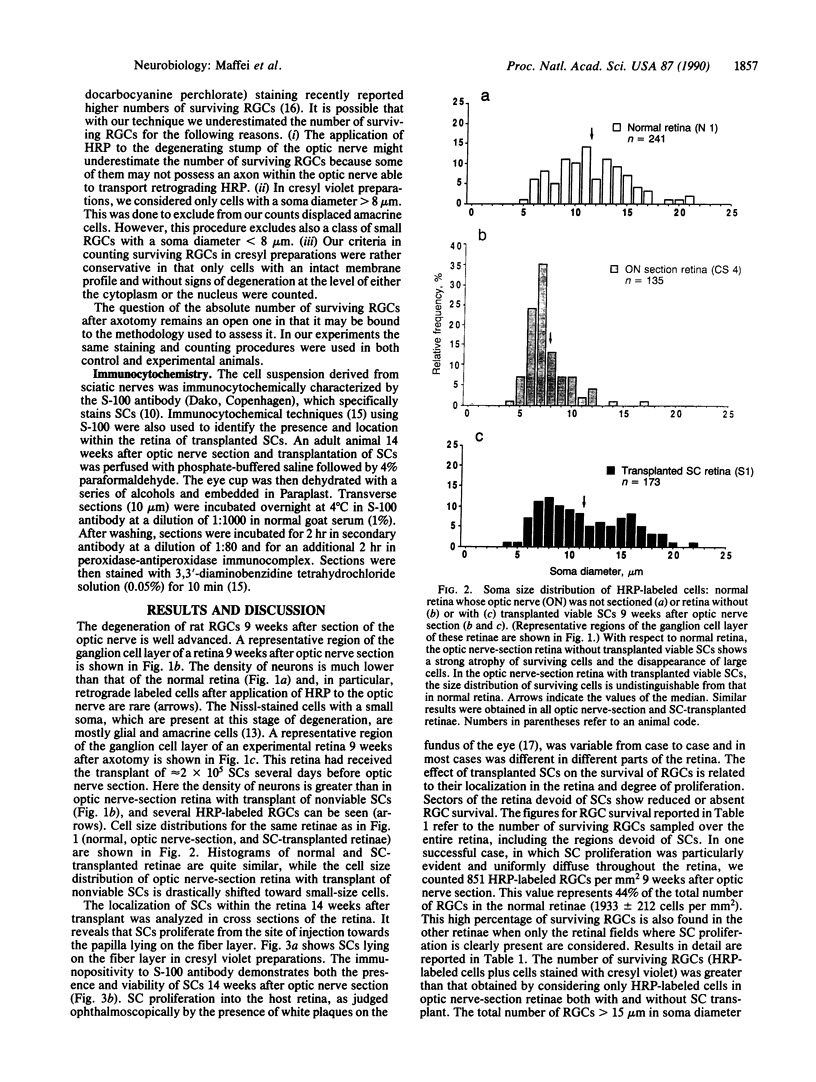

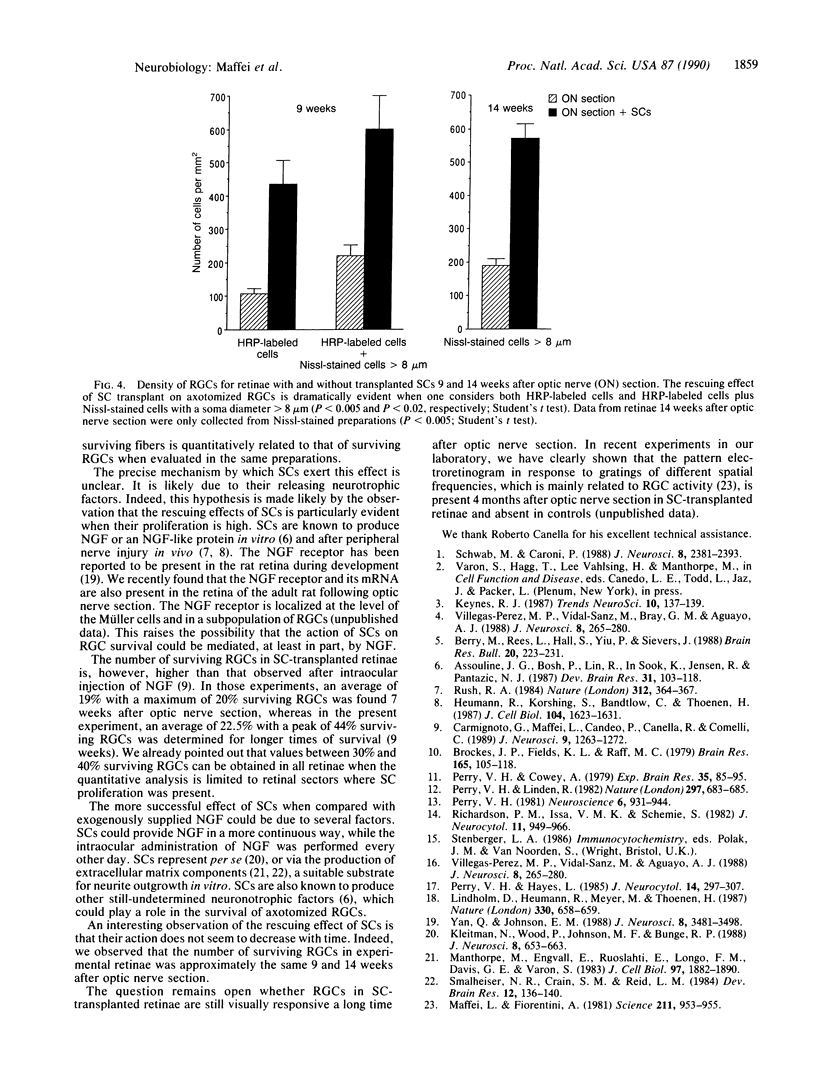
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assouline J. G., Bosch P., Lim R., Kim I. S., Jensen R., Pantazis N. J. Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Brain Res. 1987 Jan;428(1):103–118. doi: 10.1016/0165-3806(87)90087-3. [DOI] [PubMed] [Google Scholar]
- Berry M., Rees L., Hall S., Yiu P., Sievers J. Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Res Bull. 1988 Feb;20(2):223–231. doi: 10.1016/0361-9230(88)90182-7. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Maffei L., Candeo P., Canella R., Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989 Apr;9(4):1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N., Wood P., Johnson M. I., Bunge R. P. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J Neurosci. 1988 Feb;8(2):653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Heumann R., Meyer M., Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987 Dec 17;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Mafei L., Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981 Feb 27;211(4485):953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Cowey A. The effects of unilateral cortical and tectal lesions on retinal ganglion cells in rats. Exp Brain Res. 1979 Mar 9;35(1):85–95. doi: 10.1007/BF00236786. [DOI] [PubMed] [Google Scholar]
- Perry V. H. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience. 1981;6(5):931–944. doi: 10.1016/0306-4522(81)90174-3. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hayes L. Lesion-induced myelin formation in the retina. J Neurocytol. 1985 Apr;14(2):297–307. doi: 10.1007/BF01258454. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982 Jun 24;297(5868):683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Shemie S. Regeneration and retrograde degeneration of axons in the rat optic nerve. J Neurocytol. 1982 Dec;11(6):949–966. doi: 10.1007/BF01148310. [DOI] [PubMed] [Google Scholar]
- Rush R. A. Immunohistochemical localization of endogenous nerve growth factor. Nature. 1984 Nov 22;312(5992):364–367. doi: 10.1038/312364a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988 Jul;8(7):2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser N. R., Crain S. M., Reid L. M. Laminin as a substrate for retinal axons in vitro. Brain Res. 1984 Jan;314(1):136–140. doi: 10.1016/0165-3806(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988 Sep;8(9):3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]









