Abstract
It is well known that a tip-focused intracellular Ca2+ gradient and the meshwork of short actin filaments at the tip region are necessary for pollen tube growth. However, little is known about the connections between the two factors. Here, a novel Ca2+-dependent actin-binding protein with molecular mass of 41 kD from lily (Lilium davidii) pollen (LdABP41) was isolated and purified with DNase I chromatography. Our purification procedure yielded about 0.6 mg of LdABP41 with >98% purity from 10 g of lily pollen. At least two isoforms with isoelectric points of 5.8 and 6.0 were detected on two-dimensional gels. The results of N-terminal sequencing and mass-spectrometry analysis of LdABP41 showed that both isoforms shared substantial similarity with trumpet lily (Lilium longiflorum) villin and other members of the gelsolin superfamily. Negative-stained electron microscope images showed that LdABP41 severed in vitro-polymerized lily pollen F-actin into short actin filaments in a Ca2+-sensitive manner. Microinjection of the anti-LdABP41 antibody into germinated lily pollen demonstrated that the protein was required for pollen tube growth. The results of immunolocalization of the protein showed that it existed in the cytoplasm of the pollen tube, especially focused in the tip region. Our results suggest that LdABP41 belongs to the gelsolin superfamily and may play an important role in controlling actin organization in the pollen tube tip by responding to the oscillatory, tip-focused Ca2+ gradient.
Pollen tube growth is a key process in the sexual reproduction of higher plants. It is highly polarized and requires both spatial and temporal coordination of many cellular functions, including ion fluxes, cytoskeleton organization and dynamics, vesicular trafficking, exocytosis, endocytosis, and cell wall synthesis (for review, see Taylor and Hepler, 1997; Franklin-Tong, 1999). It is widely accepted that the actin cytoskeleton plays a major role in modulation of pollen tube growth. Actin filaments, together with myosin, are a crucial element to support intracellular trafficking of organelles and secretory vesicles along actin cables that are oriented axially throughout the shank of elongating pollen tubes (Cai et al., 1997; Vidali and Hepler, 2000; Hepler et al., 2001). Although the presence and exact distribution of F-actin in the pollen tube apex has been somewhat controversial, accumulating evidence indicates that a highly dynamic array of actin filaments in the tip region may play a primary role in polarized growth. Early studies using chemically fixed cells and fluorescent-phalloidin staining indicate that there is a dense actin network at the extreme apex of pollen tubes (Pierson, 1988; Derksen et al., 1995). However, investigations with rapid freezing, freeze substitution microscopy techniques suggest that the extreme apex of pollen tubes contains little F-actin, but only occasional sparse or fine actin filaments at the extreme apex (Doris and Steer, 1996). Results obtained by microinjection of trumpet lily (Lilium longiflorum) pollen tubes with fluorescent phalloidin (Miller et al., 1996) and expression of the actin-binding region of mouse talin fused with the green-fluorescent protein in tobacco (Nicotiana tabacum) pollen tubes show that the extreme apex of the pollen tube contains little F-actin. Instead, in the subapical region there is a dense network of short actin filaments, which are somewhat disorganized (Kost et al., 1998). In addition, a collar or dense meshwork of F-actin at about 5 μm behind the tip is observed in chemically fixed maize (Zea mays), poppy (Papaver rhoeas), and trumpet lily pollen tubes (Gibbon et al., 1999; Geitmann et al., 2000; Vidali and Hepler, 2001). Recently, a population of dynamic tip-localized F-actin, termed short actin bundles (SABs), has been investigated in tobacco pollen tubes, and the dynamics of SABs are associated with those of the subapical actin ring or actin collar (Fu et al., 2001). The shorter and finer actin cables in the subapical region are perhaps used to generate the thicker and longer actin bundles in the distal regions of pollen tubes (Chen et al., 2002; Cheung and Wu, 2004). Briefly, the dynamics of SABs and subapical actin filaments appear to be critical for pollen tube growth.
It has also been shown that there is a similar polarized organization of cytosolic free Ca2+ concentration [Ca2+]i in the form of a steep tip-focused intracellular gradient (Rathore et al., 1991; Miller et al., 1992; Holdaway-Clarke et al., 1997), which is essential for pollen tube growth (Holdaway-Clarke and Hepler, 2003). The distribution of Ca2+ in the tip region overlaps with the actin filament gradient in pollen tubes. The precise function of the [Ca2+]i gradient at the pollen tube tip remains to be elucidated. It is additionally pertinent that elevation of [Ca2+]i concentration results in fragmentation of F-actin in trumpet lily pollen (Kohno and Shimmen, 1987) and depolymerization of F-actin during the self incompatibility (SI) response of poppy pollen (Geitmann et al., 2000; Snowman et al., 2002). These depolymerizing and severing activities are almost certainly brought about by the action of specific actin-binding proteins (Staiger, 2000; Holdaway-Clarke and Hepler, 2003; Staiger and Hussey, 2004).
The gelsolin superfamily is the only known Ca2+-dependent actin filament-severing protein that has been well characterized in vertebrates and lower eukaryotic cells (for review, see Dos Remedios et al., 2003; McGough et al., 2003). Several members of this family have been identified in plants. Gelsolin-like proteins have been identified by immunoblotting in maize and lily (Lilium davidii) pollen (Wu and Yan, 2000; Tao and Ren, 2003). At least five villin isoforms (AtVLNs) are present in the Arabidopsis (Arabidopsis thaliana) genome (Klahre et al., 2000; Staiger and Hussey, 2004). P-135-ABP and P-115-ABP, actin-bundling proteins purified from trumpet lily pollen, are villin-like proteins based on primary-sequence analysis (Vidali et al., 1999; Yokota et al., 2003). However, these latter proteins do not have obvious severing or capping activities (Yokota et al., 1998, 2000, 2003). A fragmin-like protein has also been identified from Mimosa pudica, but it has not been purified to homogeneity and little is known about its biochemical properties and function (Yamashiro et al., 2001). Recently, a gelsolin-like 80-kD protein from poppy pollen (PrABP80) was isolated and characterized biochemically (Huang et al., 2004). Poppy gelsolin shows Ca2+-regulated severing, as verified indirectly by fluorimetric assays of actin polymerization and depolymerization and directly by fluorescence light microscopy of single actin filaments. PrABP80 can also nucleate the formation of new actin filaments during dynamic-assembly reactions and caps the barbed ends of actin filaments. This protein has been proposed to play a central role during the Ca2+-mediated depolymerization of actin during SI in poppy pollen. Nevertheless, the full repertoire of actin-binding proteins that account for linking high Ca2+ concentration and organization of actin cytoskeleton at the tip of the pollen tubes remains to be elucidated.
In this study, we isolated and purified a 41-kD Ca2+-dependent actin-binding protein from lily pollen (LdABP41) with DNase I chromatography. This scheme is based on the association between actin and the protein in the presence of Ca2+ and dissociation in the absence of Ca2+. We also characterized the in vitro activities and in vivo function of LdABP41 in pollen tube growth.
RESULTS
Purification of 41-kD Ca2+-Dependent Actin-Binding Protein from Lily Pollen
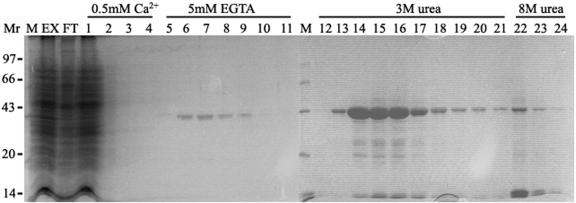
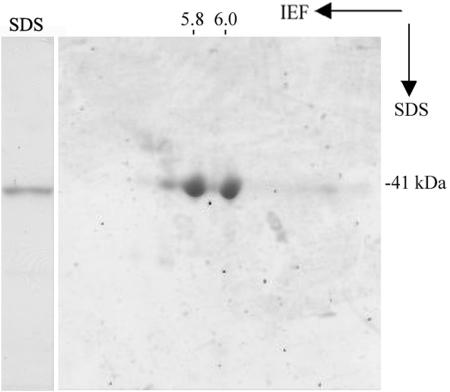
To identify Ca2+-dependent actin-binding proteins from pollen, DNase I chromatography was employed. This purification is based on the ability of DNase I to bind with high affinity to G-actin even if the G-actin is associated with an actin-binding protein (Bretscher and Weber, 1980; Harris and Gooch, 1981; Kurth et al., 1983). By changing the washing and elution conditions, assuming an association between actin and actin-binding proteins in the presence of Ca2+ and dissociation in the absence of Ca2+, a Ca2+-dependent actin-binding protein with molecular mass of 41 kD was isolated and purified from lily pollen. The purification procedure from a typical experiment is shown in Figure 1. Crude extract of lily pollen (Fig. 1, lane EX) was the high-speed supernatant obtained by homogenization and differential centrifugation. Most of the proteins found in the crude extract flowed through the DNase I column (Fig. 1, lane FT). Extensive washes with Ca2+-containing buffer removed proteins not bound specifically to G-actin from the column (Fig. 1, lanes 1–4). Subsequently, with the application of EGTA-containing buffer, a 41-kD Ca2+-dependent actin-binding protein was eluted from the column (Fig. 1, lanes 5–11). Three molar urea was employed to elute the remaining proteins (Fig. 1, lanes 12–21) and 8 m urea was used to regenerate the column (Fig. 1, lanes 22–24). The fraction eluted by 3 m urea (urea-eluate) was composed mainly of a 43-kD protein. Results from immunoblot analysis showed that the protein was actin (data not shown). The typical purification yielded about 0.6 mg of the 41-kD protein with a purity >98% from 10 g of lily pollen (Fig. 2A), representing 0.1% of total lysate protein. According to the actin amount from the urea-eluate (about 6 mg), it is estimated that the ratio of the 41-kD protein to actin is 1:10. The two-dimensional (2-D) PAGE analysis showed that the 41-kD protein had two main isoforms of pI 5.8 and pI 6.0 (Fig. 2B).
Figure 1.
Purification of a 41-kD Ca2+-dependent actin-binding protein from lily pollen. Coomassie Blue-stained gel of a representative purification procedure through DNase I column: M, marker; EX, clarified pollen extracts; FL, flow-through from the DNase I-Sepharose column; 1 to 4, buffer wash; 5 to 11, fractions of eluted target protein using EGTA buffer; 12 to 21, fractions of 3 m urea wash to remove most of the remaining actin; 21 to 24, fractions of 8 m urea wash to remove most of the remaining profilin. Molecular mass standards are given at left in kilodaltons.
Figure 2.
Purified LdABP41 has two isoforms. Coomassie Blue-stained 2-D PAGE analysis of the 41-kD proteins. The values of the pI are shown on the top.
N-Terminal Sequence and Tandem Mass Spectrometry Analysis of 41-kD Protein
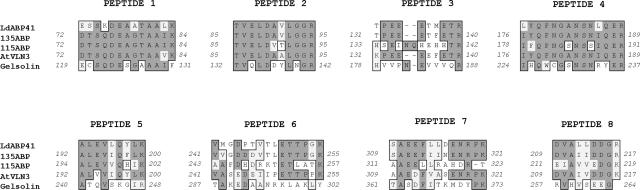
To further understand the relevance of the LdABP41 isoforms of pI 5.8 and pI 6.0 and gain insight about their identity, we analyzed the two protein spots obtained from 2-D PAGE. The individual proteins were excised from the gel, digested with trypsin, and subjected to mass spectrometry (MS). Peptide mass fingerprinting of the two proteins by matrix-assisted laser-desorption ionization time of flight (MALDI-TOF) yielded fragment profiles that gave best matches to an actin-bundling protein, 135-ABP, from trumpet lily. This analysis also identified other villin/gelsolin family members from plants (not shown). N-terminal sequence analysis by Edman degradation of the mixed protein yielded a sequence of PAFQGVGQRLGTEI, which is a perfect match with residues 9 to 23 of 135-ABP. To obtain additional sequence information, the tryptic digests of pI 6.0 and pI 5.8 spots were subjected to electrospray ionization-tandem MS (ESI-MS/MS). A total of 12 peptide fragments was analyzed and the de novo sequence results given in Table I. All 12 peptides shared greatest identity with 135-ABP from lily, with percent identities ranging from 54% to 91%. These sequences were less similar to 115-ABP, another trumpet lily villin (Table I) and to Arabidopsis VILLIN3 (Fig. 3). The sequence conservation with human plasma gelsolin was less than 50% identity for these polypeptides. Interestingly, all sequences aligned best with the first three gelsolin-homology domains (G1–G3) of the different villin/gelsolin family members. This observation, along with the Mr of these polypeptides, suggested that LdABP41 isoforms are fragmin/severin/CapG-like proteins. Finally, four peptide masses and the sequences obtained from them were identical between the pI 6.0 and pI 5.8 proteins (peptide 2 = peptide 9, 3 = 10, 4 = 11, and 7 = 12). This is strong evidence suggesting that the protein primary sequences may be identical and that the mobility difference observed on 2-D gels may be due to posttranslational modification.
Table I.
LdABP41 peptides determined by ESI-MS/MS
| Peptide Mass-to-Charge Ratio
|
Peptide Molecular Mass (D)
|
De Novo Sequence Determined by ESI-MS/MSa
|
Sequence Matchesb
|
Domain Matchesc
|
Sequence Identity
|
|||
|---|---|---|---|---|---|---|---|---|
| 135-ABP | 115-ABP | Gelsolin | ||||||
| pI 5.8 | % | |||||||
| 1 | 660.8 | 1,319.6 | ESSKDEAATAALK | DTSQDEAGTAAIK | G1 | 54 | 54 | 38 |
| 2 | 565.3 | 1,128.6 | TVELDAVLGGR | TVELDAVLGGR | G1/G2 | 91 | 73 | 50 |
| 3 | 619.8 | 1,237.6 | TPEEETFETR | TPEEETFETR | G2 | 80 | 25 | 8 |
| 4 | 551.9 | 1,652.8 | LYQFNGANSNLQER | IYQFNGANSNIQER | G2 | 64 | 50 | 33 |
| 5 | 538.8 | 1,075.6 | ALEVLQYLK | ALEVIQFLK | G2 | 67 | 56 | 20 |
| 6 | 515.9 | 1,544.8 | VMGDPTVTLETTPGK | VVGDDDVTLETTPGK | G3 | 67 | 27 | 0 |
| 7 | 516.6 | 1,546.8 | SAEEFLLDENRPK | SAEEFIINENRPK | G3 | 54 | 23 | 15 |
| pI 6.0 | ||||||||
| 8 | 655.3 | 1,962.9 | DVALLDDGR | DVAIIDDGR | G2/G3 | 78 | 33 | 22 |
| 9 | 565.3 | 1,128.6 | TVELDAVLGGR | TVELDAVLGGR | G1/G2 | 91 | 73 | 50 |
| 10 | 619.8 | 1,237.6 | VVEEETOETR | TPEEETFETR | G2 | 60 | 25 | 8 |
| 11 | 827.4 | 1,652.8 | LYQFNGANSNLGAER | IYQFNGANSNIQER | G2 | 86 | 50 | 33 |
| 12 | 773.9 | 1,545.8 | SAEEOLLNENRPK | SAEEFIINENRPK | G3 | 62 | 23 | 23 |
Boldface letters are single amino acid code for fragments that were sequenced by MS/MS. The O represents oxidized methionine. The presence of the putative b or y fragment ions (or both; see Papayannopoulos, 1995 for fragment ion nomenclature) for boldface amino acid residues were observed in the experimental MS/MS spectrum and are within 175 ppm mass tolerance. Plain text letters do not fit these criteria.
Sequences shown were the best match primary amino acid sequence from 135-ABP (GenBank no. AAD54660). The entire de novo sequence was used in BLAST searches (http://dove.embl-heidelberg.de/Blast2/msblast.html).
The gelsolin subdomain for which the best match was obtained (e.g. G1–G6) is given. These were determined by domain analysis of the human plasma gelsolin sequence described by Burtnick et al. (1997).
Figure 3.
Sequence similarity between LdABP41 and villin/gelsolin family members. Peptide sequences for LdABP41 from tryptic digests of gel-excised protein subjected to ESI-MS/MS were obtained as described in the text. Alignments of the de novo sequences determined from eight fragments in comparison with other gelsolins and villins are shown. Peptides 1 to 7 were from pI 5.8 isoform and peptide 8 was from pI 6.0. Four other peptide sequences obtained from the pI 6.0 isoform were identical to peptides 2, 3, 4, and 7 of the 5.8 isoform (not shown). LdABP41 shares 54% to 91% amino acid sequence identity with corresponding regions of trumpet lily 135-ABP and ≤50% identity with human gelsolin (see Table I for additional information). Accession numbers are as follows: 135-ABP from trumpet lily (AF088901), 115-ABP from trumpet lily (AB097407), Arabidopsis VILLIN3 (AtVLN3; AF081203), and human plasma gelsolin (CAA28000). Alignments were created using the ClustalW algorithm with MacVector software (version 7.1.1, Accelrys, Madison, WI).
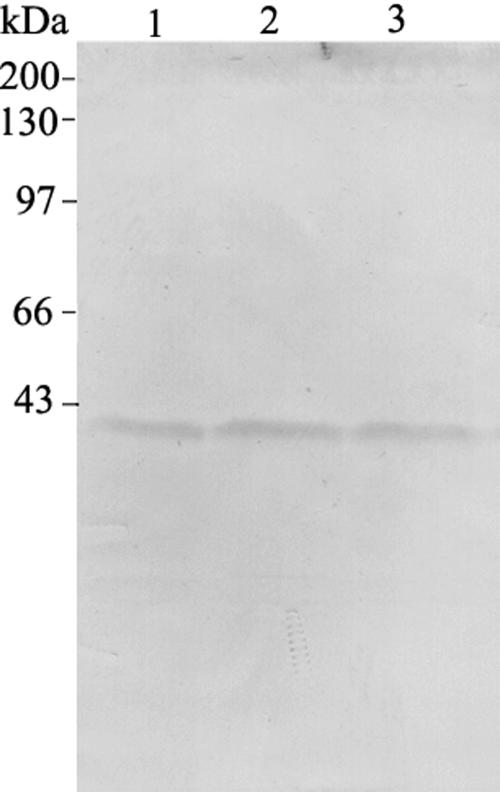
To investigate whether the 41-kD proteins might be proteolytic products from lily villin (135-ABP) generated during the extracting procedure, immunoblot analysis of the crude extracts from pollen grains with different extraction times was performed. The purified anti-LdABP41 antibody recognized only the 41-kD protein. Furthermore, the amount of the 41-kD protein slightly increased with increasing extraction time (Fig. 4). The 41-kD Ca2+-dependent actin-binding protein is referred to as LdABP41 hereafter.
Figure 4.
LdABP41 isoforms are not proteolytic products arising from protein extraction. Anti-LdABP41 immunoblot of LdABP41 in crude extract. Lane 1, Pollen grains were extracted in extraction buffer for 5 min; lane 2, pollen grains were extracted in extraction buffer for 15 min; lane 3, pollen grains were extracted in extraction buffer for 30 min. Molecular mass standards are given at left in kilodaltons.
LdABP41 Has a Severing Effect on Actin Filaments
To examine the ability of LdABP41 protein to act on actin filaments, sedimentation assays were performed. Lily pollen actin filaments were incubated with or without substoichiometric amounts of purified LdABP41 for 60 min at 20°C in the presence of Ca2+ or EGTA. G-actin and short actin fragments were separated from F-actin through ultracentrifugation and were left in the supernatant, whereas F-actin was sedimented in the pellet. The supernatants and pellets were analyzed by SDS-PAGE (Fig. 5A). Compared to the control (Fig. 5A, lane 1), samples with LdABP41 contained less actin in the pellets (Fig. 5A, top, lanes 3–5) and more actin in the supernatant fractions (Fig. 5A, bottom, lanes 3–5) in the presence of 0.2 mm Ca2+. The higher the ratio of LdABP41 to actin, the more actin appeared in the supernatant and the less actin appeared in the pellets. Furthermore, no detectable LdABP41 was found to sediment with F-actin in the pellets. However, the effect of LdABP41 was minimized when Ca2+ was chelated with 2 mm EGTA (Fig. 5A, lane 6). Two millimolar EGTA alone had no effects on the amount of actin in pellet or supernatant compared to control (Fig. 5A, lane 2). These results are consistent with calcium-mediated severing of actin filaments by LdABP41.
Figure 5.
Severing activity of LdABP41 on actin filaments. A, Severing activities of LdABP41 on actin filaments from lily pollen were determined by high-speed cosedimentation assays in the presence or absence of Ca2+. Lanes 1 to 6 (top section), Filamentous actin sedimenting in the pellets at the LdABP41:actin ratio of 0:1, 0:1, 0.1:1, 0.05:1, 0.02:1, and 0.05:1, respectively. Lanes 1 to 6 (bottom section), G-actin remaining in the supernatants at the LdABP41:actin ratio corresponding to the top lanes; lanes 1 and 3 to 5, in the presence of 0.2 mm Ca2+; lanes 2 and 6, in the absence of Ca2+ (plus 2 mm EGTA). B, Electron microscopic examination of severing effects of purified LdABP41 on actin filaments. Lily pollen F-actin after a 30-min incubation without (a) or with LdABP41 (b, c, and d) in the presence (a, b, c, and d) and absence (e) of 0.2 mm Ca2+. a, F-actin in the presence of Ca2+; b, a 0.02:1 molar ratio of LdABP41 to F-actin in the presence of Ca2+; c, a 0.05:1 molar ratio of LdABP41 to F-actin in the presence of Ca2+; d, a 0.1:1 molar ratio of LdABP41 to F-actin in the presence of Ca2+; e, a 0.05:1 molar ratio of LdABP41 to F-actin in the absence Ca2+ (plus 2 mm EGTA). Bar = 500 nm.
Results from negative-stain electron microscopy lend further support to the argument that actin filaments are fragmented in the presence of LdABP41 (Fig. 5B). According to the average length measured from more than 50 actin filaments, the filament length was reduced with an increasing of the ratio of LdABP41 to actin. The average length of actin filaments in the control without LdABP41 was 1569.8 ± 111.2 nm (Fig. 5B, a); in the presence of 2% of LdABP41 it was 1253.5 ± 109.8 nm (Fig. 5B, b); in the presence of 5% of LdABP41 it was 384.9 ± 24.9 nm (Fig. 5B, c); and in the presence of 10% of LdABP41 it was 209.4 ± 15.8 nm (Fig. 5B, d). However, in the presence of both 5% of LdABP41 and EGTA, the average filament length increased to 932.2 ± 96.6 nm (Fig. 5B, e), indicating that the filament-severing activity of LdABP41 was partially abolished by the addition of EGTA. Therefore, our results demonstrate that the LdABP41 protein has a severing activity on plant actin filaments in a Ca2+-dependent manner.
LdABP41 Promotes the Nucleation of Actin Polymerization
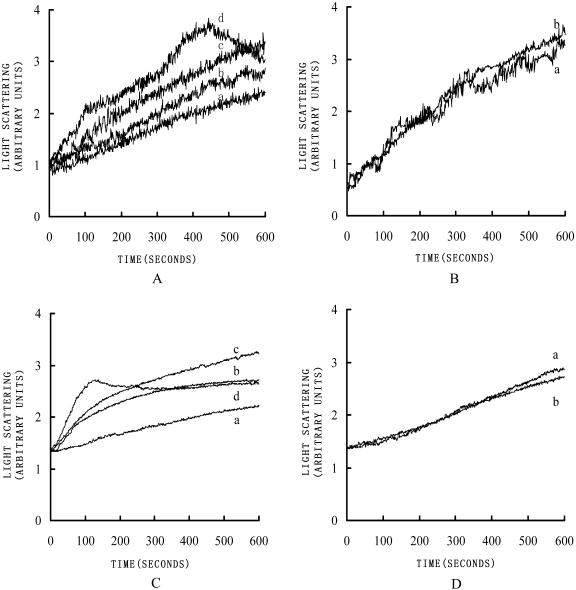
To examine the effect of LdABP41 on the dynamics of actin polymerization, assays of actin assembly kinetics were performed. Actin polymerization was measured by 90° light scattering (Ren et al., 1997). When 10 μm lily pollen G-actin was polymerized at room temperature by the addition of MgCl2, KCl, and ATP to 5 mm, 50 mm, and 0.5 mm, respectively, there appeared a short lag period followed by a rapid actin polymerization (Fig. 6A, a). When LdABP41 and actin were mixed in a ratio of 0.005:1 in the presence of 0.2 mm Ca2+, the lag period was slightly shortened (Fig. 6A, b) indicating that the nucleation of the G-actin polymerization process was accelerated. By increasing of the ratio of LdABP41:actin to 0.01:1, the lag phase was shortened even further (Fig. 6A, c). Increasing the ratio of LdABP41:actin to 0.02:1 almost completely eliminated the lag phase (Fig. 6A, d), yet the curve declined after 400 s indicating there could be some monomer binding occurring at higher ratios of LdABP41 to actin filaments. However, when LdABP41 was added in the presence of both micromolar amounts of Ca2+ and EGTA, the nucleation activity of the LdABP41 was totally abolished (Fig. 6B). The nucleation activity of the LdABP41 on rabbit skeletal-muscle actin has also been tested and shows similar results (Fig. 6, C and D). These results provide evidence for the ability of LdABP41 to promote nucleation of actin polymerization and the activity is Ca2+ dependent.
Figure 6.
LdABP41 nucleates actin filament assembly. Time courses for actin polymerization were measured by 90° light scattering. Ten micromolar G-actin was polymerized by the addition of salts (as describe in the text) in the presence of various ratios of LdABP41 to actin. A, LdABP41 at various ratios to pollen actin in the presence of Ca2+, 0:1 (a), 0.005:1 (b), 0.01:1 (c), 0.02:1 (d). B, LdABP41 at various ratios to pollen actin in the absence of Ca2+ (plus 2 mm EGTA), 0:1 (a), 0.01:1 (b). C, LdABP41 at various ratios to rabbit skeletal muscle actin in the presence of Ca2+, 0:1 (a), 0.005:1 (b), 0.01:1 (c), 0.02:1 (d). D, LdABP41 at various ratios to rabbit skeletal muscle actin in the absence of Ca2+ (plus 2 mm EGTA), 0:1 (a), 0.01:1 (b).
The Effects of LdABP41 on the Growth Rate of Lily Pollen Tubes
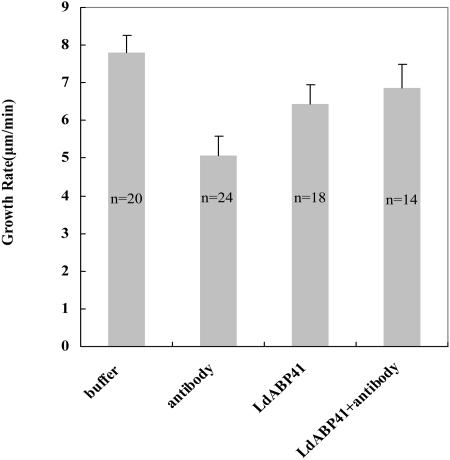
To assess directly the function of LdABP41 in pollen tube growth, we microinjected purified anti-LdABP41 antibody, LdABP41 plus purified anti-LdABP41 antibody, LdABP41 and injection buffer, respectively, into lily pollen grains cultured in slowly flowing germination solution, and then measured the elongation rate of the pollen tubes. Because mechanical stimuli or damage could be caused by the injection during the first 10 min (Lin and Yang, 1997), the elongation rate was calculated from 10 min to 30 min after the injection. Injection of buffer was used as a control. Compared with buffer-injected pollen tubes (Fig. 7), the elongation rate of pollen tubes injected with anti-LdABP41 antibody was considerably lower (Fig. 7). However, the antibody-induced inhibition of pollen tube elongation was abolished to some extent when the antibody was premixed with the LdABP41 prior to microinjection (Fig. 7). The elongation rate of LdABP41-injected pollen tubes was also reduced compared with that of pollen injected with buffer (Fig. 7). In addition, antibody injections also induced a distinct morphology of pollen tubes: About one-half were much wider than untreated pollen tubes or buffer-injected pollen tubes.
Figure 7.
Inhibition of pollen tube elongation by microinjected anti-LdABP41 antibody. Pollen tubes cultured in a germination solution were microinjected with injection buffer, anti-LdABP41 antibody, anti-LdABP41 antibody plus purified LdABP41, and the purified LdABP41, respectively, using the procedure described in “Materials and Methods.” Elongation rates of pollen tubes were measured from 10 min after injection to 30 min. Columns represent the average elongation rate calculated by the deletion of one maximum and minimum from each treatment (mean ± se) with n representing the number of determinations. The elongation rate for antibody injection was significantly different from control or LdABP41+antibody (P < 0.05).
Localization of LdABP41 in Lily Pollen Tubes
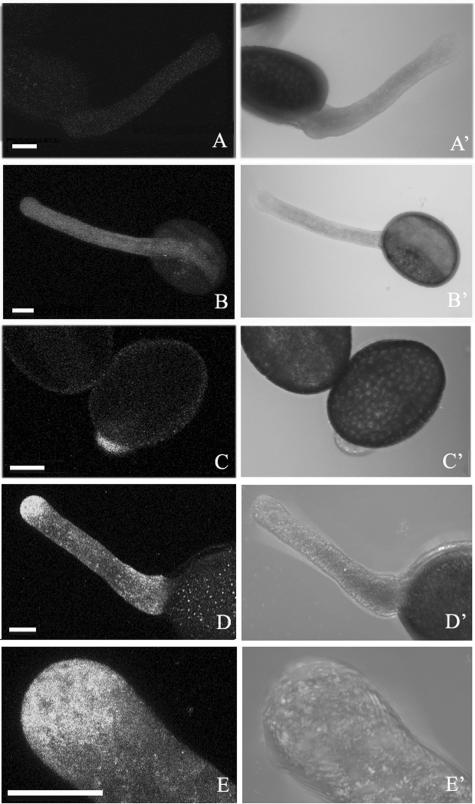
To investigate the function of LdABP41 in vivo, we analyzed the subcellular distribution of the protein in germinated pollen using an affinity-purified, polyclonal anti-LdABP41 antibody that strongly cross-reacts with LdABP41 protein in western blots (Fig. 2). Confocal images of pollen at an early stage of germination showed dense labeling of LdABP41 at the tube tip (Fig. 8, C and C′). In germinated pollen tubes, LdABP41 was localized in a punctate pattern throughout the cytoplasm of the pollen tubes, but especially focused on the tip region (Fig. 8, D and D′). However, there was no obvious localization of LdABP41 in the pollen grain. Controls without the first antibody did not give any particular localization (Fig. 8, A and A′) or with the anti-profilin antibody labeled uniformly along the length of tubes (Fig. 8, B and B′).
Figure 8.
Localization of LdABP41 in lily pollen. Germinated lily pollen labeled for LdABP41 was examined by confocal microscopy. No primary antibody (A and A′) or anti-profilin antibody (B and B′) were used as controls. The distribution of LdABP41 was shown in recently germinated pollen grains (C and C′) and pollen tubes (D and D′) or with higher magnification (E and E′); both fluorescent images (C and D) and transmission images (C′ and D′) show the localization of LdABP41 at the tip of germinated pollen tube (C) and a highest concentration at the tip region of pollen tube (D); higher magnification images (E and E′) showed punctate distribution pattern for LdABP41 in pollen tube. Bars = 20 μm.
DISCUSSION
LdABP41 from Lily Pollen Is a New Member of the Gelsolin Superfamily
The gelsolin superfamily, comprising the only known Ca2+-dependent actin filament-severing proteins, is a group of multifunctional actin-binding proteins that is well studied in vertebrates and lower eukaryotic cells (for review, see Dos Remedios et al., 2003). However, little is known about the biochemical properties and function of the members of gelsolin superfamily in plant cells (for review, see McGough et al., 2003; Staiger and Hussey, 2004). The gelsolin family is quite large and diverse. Most of the members of the family are around 80 kD (gelsolin, adseverin), whereas some members with C-terminal extensions (villin, advillin) are around 90 kD (in vertebrates) or 115 to 135 kD (in pollen). Some members are around 41 kD (CapG, severin, fragmin, earthworm actin modulator), which is only about half of the size of cytoplasmic gelsolin, and they are composed of just three, rather than six, gelsolin homology domains (Giebing et al., 1997; Friederich and Louvard, 1999). Forty-one-kilodalton proteins of the family have traditionally been considered ancestral to the larger protein of the family (Folger et al., 1999). In this study, we have provided the first biochemical and cytological evidence, to our knowledge, for the existence of a 41-kD gelsolin superfamily member in lily pollen. A 2-D gel analysis shows that the protein contains two protein bands with pI value of 5.8 and 6.0. To distinguish whether the two bands are proteolytic products from a larger protein, MS analysis of the two bands has been performed. The results show that both of the two bands have greatest similarity to the N-terminal part of 135-ABP, a 135-kD gelsolin/villin superfamily protein purified from trumpet lily pollen (Vidali et al., 1999). Therefore, it is unlikely that they originate from the N-terminal and C-terminal parts of lily villin.
To examine further whether LdABP41 proteins derive from a proteolytic breakdown product of lily villin during the process of protein extraction, immunoblot analysis of crude extract of pollen grains at different extraction times was performed. If LdABP41 came from a protein of higher molecular mass, the anti-LdABP41 antibody should be able to recognize larger protein bands, and the amount of those proteins should decrease and LdABP41 should increase during extended extraction. However, our experiments demonstrate that purified anti-LdABP41 antibody does not recognize any other proteins in the crude extract, but only a 41-kD protein band, and the amount of LdABP41 does not significantly change with increasing extraction time. The slight increase in the amount of LdABP41 can be explained as more thorough extraction of proteins from the pollen. From the results, it can be concluded that the two bands are isovariants of a new member of gelsolin/villin superfamily.
In addition, our results show that LdABP41 protein has G-actin-binding, actin filament-severing, and actin filament-nucleating activities. All of these activities are Ca2+-sensitive. These results are consistent with the results obtained for gelsolin, severin, and fragmin (for review, see Dos Remedios et al., 2003; McGough et al., 2003), but its apparent molecular mass on SDS-PAGE (41 kD) is equivalent with that of severin and fragmin. The LdABP41 protein also has a similar pI with these proteins (fragmin, 5.7; trumpet lily villin, 5.8; gelsolin, 5.9). Moreover, LdABP41 does not have the actin filament-bundling activity demonstrated for trumpet lily villins (Yokota and Shimmen, 1999; Yokota et al., 2003). All of these data support that LdABP41 is a new member of the gelsolin/villin superfamily in plant cells.
Potential Cross-Talk between SABs and Calcium Via LdABP41 in Pollen Tube Growth
The role of the actin cytoskeleton in growing pollen tubes is always the focus of the study of actin dynamics, but the mechanism remains controversial. Extensive axial actin cables are found in the shank of pollen tubes, but in the tip region of pollen tubes, instead of actin cables, SABs have been observed (Fu et al., 2001). Actin filaments are not only involved in the transport of secretory vesicles for cell elongation, but also play a role directly in the tip growth of pollen tubes. However, the mechanism of actin cytoskeleton function in pollen tube growth is still not clear. It is well known that cytosolic Ca2+ is high at the extreme tip of the pollen tubes, creating a gradient that is necessary for the pollen tube growth (Franklin-Tong, 1999; Holdaway-Clarke and Hepler, 2003). Proteins from the gelsolin superfamily are the most potent Ca2+-dependent actin filament-severing proteins identified to date (Sun et al., 1999). One model is that gelsolin family members enhance the rate of actin polymerization by increasing the number of filament ends and by stabilizing the actin nuclei for polymerization. Although it is postulated that villin may also respond to the apical calcium gradient in pollen, fragmenting microfilaments and thus locally facilitating actin remodeling (Vidali et al., 2001; Holdaway-Clarke and Hepler, 2003), a Ca2+-sensitive severing activity of trumpet lily villins has not been detected (Yokota et al., 2000, 2003). Recently, a gelsolin-like 80-kD protein has been purified from poppy pollen (PrABP80) and shows calcium-regulated actin filament severing, nucleation, and capping activities (Huang et al., 2004), indicating the existence of other gelsolin/villin superfamily proteins in plant cells. Its presence and activity in poppy pollen are consistent with a role in the massive, calcium-induced depolymerization of actin during the SI response (Snowman et al., 2002). The results of our assays show that the LdABP41 severs in vitro-polymerized lily pollen F-actin into short actin filaments in a Ca2+-sensitive manner.
Results from the microinjection of purified antiserum of the protein into geminated lily pollen grain demonstrate that LdABP41 protein is required for pollen tube growth. In addition, the results of immunofluorescence localization show that LdABP41 appears to be localized on some kind of vesicles throughout the cytoplasm of the pollen tube, and the fact that it focused preferentially in the tip region of pollen tubes suggests that LdABP41 might localize to vesicles needed for the tip growth. In keeping with the tip-focused location of LdABP41, the short actin filaments therein, the high concentration of calcium at the tip of the tube, and the fact that the introduction of anti-LdABP41 antibody prevents pollen tube growth, it is strongly suggested that LdABP41 may play an important role in the pollen germination and pollen tube growth through regulating the dynamics of actin cytoskeleton. Identification of LdABP41 strongly supports the universal existence of the 40-kD actin-modulating protein previously found just in Dictyostelium amoebae (severin), Physarum slime molds (fragmin), and macrophages (CapG). Although bioinformatics of Arabidopsis and rice (Oryza sativa) shows no G1–G3 genes in these organisms, there is a splice variant for Arabidopsis villin 1 (Huang et al., 2004) that would produce a G1–G3. Our results should lead to a better understanding of the modulation of actin cytoskeleton corresponding to the tip growth of pollen tubes. For a more comprehensive assessment of the function of LdABP41, cloning the gene and making transgenic plants or knock-out mutations are needed.
MATERIALS AND METHODS
Plant Material
Mature lily (Lilium davidii cv Duch.) pollen grains were collected from Lanzhou, Gansu Province in northwest China. After being air dried, the pollen was stored at −20°C before use.
Preparation of Deoxyribonuclease I Affinity Chromatography Column
Sixty milligrams of deoxyribonuclease (DNase I, Sigma, St. Louis, type IV) was coupled to 2.5 g of cyanogen bromide-activated Sepharose 4B (Pharmacia Biotech, Piscataway, NJ) according to Schafer et al. (1998).
Purification of Ca2+-Sensitive Actin-Binding Protein LdABP41
Isolation of Ca2+-sensitive actin-binding protein from lily pollen was performed as described by Yamashiro et al. (2001) with some modifications. Ten grams of lily pollen in 50 mL extraction buffer (0.1 m Tris, 0.4 m sorbitol, 32 mg/mL polyvinylpyrrolidone-10, 0.5 mm CaCl2, 50 mm NaF, 10% glycerol, 0.5 mm ATP, 5 mm dithiothreitol [DTT], 0.5 mm phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, pH 8.0) was ground in a mortar for 20 min at 4°C and centrifuged at 100,000g for 1 h. The supernatant was loaded onto the DNase I affinity column preequilibrated with the extraction buffer and washed with 50 column volumes of Ca2+ buffer (0.1 m Tris, 0.5 mm CaCl2, 10% glycerol, 0.5 mm ATP, 5 mm DTT, 0.5 mm phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, pH 7.5). Then, 20 mL of the EGTA buffer (0.1 m Tris, 0.5 mm EGTA, 0.5 mm ATP, 5 mm DTT, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin, pH 7.5) was used to elute Ca2+-sensitive actin-binding proteins from the affinity column. The EGTA eluate was collected as 1-mL fractions. Twenty milliliters of the same solution containing 3 m and 8 m urea was used to elute actin (not for the use of the following experiments) and regenerate the DNase I resin, respectively. The fractions containing >0.1 mg/mL LdABP41 were pooled and dialyzed against 500 mL of HEPES buffer (5 mm HEPES-KOH, pH 7.2, 0.1 mm DTT, 50 μM MgCl2, 0.1 mm ATP) for three times and stored at 4°C before use. At the same time, 20 μL of the fractions containing target protein were analyzed by 12.5% (w/v) SDS-PAGE. Protein concentration was measured as Bradford (1976) using bovine serum albumin (BSA) as standard.
Preparation of Actin
Actin for polymerization experiments was prepared from lily pollen by the method of Ren et al. (1997). Rabbit skeletal-muscle G-actin was prepared by the method of Spudich and Watt (1971) and followed by a modified-profilin-affinity chromatography (Ren et al., 1997); 5 mg of G-actin obtained from traditional method was mixed with 10 mg of recombinant human profilin for 0.5 h and then applied to a Poly-l-Pro-Sepharose affinity column. The elution with just one actin band was collected and subjected to one polymerization-depolymerization cycle. Purified globular or monomeric actin (G-actin) was stored in G-buffer (5 mm Tris, 0.2 mm CaCl2, 0.01% NaN3, 0.5 mm DTT, 0.4 mm ATP, pH 7.0) at −80°C before use. For F-actin preparation, 50 mm KCl, 5 mm MgCl2, 0.5 mm ATP in G-actin were added to the G-actin sample and incubated at 4°C for 16 h.
Preparation of Antiserum and Antibody Purification
A rabbit polyclonal antiserum raised against purified LdABP41 was obtained by injection of 250 μg protein in complete Freund's adjuvant at each of six dorsal sites and was followed by three times of equivalent challenge incubation at 2-week intervals. Positive sera were stored at −80°C. The polyclonal antibodies were affinity purified according to the method of Lin and Yang (1997). The purified LdABP41 was separated on 12% SDS-PAGE gels and transferred to nitrocellulose membrane. The purified protein was excised and blocked for 1 h in Tris-buffered saline (TBS) containing 3% (w/v) BSA and 0.5% (v/v) Tween 20. Individual strips were incubated overnight at room temperature in rabbit serum diluted 1:50 with TBS containing 0.5% (v/v) Tween 20. The nitrocellulose was washed three times with 10 mL of TBS plus Tween 20 for 5 min with gentle agitation to remove unbound antibodies. Bound antibodies were eluted with 2 mL of Gly elution buffer (0.1 m Gly-HCl, pH 2.5, 0.5 m NaCl, 0.05% Tween 20) for 3 min on ice with gentle mixing. Eluted antibodies were transferred immediately to a test tube containing 0.3 mL of 1 m Tris, pH 8.0, for neutralization. Elution and neutralization were repeated once for each nitrocellulose strip, followed by a 2-mL TBS plus Tween 20 rinse. The two eluates and the rinse were combined and supplemented with 0.75 mL of 10% (w/v) BSA and 75 μL of 5% NaN3, aliquoted, and stored at −80°C. This purified antibody was used for immunofluorescence localization and microinjection.
Protein Identification by Peptide Mass Fingerprinting and N-Terminal Sequencing
Two-dimensional gel electrophoresis was carried out as described by Garrels (1979). The first dimension gels contained 1.6% ampholines with pH 5 to 7 and 0.4% ampholines in the pH range 3.5 to 10. The second dimension gels were 12% SDS-PAGE mini-slab gels. Spots of interest were excised from the gel followed by destaining, reduction, alkylation, and hydrolysis with modified porcine trypsin as described by Hellman et al. (1995). Samples for MALDI-TOF analysis were prepared by mixing a small aliquot of the digestion supernatant 1:1 with 4-hydroxy-α-cyano-cinnamic acid. Peptide mass fingerprinting was performed on the MALDI-TOF (Autoflex, Bruker Instruments, Billerica, MA). A peak list was compiled with the mass-to-charge ratio software (Proteometrics, New York) and used for peak selection. The peptide masses were used in a search against the National Center for Biotechnology Information database using Mascot software (Matrix Science, London).
To determine the N-terminal amino acid sequence, a single band with molecular mass of 41 kD obtained from SDS-PAGE of the lily pollen was transferred onto polyvinylidene difluoride membrane using a semidry blotting system (Bio-Rad Laboratories, Hercules, CA) and applied to a Beckman LF3000 amino acid sequence analyzer (Beckman Instruments, Fullerton, CA) for analysis.
For de novo sequence analysis by ESI-MS/MS, individual protein spots from 2-D gels were harvested and prepared as described previously (Huang et al., 2004). Mass spectra were collected on a QSTAR Pulsar i (ABI, Sunnyvale, CA) quadropole-time of flight MS equipped with nano-ESI source by the Mass Spectrometry Consortium for the Life Sciences at the University of Minnesota. Sequence analysis was performed with BioAnalyst software (ABI) and MS-BLAST searches performed at http://www.bork.embl-heidelberg.de.
Electron Microscopy
The purified LdABP41 at 0.5 and 1 μm, and lily F-actin (10 μm), were mixed and incubated for 30 min at 25°C in the presence of 0.5 mm CaCl2 or 2 mm EGTA. Samples were mounted on grids covered with carbon-coated collodion film and negatively stained with 1% uranyl acetate. Electron micrographs were taken at a direct magnification of ×40,000 with a transmission electron microscope (Hitachi-H600; Tokyo). Negative staining of actin filaments without purified Ca2+-sensitive actin-binding protein was performed as a control.
Sedimentation Analysis of Actin Filaments
LdABP41 was transferred to HEPES buffer as described above and mixed with F-actin of 0.4 mg/mL to a final concentration of 0.5 and 1 μm of LdABP41, respectively, at 25°C in the presence of 0.5 mm CaCl2 or 2 mm EGTA for 1 h. Finally the mixtures were centrifuged for 1 h at 100,000g and the supernatants (actin monomers or short fragments) and pellets (actin filaments) were analyzed by SDS-PAGE. The gel was stained with Coomassie Brilliant Blue and photographed by using a CCD camera. F-actin without the purified LdABP41 was used as control.
Dynamic Assay of Actin Polymerization
The purified LdABP41 was exchanged to HEPES buffer with a 10-kD-cutoff ultrafiltration tube (4206, Millipore, Bedford, MA) and was mixed with G-actin to final concentrations of 200, 100, 50 nm, and 10 μm, respectively. The actin was then polymerized by addition of KCl, MgCl2, and ATP to final concentrations of 50, 5, 0.5 mm, respectively, in the presence of 0.2 mm CaCl2 or 2 mm EGTA. Dynamic polymerization was monitored by 90° light scattering over a 10-min period at room temperature with a spectrophotometer (Fluoro Max-II; Beckman Instruments) set for excitation and emission wavelengths of 450 nm.
Immunoblotting
Immunoblot analysis of the crude extract from lily pollen grains was performed by the following procedure: 1 g of pollen was added to 10 mL protein isolation buffer (as mentioned above) and hand-drilled for about 5, 15, and 30 min, respectively. The grindate was centrifuged at 100,000g for 5 min and the supernatant transfered to SDS-PAGE. Western transfer was performed using the Hoefer western-blotting system (Pharmacia Biotech). Purified LdABP41 antibody (as mentioned above) was used at dilutions of 1:1,000 and horseradish peroxidase-labeled anti-rabbit IgG antibody (Pharmacia Biotech) was used at dilution of 1:3,000.
Microinjection of Lily Pollen
A total of 0.2 g of lily pollen, stored at −20°C, was stirred in 50 μL germination solution (15% Suc, 0.01% H3BO3, 0.01% KNO3, 0.02% MgSO4, 0.03% Ca[NO3]2) for 3 min to be degreased. Then it was suspended in 30 mL germination solution to germinate at room temperature. Approximately 45 min after germination, pollen tubes of about 50 μm long were chosen for microinjection. Microinjection was performed according to the method of Lin and Yang (1997) with some modifications. To reduce the effect on pollen tube growth caused by microinjection, we chose pollen grains but not pollen tubes for microinjection. The micropipette tip remained in the pollen after injection for a few minutes before we pulled it out from the pollen. To keep the germination solution fresh, the injected cell was kept in a container that contained about 1 mL germination solution, which was driven by a constant flow pump during and after the microinjection. Approximately 0.3 nL of agents were injected into each pollen grain. The lengths of pollen tubes were measured at the time of injection and 30 min after injection for the calculation of the rate of pollen tube elongation. Four kinds of agents, LdABP41 (5 μm), affinity-purified polyclonal anti-LdABP41 antibody (4 μm), Tris-HCl buffer; the mixture containing 1.7 μm purified LdABP41, and 3.6 μm antibody were injected into pollen grains, respectively.
Immunostaining of Pollen Tube and Confocal Microscopy
Lily pollen grains were germinated according to method mentioned above and fixed in 4% formaldehyde in PIPES buffer (pH 6.8) for 1 h with constant agitation. After rinsing in PIPES buffer three times, 15 min each, and digesting with 0.5% cellulase and 0.5% pectinase in PIPES, the pollen was permeabilized with 3% Triton X-100 solution for 30 min. Then, the pollen was blocked with 3% BSA in phosphate-buffered saline (PBS) for 2 h and incubated in polyclonal affinity-purified anti-LdABP41 antibody diluted at 1:100 in PBS overnight at 4°C, washed in PBS, and incubated in fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody, diluted at 1:500 for 2 h. After washing with PBS, the stained pollen grains were mounted in 50% glycerol and viewed by means of laser-scanning confocal microscope (Olympus FV300, Tokyo) mounted on an inverted microscope (Olympus IX-70) using ×40 oil immersion objective. Samples were excited with the blue line (488 nm) of an argon laser beam, and the image was collected by Olympus Fluoview 4.0 software. Sample without primary antibody was used as control.
Acknowledgments
We thank Dr. Ming Yuan (China Agricultural University) and Noni Franklin-Tong (University of Birmingham, UK) for critical reading and comments on the manuscript.
This work was supported by the National Natural Science Foundation for Distinguished Young Scholars (grant no. 30325005 to H.R.) and by the Major State Basic Research Developmental Program of China (grant no. G1999011701 to H.R.). Work in the lab of C.J.S. was supported, in part, by the U.S. Department of Energy, Energy Biosciences Division (DE–FG02–04ER15526).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.046326.
References
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Weber K (1980) Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20: 839–847 [DOI] [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC (1997) The crystal structure of plasma gelsolin: implications for actin severing, capping and nucleation. Cell 90: 661–670 [DOI] [PubMed] [Google Scholar]
- Cai G, Moscatelli A, Cresti M (1997) Cytoskeletal organization and pollen tube growth. Trends Plant Sci 2: 86–91 [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu H-M, Cheung AY (2002) The regulation of actin organization by actin depolymerizing factor (ADF) in elongating pollen tubes. Plant Cell 14: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu H-M (2004) Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16: 257–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J, Rutten T, Van Amstel T, de Win A, Doris F, Steer M (1995) Regulation of pollen tube growth. Acta Bot Neerl 44: 93–119 [Google Scholar]
- Doris FP, Steer MW (1996) Effects of fixatives and permeabilisation buffers on pollen tubes: implications for localisation of actin microfilaments using phalloidin staining. Protoplasma 195: 25–36 [Google Scholar]
- Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83: 433–473 [DOI] [PubMed] [Google Scholar]
- Folger PA, Berg WJ, DeJesus Z, Fong Y, Pardee JD (1999) A mammalian severin replaces gelsolin in transformed epithelial cells. Cancer Res 59: 5349–5355 [PubMed] [Google Scholar]
- Franklin-Tong VE (1999) Signaling and the modulation of pollen tube growth. Plant Cell 11: 727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E, Louvard D (1999) Villin. In T Kries, R Vale, eds, Guidebook to the Cytoskeletal and Motor Proteins. Oxford University Press, New York, pp 175–179
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels JI (1979) Two dimension gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem 254: 7961–7977 [PubMed] [Google Scholar]
- Geitmann A, Snowman BN, Emons AMC, Franklin-Tong VE (2000) Alterations in the actin cytoskeleton of pollen tubes are induced by the self-incompatibility reaction in Papaver rhoeas. Plant Cell 12: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Kovar DR, Staiger CJ (1999) Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11: 2349–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebing T, Obermann WMJ, Fuerst D, D'Haese J (1997) C-terminal deleted fragments of 40-kDa earthworm actin modulator still show gelsolin activities. FEBS Lett 417: 191–195 [DOI] [PubMed] [Google Scholar]
- Harris HE, Gooch J (1981) An actin depolymerizing protein from pig plasma. FEBS Lett 123: 49–53 [DOI] [PubMed] [Google Scholar]
- Hellman U, Wernstedt C, Gonez J, Heldin CH (1995) Improvement of an “in-gel” digestion procedure for the micropreparation of intenal protein fragment for amino acid sequencing. Anal Biochem 224: 451–455 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijó JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159: 539–563 [DOI] [PubMed] [Google Scholar]
- Huang S, Blanchoin L, Chaudhry F, Franklin-Tong VE, Staiger CJ (2004) A gelsolin-like protein from Papaver rhoeas pollen (PrABP80) stimulates calcium-regulated severing and depolymerization of actin filaments. J Biol Chem 279: 23364–23375 [DOI] [PubMed] [Google Scholar]
- Klahre U, Friederich E, Kost B, Louvard D, Chua N-H (2000) Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis. Plant Physiol 122: 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Shimmen T (1987) Ca2+-induced fragmentation of actin filaments in pollen tubes. Protoplasma 141: 177–179 [Google Scholar]
- Kost B, Spielhofer P, Chua N-H (1998) A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J 16: 393–401 [DOI] [PubMed] [Google Scholar]
- Kurth MC, Wang L-L, Dingus J, Bryan J (1983) Purification and characterization of a gelsolin-actin complex from human platelets: evidence for Ca2+-insensitive functions. J Biol Chem 258: 10895–10903 [PubMed] [Google Scholar]
- Lin Y, Yang Z (1997) Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for rho-type GTPases in the control of tip growth. Plant Cell 9: 1647–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Staiger CJ, Min JK, Simonetti K (2003) The gelsolin family of actin regulatory proteins: Modular structures, versatile functions. FEBS Lett 552: 75–81 [DOI] [PubMed] [Google Scholar]
- Miller DD, Callaham DA, Gross DJ, Hepler PK (1992) Free Ca2+ gradient in growing pollen tubes of Lilium. J Cell Sci 101: 7–12 [Google Scholar]
- Miller DD, Lancelle SA, Hepler PK (1996) Actin microfilament do not form a dense meshwork in Lilium longiflorum pollen tube tips. Protoplasma 195: 123–132 [Google Scholar]
- Papayannopoulos IA (1995) Interpretation of collision-induced dissociation tandem mass spectra of peptides. Mass Spectrom Rev 14: 49–71 [Google Scholar]
- Pierson ES (1988) Rhodamine-phalloidin staining of F-actin in pollen after dimethyl sulphoxide permeabilisation: a comparison with the conventional formaldehyde preparation. Sex Plant Reprod 1: 83–87 [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR (1991) A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev Biol 148: 612–619 [DOI] [PubMed] [Google Scholar]
- Ren HY, Gibbon BC, Ashworth SL, Sherman DM, Yuan M, Staiger CJ (1997) Actin purified from maize pollen functions in living plant cells. Plant Cell 9: 1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Jennings PB, Cooper JA (1998) Rapid and efficient purification of actin from non-muscle sources. Cell Motil Cytoskeleton 39: 166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ (2002) Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA, Watt S (1971) The regulation of rabbit skeletal muscle contraction. J Biol Chem 246: 4866–4871 [PubMed] [Google Scholar]
- Staiger CJ (2000) Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 257–288 [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Hussey PJ (2004) Actin and actin-modulating proteins. In PJ Hussey, ed, The Plant Cytoskeleton in Cell Differentiation and Development. Blackwell Scientific Publications, Oxford, pp 32–80
- Sun HQ, Yamamoto M, Mejillano M, Yin HL (1999) Gelsolin, a multifunctional actin regulatory protein. J Biol Chem 274: 33179–33182 [DOI] [PubMed] [Google Scholar]
- Tao Z, Ren H (2003) Regulation of gelsolin to plant actin filaments and its distribution on pollen. Sci China Ser C Life Sci 46: 379–388 [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48: 461–491 [DOI] [PubMed] [Google Scholar]
- Vidali L, Hepler PK (2000) Actin in pollen and pollen tubes. In CJ Staiger, F Baluska, D Volkmann, PW Barlow, eds, Actin: A Dynamic Framework for Multiple Plant Cell Functions. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 323–345
- Vidali L, Hepler PK (2001) Actin and pollen tube growth. Protoplasma 215: 64–76 [DOI] [PubMed] [Google Scholar]
- Vidali L, Holdaway-Clarke TL, Hepler PK (2001) The calcium/cytoskeleton connection in pollen tube growth. In A Geitmann, M Cresti, IB Heath, eds, Cell Biology of Plant and Fungal Tip Growth. IOS Press, Amsterdam, The Netherlands, pp 27–35
- Vidali L, Yokota E, Cheung AY, Shimmen T, Hepler PK (1999) The 135 kDa actin-bundling protein from Lilium longiflorum pollen is the plant homologue of villin. Protoplasma 209: 283–291 [Google Scholar]
- Wu W, Yan LF (2000) Immunochemical identification of gelsolin by western blotting in maize pollen. Chinese Sci Bull 45: 256–258 [Google Scholar]
- Yamashiro S, Kameyama K, Kanzawa N, Tamiya T, Mabuchi I, Tsuchiya T (2001) The gelsolin/fragmin family protein identified in the higher plant Mimosa pudica. J Biochem 130: 243–249 [DOI] [PubMed] [Google Scholar]
- Yokota E, Muto S, Shimmen T (2000) Ca2+-calmodulin suppresses the F-actin binding activity of a 135-kDa actin-bundling protein isolated from lily pollen tubes. Plant Physiol 123: 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Shimmen T (1999) The 135-kDa actin-bundling protein from lily pollen tubes arranges F-actin into bundles with uniform polarity. Planta 209: 264–266 [DOI] [PubMed] [Google Scholar]
- Yokota E, Takahara K, Shimmen T (1998) Actin-bundling protein isolated from pollen tubes of lily: biochemical and immunocytochemical characterization. Plant Physiol 116: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Vidali L, Tominaga M, Tahara H, Orii H, Morizane Y, Hepler PK, Shimmen T (2003) Plant 115-kDa actin-filament bundling protein, P-115-ABP, is a homologue of plant villin and is widely distributed in cells. Plant Cell Physiol 44: 1088–1099 [DOI] [PubMed] [Google Scholar]