Abstract
Background
Patients with prior invasive fungal infection (IFI) increasingly proceed to allogeneic hematopoietic cell transplantation (HSCT), however, little is known about the impact of prior IFI on survival.
Methods
Patients with pre-transplant IFI (cases; n=825) were compared to controls (n=10,247). A subset analysis assessed outcomes in leukemia patients pre- and post-2001.
Results
Cases were older with lower performance status (KPS), more advanced disease, higher likelihood of acute myeloid leukemia (AML), and having received cord blood, reduced intensity conditioning (RIC), mold-active fungal prophylaxis and more recently transplanted. Aspergillus spp. and Candida spp. were the most commonly identified pathogens. 68% of patients had primarily pulmonary involvement. Univariate and multivariable analysis demonstrated inferior progression-free (PFS) and overall (OS) survival for cases. At 2 years, cases had higher mortality and shorter PFS with significant increases in non-relapse mortality (NRM) but no difference in relapse. One year probability of post-HSCT IFI was 24% (cases) and 17% (control, p <0.001). The predominant cause of death was underlying malignancy; infectious death was higher in cases (13 vs 9%). In the subset analysis, patients transplanted before 2001 had increased NRM with inferior OS and PFS compared to later cases.
Conclusions
Pre-transplant IFI is associated with lower PFS and OS after allogeneic HSCT but significant survivorship was observed. Consequently, pre-transplant IFI should not be a contraindication to allogeneic HSCT in otherwise suitable candidates.
Introduction
Invasive fungal infections (IFI) historically portend a poor prognosis in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). An observational study of the Transplant Associated Infection Surveillance Network (TRANSNET) suggests that post-HSCT IFI remains problematic, with cumulative incidence rates varying between 5.8–7.7% in the allogeneic transplant setting1. Historically, prior mold infection was considered a relative contraindication to transplantation. Although the risk of reactivation of fungal infection in the setting of HSCT is high, HSCT may be the only remaining treatment option in the face of an otherwise fatal hematologic malignancy. With the advent of novel broader spectrum antifungal agents, prophylaxis and treatment of fungal infections have significantly improved. Likewise, use of granulocyte colony-stimulating growth factors has decreased the length of neutropenia, a major risk factor for IFI2– 7. Additionally, reduced intensity conditioning (RIC) transplantation may help to minimize opportunistic infections and to maximize graft-versus-tumor effects8, 9. These advances in HSCT and its associated supportive care have enabled patients to successfully receive a HSCT despite having previously documented fungal infection8, 10–12
To date, there are limited data from large multicenter cohorts comparing outcomes in patients who have undergone allogeneic HSCT with known prior yeast or mold infection to a matched cohort without previous IFI. Fukuda et al. reported the outcomes of 45 patients with pre-transplant invasive aspergillosis (IA) treated in the pre-mold active azole era and demonstrated that post-transplant recurrent IA was seen in 29% and that the cases had lower overall survival than controls. Notably, those patients with >1 month of antifungal therapy, received RIC or those that had resolution of radiographic disease findings had better outcomes11. Martino et al. reported a retrospective survey of 129 patients with a history of proven or probable IA of whom 44% had undergone RIC12. They observed a 22% post-transplant progression rate for IA at 2 years, which occurred more frequently in those patients undergoing conventional myeloablative HSCT, for patients with grade II–IV acute graft versus host disease (GVHD), for patients receiving bone marrow or cord blood allografts, and for patients with CMV disease. Most recently, a report from the EBMT analyzed the long term outcomes of pre-existing IA on transplant outcomes of patients with acute leukemia only13. Data were available from 1152 pts with a median follow-up of 52 months. There was no significant impact of the pre-existing IA on overall survival, relapse free survival, non-relapse mortality, acute or chronic GVHD, although there was a trend toward lower overall survival and higher non-relapse mortality13. Herein, we explore the CIBMTR data base on the influence of participating center documented preexisting IFI on clinical outcomes after HSCT and the risk factors associated with fungal disease progression post-transplant. We also examine the impact of changes in supportive care over the past decade versus earlier time periods to determine if the evolution of conditioning regimen and advances in supportive care translate into improved clinical outcomes in patients with pre-existing IFI undergoing HSCT.
Materials and Methods
Data Source and Patients
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is a research affiliate of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) established in 2004. It comprises a voluntary working group of more than 500 transplantation centers worldwide that contribute data on consecutive allogeneic and autologous HCT procedures to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, Minnesota. Participating centers report longitudinal data on all transplants and compliance is monitored by on-site audits. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Studies conducted by the CIBMTR are performed under guidance and review of the Institutional Review Board of the National Marrow Donor Program. Transplant essential data, collected for consented patients participating in CIBMTR data collection, include demographic, disease type and stage, survival, relapse, graft type, the presence of GVHD, and cause of death data. A subset of CIBMTR participants are selected for comprehensive research level data collection by weighted randomization.
Subject Eligibility
The primary analysis includes all adult and pediatric patients receiving a first allogeneic HSCT for acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), or myelodysplasia (MDS) between 2001 and 2009. Patients with severe aplastic anemia or lymphoma were excluded, recognizing the difference in natural history. The secondary analysis includes only patients with AML or ALL receiving a first allogeneic transplant following myeloablative conditioning between 1995 and 2009, comparing outcomes pre- and post-2001 due to improvements in supportive care and as a surrogate for emergence of novel antifungal agents with recognition that FDA approval for caspofungin and voriconazole was 2001 & 2002, respectively.
Cases
Patients reported to the CIBMTR as having a documented or suspected IFI in the 12 months prior to allogeneic transplantation were considered as cases14–16. This information is reported as an event but diagnostic criteria used to determine IFI are not captured. Data on the organism, if available, and the site of infection are reported by the transplant center. Patients for whom CIBMTR forms indicated the site of infection was oral cavity or genitalia only were excluded from analysis, recognizing that these situations likely represented mucosal disease or colonization rather than true infection. Patients with an IFI reported more than 12 months prior to transplant were excluded.
Controls
All patients reported as “No” IFI in the 12 months prior to transplant and who were transplanted at the same centers were considered as contemporary controls. Any patients without any response (missing data) to the question of pre-transplant IFI were excluded. This approach was instituted to provide balance regarding selection criteria for HSCT as well as minimize ascertainment bias of documented/suspected IFI rather than draw controls from centers that may have different selection criteria and thus present an unintended bias toward better risk patients.
Endpoints
Non-relapse mortality (NRM) was the primary outcome. Secondary outcomes included relapse, progression-free survival (PFS), overall survival (OS), acute GVHD, and chronic GVHD. Each outcome was analyzed in both the primary and secondary analysis. NRM was defined as death within the first 28 days of transplant for any cause or death without relapse or progression of the primary malignancy. NRM was assessed at Day 100, 1 year and 2 years after HSCT. Relapse was defined as the recurrence of malignancy from a remission state. OS was determined from the date of HSCT to the time of death or last follow-up. Death was considered a competing risk for assessment of cumulative incidence of relapse, acute and chronic GVHD. For PFS, patients were considered treatment failures at the time of relapse of underlying malignancy or death from any cause.
Statistical Analysis
Patient-, disease- and transplant-related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon test for continuous variables. The probabilities of PFS and OS were calculated using the Kaplan-Meier estimator. Probability estimates for other endpoints were generated using cumulative incidence functions to account for competing risks.
Multivariable analyses for outcomes at 2 years were performed using pseudo-value approach to adjust for other potentially significant covariates and determine if there is an interaction with the main effect variable of the presence or absence of a pre-transplant IFI 17, 18. Other risk factors include age, CMV serostatus, disease, disease stage, conditioning intensity, graft type, degree of HLA match, GVHD prophylaxis, peri-transplant T cell depletion and post-transplant use of growth factor. Interaction between the main effect and significant covariates were tested and reported to assess for differences between cases and controls. Multivariable models were built using a backward variable selection method. All P-values are 2-sided, the level of significance (alpha) of 0.05 was used throughout. All analyses were carried out using SAS Version 9.3 statistical software (SAS Institute, Cary, NC).
Results
Patient Characteristics
Patient, disease and transplant characteristics are presented in Table 1 for the primary analysis of the 825 patients with reported prior IFI transplanted between 2001 and 2009 and the 10,247 controls transplanted at the same center without reported prior IFI. The fungal pathogens that were identified as IFI included 199 Candida spp. infections, 281 Aspergillus spp. infections, and 50 “other” fungal infections, including Mucormycosis, Fusarium and Cryptococcus. Infections reported as “suspected” were identified in 295 patients and are included. Five hundred fifty-seven (68%) patients had pulmonary infections while 261 (32%) had only extra-pulmonary involvement. The median time from infection to transplant was approximately 3.5 months (range <1–12 months); thus, more than 50% of the patients experienced their IFI more than 100 days prior to HSCT. For both cases and controls, the time from diagnosis of hematologic malignancy to transplant were 7 (<1 – 310) and 8 (<1 – 607) months (p=0.277), respectively. Details of the status of the IFI at time of transplant were not available in the CIBMTR database. As expected, significant differences between the cases and the controls existed. Cases were older, more likely to have compromised performance status and more advanced acute leukemia, and to have received mold active secondary fungal prophylaxis. Cases were more likely to receive cord blood as a stem cell source, to receive reduced intensity/non-myeloablative conditioning regimens and transplants in more recent years, and to receive non-methotrexate-containing GVHD prophylaxis regimens19. No significant differences were found in donor/recipient CMV status, gender, use of growth factor support, and use of steroid-containing GVHD prophylaxis between the case and control cohorts.
Table 1.
Characteristics of patients who underwent allogeneic transplants for AML, ALL, CML, MDS and had Invasive fungal infection within 12 months prior to transplant vs. those who had no documented invasive fungal infection within 12 months prior to transplant, reported to the CIBMTR, from 2001 to 2009
| Variable | Pre-tx invasive fungal infection N (%) |
All others N (%) |
p_value |
|---|---|---|---|
| Patient related | |||
| Number of patients | 825 | 10247 | |
| Number of centers | 158 | 158 | |
| Age, median(range), years (age) | 44 (1 – 74) | 39 (<1 – 79) | <0.001 |
| Age at transplant, years | <0.001 | ||
| <=10 | 81 (10) | 1039 (10) | |
| 11–20 | 96 (12) | 1313 (13) | |
| 21–30 | 97 (12) | 1417 (14) | |
| 31–40 | 89 (11) | 1495 (15) | |
| 41–50 | 150 (18) | 1848 (18) | |
| >50 | 312 (38) | 3135 (31) | |
| Gender | 0.937 | ||
| Male | 465 (56) | 5761 (56) | |
| Female | 360 (44) | 4486 (44) | |
| Lansky/Karnofsky score at transplant | <0.001 | ||
| <90 | 282 (34) | 2507 (24) | |
| >=90 | 504 (61) | 7294 (71) | |
| Missing | 39 (5) | 446 (4) | |
| Disease-related | |||
| Disease | <0.001 | ||
| AML | 609 (74) | 5310 (52) | |
| ALL | 171 (21) | 2548 (25) | |
| CML | 22 (3) | 1578 (15) | |
| MDS | 23 (3) | 811 (8) | |
| Disease stage at transplant | <0.001 | ||
| AML/ALL/CML/MDS Early | 378 (46) | 5335 (52) | |
| AML/ALL/CML Intermediate | 218 (26) | 2701 (26) | |
| AML/ALL/CML/MDS Advanced | 229 (28) | 2211 (22) | |
| Time from Infection to transplant (months) | |||
| 0–2 | 211 (26) | ||
| 2–6 | 479 (58) | ||
| 6–12 | 135 (16) | ||
| Time from Infection to transplant, median(range), days | 105 (7 – 362) | ||
| Time from Infection to transplant | |||
| 0–29 days | 49 (6) | ||
| 30–59 days | 154 (19) | ||
| 60–99 days | 186 (23) | ||
| 100–179 days | 291 (35) | ||
| 180–365 days | 145 (18) | ||
| Type of fungal infection | |||
| Aspergillus | 281 (34) | ||
| Mucormycosis | 9 (1) | ||
| Other Mold infection | 19 (2) | ||
| Candida albicans | 56 (7) | ||
| Other Candida | 143 (17) | ||
| Suspected fungal infection | 295 (36) | ||
| Other | 22 (3) | ||
| Infection Location | |||
| Localized extrapulmonary | 261 (32) | ||
| Pulmonary | 557 (68) | ||
| Combine Pulmonary and extrapulmonary | 7 (<1) | ||
| Received antifungal prophylaxis | <0.001 | ||
| None | 108 (13) | 1555 (15) | |
| Amphotericin +/− others | 136 (16) | 885 (9) | |
| Fluconazole +/− others | 279 (34) | 5764 (56) | |
| Itraconazole +/− others | 54 (7) | 674 (7) | |
| Voriconazole +/− others | 175 (21) | 994 (10) | |
| Posiconazole +/− others | 23 (3) | 163 (2) | |
| Echinocandin +/− others | 50 (6) | 212 (2) | |
| Transplant-related | |||
| Donor/Recipient CMV status | 0.268 | ||
| +/+ | 303 (37) | 3652 (36) | |
| +/− | 81 (10) | 1041 (10) | |
| −/+ | 215 (26) | 2631 (26) | |
| −/− | 187 (23) | 2563 (25) | |
| Missing | 39 (5) | 360 (4) | |
| Donor/recipient gender match | 0.002 | ||
| Male-Male | 289 (35) | 3541 (35) | |
| Male-Female | 195 (24) | 2456 (24) | |
| Female-Male | 150 (18) | 2024 (20) | |
| Female-Female | 133 (16) | 1802 (18) | |
| Donor gender missing | 58 (7) | 424 (4) | |
| Donor/recipient HLA match | 0.003 | ||
| Cord blood | 124 (15) | 1196 (12) | |
| HLA-identical siblings | 356 (43) | 4340 (42) | |
| Other related | 34 (4) | 345 (3) | |
| Well matched unrelated | 180 (22) | 2722 (27) | |
| Partially matched unrelated | 87 (11) | 1149 (11) | |
| Mismatched unrelated | 19 (2) | 290 (3) | |
| Unrelated (HLA match information missing) | 25 (3) | 205 (2) | |
| GVHD prophylaxis | <0.001 | ||
| t-cell depletion | 47 (6) | 601 (6) | |
| FK506+MTX +/− other | 222 (27) | 2969 (29) | |
| FK506 +/− other | 98 (12) | 1289 (13) | |
| MTX+CsA +/− other | 252 (31) | 3589 (35) | |
| CsA +/− other | 206 (25) | 1799 (18) | |
| Conditioning regimen | <0.001 | ||
| Myeloablative | 506 (61) | 7276 (71) | |
| Non-myeloablative/RIC | 319 (39) | 2971 (29) | |
| Lymphocyte depleting agents (ATG/alemtuzumab as conditioning or GVHD prophylaxis) | 0.034 | ||
| ATG alone | 270 (33) | 2920 (28) | |
| Alemtuzumab alone | 31 (4) | 437 (4) | |
| No ATG or Alemtuzumab | 524 (64) | 6890 (67) | |
| G-CSF, GM-CSF | 0.512 | ||
| No | 466 (56) | 5667 (55) | |
| Yes | 359 (44) | 4580 (45) | |
| Steroid containing GVHD prophylaxis | 0.703 | ||
| No | 790 (96) | 9840 (96) | |
| Yes | 35 (4) | 407 (4) | |
| Graft type | 0.005 | ||
| Bone Marrow | 193 (23) | 2752 (27) | |
| Peripheral blood | 508 (62) | 6299 (61) | |
| Cord blood | 124 (15) | 1196 (12) | |
| Year of transplant | <0.001 | ||
| 2001–2002 | 146 (18) | 1969 (19) | |
| 2003–2004 | 123 (15) | 1972 (19) | |
| 2005–2006 | 174 (21) | 2571 (25) | |
| 2007–2008 | 236 (29) | 2546 (25) | |
| 2009 | 146 (18) | 1189 (12) | |
| Median follow-up of survivors, months | 37 (2 – 120) | 48 (1 – 128) | |
Abbreviations: AML = acute myelogenous leukemia; ALL = acute lymphoblastic leukemia; CML = chronic myelogenous leukemia; GVHD = graft versus host disease; MTX = methotrexate; CsA = cyclosporine; FK506 = tacrolimus; BM = bone marrow; PB = peripheral blood; CB = cord blood; TBI = total body irradiation.
Other mould infections included: Fusarium species and Cryptococcus species
Other infections included: Histoplasmosis and other fungus
Survival, Non-relapse Mortality and Relapse Outcomes
Univariate probabilities of outcomes of interest after HSCT between subjects with pre-existing IFI and those without IFI are summarized in Table 2. In nearly all outcomes measured, statistical differences between the cases and the controls at 1, 3, and 5 years after HSCT were noted. There were no differences in univariate transplant outcomes for OS, TRM, DFS, RR, aGVHD or cGVHD (data not shown) when comparing Aspergillus spp. cases, Candida spp. cases, Other fungal cases, and Suspected IFI so all pre-transplant IFIs were combined for the univariate analysis. OS was 30% (95% Confidence interval (CI): 26 – 34%) at 5 years in patients with pre-transplant IFI versus 45% (95% CI: 44 – 46%) in the control population (p < 0.0001) (Figure 1). The lower OS was a composite reflection of higher relapse rates and higher NRM in the cases. Interestingly, acute GVHD by 100 days and chronic GVHD at 1, 3, and 5 years were statistically less frequent in the patients with reported pre-existing IFI (aGVHD: cases 34%, controls 39%, p=0.0022; cGVHD at 5 years: cases 36%, controls 45%, p <0.0001) (Table 2). Additionally, reported post-transplant IFI occurred more commonly in the cases versus controls at 3 months, 6 months, and 1 year after HSCT. Only 144 (17%) patients with pre-HSCT IFI were subsequently reported as developing a post-transplant IFI; of these, 46 (32%) experienced relapse with the previously identified fungal pathogen, while 97 (67%) patients had fungal pathogens different from the original pathogen reported. One patient had an unidentified fungus reported.
Table 2.
Univariate outcomes of patients who underwent allogeneic transplants for AML, ALL, CML, MDS and had Invasive fungal infection within 12 months prior to transplant vs. those who had no documented invasive fungal infection within 12 months prior to transplant, reported to the CIBMTR, from 2001 to 2009
| Outcomes | Pre-tx invasive fungal infection | All others | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| N Eval | Probability (95% CI) | N Eval | Probability (95% CI) | ||
| Overall survival from transplant | 825 | 10247 | |||
| @ 1 year | 54 (48–55)% | 65(64–66) % | <.0001 | ||
| @ 3 years | 35 (31–38)% | 50 (49–51)% | <.0001 | ||
| @ 5 years | 30 (26–34)% | 45 (44–46)% | <.0001 | ||
| Relapse | 813 | 10146 | |||
| @ 1 year | 33 (29–36) % | 26(25–27) % | 0.0002 | ||
| @ 3 years | 42 (39–46) % | 35 (34–36)% | <.0001 | ||
| @ 5 years | 45 (41–49) % | 39 (38–40)% | 0.0018 | ||
| Non-relapse mortality | 813 | 10146 | |||
| @ 1 year | 23 (20–26) % | 17(17–18) % | 0.0003 | ||
| @ 3 years | 27 (24–30) % | 22 (21–23)% | 0.0046 | ||
| @ 5 years | 29 (26–32) % | 25 (24–26)% | 0.0347 | ||
| Disease free survival | 813 | 10146 | |||
| @ 1 year | 45 (41–48) % | 57(56–58) % | <.0001 | ||
| @ 3 years | 31(28–34) % | 44 (43–45)% | <.0001 | ||
| @ 5 years | 27(23–31) % | 37 (36–38)% | <.0001 | ||
| Acute GVHD (grade 2–4) | 822 | 10224 | |||
| @ 100 days | 34 (31–37) % | 39(38–40) % | 0.0022 | ||
| Chronic GVHD | 810 | 10098 | |||
| @ 1 year | 31 (28–34) % | 39(38–40) % | <.0001 | ||
| @ 3 years | 35 (32–38) % | 44 (43–45)% | <.0001 | ||
| @ 5 years | 36 (32–39) % | 45 (44–46)% | <.0001 | ||
| Fungal infection within 1 year post TX | 824 | 10168 | |||
| @ 100 days | 17 (15–20) % | 11(10–11) % | <.0001 | ||
| @ 6 months | 21 (19–24) % | 14 (13–14)% | <.0001 | ||
| @ 1 years | 24(21–27) % | 17 (16–18)% | <.0001 | ||
Figure 1.
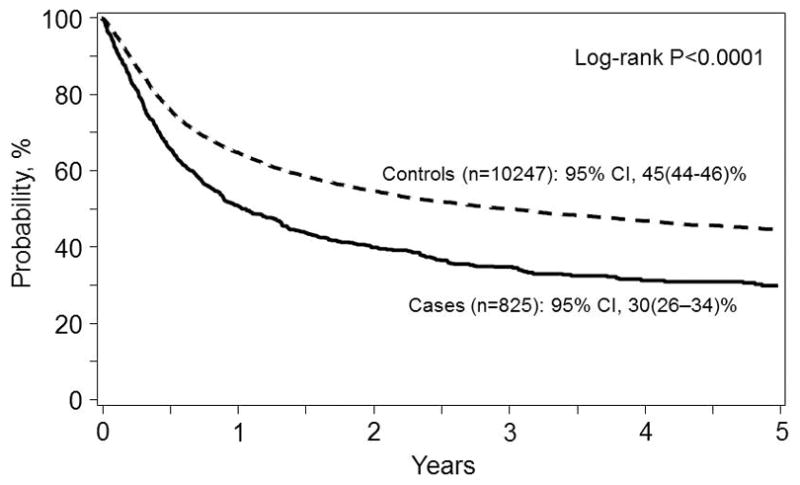
Overall survival from the time of transplant for patients with (Cases, solid line) and without (Controls, dashed line) an invasive fungal infection in the 12 months prior to allogeneic transplantation. The point-wise comparison at 5 years is shown.
As shown in Table 3, on multivariable analysis, cases with pre-transplant IFI had higher overall mortality [Relative Risk {RR} 1.33, 95%CI (1.19 – 1.48), p <0.0001] and shorter PFS at 2 years [RR of relapse or death 1.24, 95%CI (1.11 – 1.38), p <0.0001] with significant increases in NRM [RR 1.27 (1.09 – 1.49), p = 0.002] compared to the control cohort (Figure 2). Relapse rates were not significantly increased [RR=1.04, 95%CI (0.91 – 1.18), p = 0.58]. The risk of being diagnosed with aGVHD by day 100 was similar between the cases and controls [RR 0.9, 95%CI (0.80 – 1.02), p = 0.09] with lower risk of cGVHD [RR 0.81, 95%CI (0.71 – 0.93), p = 0.002] identified in the cases. Additionally, when comparing cases with the control patients at one year, there was a greater likelihood of experiencing a post-transplant IFI [RR 1.36, 95%CI (1.16 – 1.58), p = 0.001].
Table 3.
Multivariable analysis of patients who underwent allogeneic transplants for AML, ALL, CML, MDS and had Invasive fungal infection within 12 months prior to transplant vs. those who had no documented invasive fungal infection within 12 months prior to transplant, reported to the CIBMTR, from 2001 to 2009
| Variable | 2 yr OS RR (95% CI) | p | 2 yr PFS RR (95% CI) | p | 2 yr NRM RR (95% CI) | p | 2 yr Relapse RR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Main Effect | <0.0001 | <0.0001 | 0.002 | NS | ||||
| Control | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Pre-HSCT IFI | 1.33 (1.19 – 1.48) | 1.24 (1.11 – 1.38) | 1.27 (1.09 – 1.49) | 1.04 (0.91 – 1.18) | ||||
|
| ||||||||
| Age, years | <0.0001 | <0.0001 | <0.0001 | NS | ||||
| ≤ 10 | 1.00 | 1.00 | 1.00 | |||||
| 11 – 20 | 1.21 (1.06 – 1.39) | 0.0051 | 1.21 (1.06 – 1.37) | 0.0039 | 1.75 (1.41 – 2.17) | <0.0001 | ||
| 21 – 30 | 1.28 (1.12 – 1.48) | 0.0004 | 1.33 (1.17 – 1.51) | <0.0001 | 1.79 (1.43 – 2.24) | <0.0001 | ||
| 31 – 40 | 1.47 (1.28 – 1.69) | <0.0001 | 1.44 (1.27 – 1.65) | <0.0001 | 2.32 (1.86 – 2.89) | <0.0001 | ||
| 41 – 50 | 1.58 (1.38 – 1.81) | <0.0001 | 1.56 (1.37 – 1.77) | <0.0001 | 2.48 (1.99 – 3.08) | <0.0001 | ||
| > 50 | 1.81 (1.59 – 2.06) | <0.0001 | 1.70 (1.50 – 1.93) | <0.0001 | 2.86 (2.30 – 3.55) | <0.0001 | ||
|
| ||||||||
| Ex vivo TCD | 0.0008 | <0.0001 | NS | <0.0001 | ||||
| None | 1.00 | 1.00 | 1.00 | |||||
| ATG | 1.09 (1.02 – 1.18) | 0.0128 | 1.37 (1.06 – 1.22) | 0.0002 | 1.12 (1.03 – 1.22) | 0.0109 | ||
| Alemtuzumab | 1.28 (1.11 – 1.48) | 0.0006 | 1.58 (1.37 – 1.81) | <0.0001 | 1.71 (1.46 – 2.01) | <0.0001 | ||
|
| ||||||||
| D/R CMV status | <0.0001 | 0.0003 | 0.0136 | 0.0295 | ||||
| Neg/Neg | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Pos/Pos | 1.19 (1.10 – 1.29) | <0.0001 | 1.16 (1.07 – 1.25) | 0.0002 | 1.16 (1.02 – 1.31) | 0.0206 | 1.10 (1.00 – 1.21) | 0.0540 |
| Pos/Neg | 1.12 (1.00 – 1.26) | 0.0459 | 1.07 (0.96 – 1.20) | 0.1948 | 1.17 (0.99 – 1.38) | 0.0614 | 1.00 (0.87 – 1.15) | 0.9923 |
| Neg/Pos | 1.21 (1.10 – 1.32) | <0.0001 | 1.19 (1.10 – 1.29) | <0.0001 | 1.17 (1.03 – 1.33) | 0.0127 | 1.14 (1.03 – 1.26) | 0.0113 |
| Missing | 1.20 (1.12 – 1.41) | 0.0319 | 1.15 (0.98 – 1.35) | 0.0873 | 1.44 (1.15 – 1.80) | 0.0015 | 0.93 (0.75 – 1.14) | 0.4886 |
|
| ||||||||
| Disease | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| AML | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ALL | 1.10 (1.02 – 1.19) | 0.0119 | 1.17 (1.08 – 1.26) | <0.0001 | 1.36 (1.21 – 1.53) | <0.0001 | 0.95 (0.87 – 1.04) | 0.2955 |
| CML | 0.84 (0.78 – 0.93) | 0.0004 | 1.02 (0.93 – 1.11) | 0.6934 | 1.32 (1.15 – 1.50) | <0.0001 | 0.82 (0.73 – 0.92) | 0.0006 |
| MDS | 0.64 (0.56 – 0.73) | <0.0001 | 0.67 (0.59 – 0.75) | <0.0001 | 1.02 (0.85 – 1.23) | 0.8239 | 0.60 (0.52 – 0.69) | <0.0001 |
|
| ||||||||
| Disease Stage | <0.0001 | <0.0001 | 0.0286 | <0.0001 | ||||
| Early | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Intermediate | 1.27 (1.18 – 1.36) | <0.0001 | 1.24 (1.15 – 1.33) | <0.0001 | 1.15 (1.03 – 1.28) | 0.0094 | 1.24 (1.14 – 1.36) | <0.0001 |
| Advanced | 2.24 (2.01 – 2.42) | <0.0001 | 2.13 (1.98 – 2.30) | <0.0001 | 1.10 (0.97 – 12.3) | 0.1289 | 2.33 (2.13 – 2.54) | <0.0001 |
|
| ||||||||
| Donor | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| HLA ID-sib | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Other related | 1.64 (1.38 – 1.93) | <0.0001 | 1.47 (1.25 – 1.72) | <0.0001 | 1.82 (1.47 – 2.27) | <0.0001 | 0.93 (0.77 – 1.13) | 0.4968 |
| Cord | 1.32 (1.18 – 1.47) | <0.0001 | 1.21 (1.09 – 1.35) | 0.0004 | 2.01 (1.70 – 2.38) | <0.0001 | 0.77 (0.67 – 0.89) | 0.0004 |
| WM unrelated | 0.88 (0.81 – 0.95) | 0.0015 | 0.87 (0.80 – 0.93) | 0.0002 | 1.11 (0.98 – 1.25) | 0.0870 | 0.76 (0.69 – 0.84) | <0.0001 |
| Other unrelated | 1.14 (1.04 – 1.25) | 0.0038 | 1.04 (0.95 – 1.13) | 0.3625 | 1.78 (1.56 – 2.01) | <0.0001 | 0.66 (0.59 – 0.74) | <0.0001 |
|
| ||||||||
| Karnosky PS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| ≥ 90% | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| < 90% | 1.38 (1.29 – 1.47) | <0.0001 | 1.32 (1.24 – 1.41) | <0.0001 | 1.26 (1.14 – 1.39) | <0.0001 | 1.21 (1.12 – 1.31) | <0.0001 |
| Missing | 1.27 (1.04 – 1.40) | 0.0114 | 1.14 (1.00 – 1.31) | 0.0588 | 1.15 (0.94 – 1.42) | 0.1807 | 1.12 (0.95 – 1.33) | 0.1746 |
|
| ||||||||
| Conditioning | NS | <0.0001 | <0.0001 | <0.0001 | ||||
| Myeloablative | 1.00 | 1.00 | 1.00 | |||||
| RIC/NMA | 1.15 (1.07 – 1.23) | 0.79 (0.71 – 0.88) | 1.36 (1.25 – 1.47) | |||||
Figure 2.
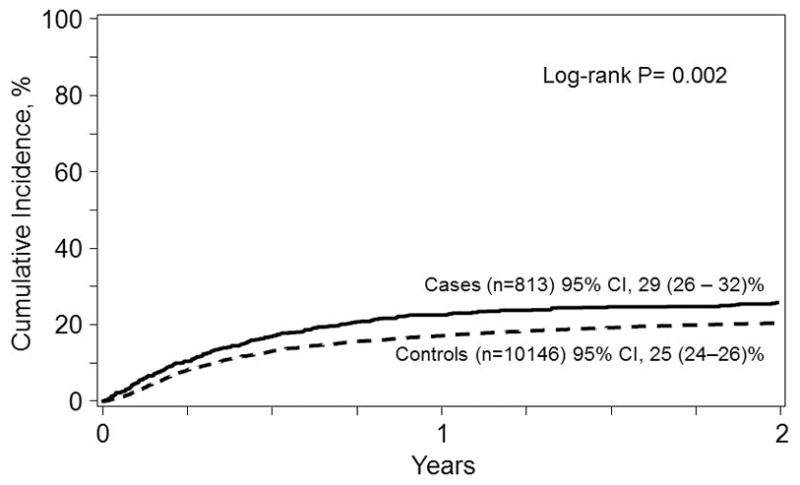
Non-relapse mortality from the time of transplant for patients with (Cases, solid line) and without (Controls, dashed line) an invasive fungal infection in the 12 months prior to allogeneic transplantation. The point-wise comparison at 5 years is shown.
Other factors negatively impacting OS and PFS include older age by decade, receipt of ATG or alemtuzumab, recipient CMV serostatus positive regardless of donor CMV serostatus, AML or ALL, more advanced disease, receiving cord blood or other related donor, and performance status <90%. OS, but not PFS, was decreased in patients receiving less than a well matched unrelated donor. PFS but not OS was improved if the patient received myeloablative conditioning. Non-relapse mortality was increased in older patients, ALL, CML, intermediate but not advanced disease status, use of either alternative related, mismatched unrelated, or cord blood as the stem cells source, and lower performance status. At 2 years, the NRM was significantly better for patients receiving RIC/NMA conditioning. Finally, the use of ATG or alemtuzumab, advanced disease stage, and RIC/NMA conditioning increased the relative risk of relapse.
The relative risk for a fungal infection of any kind at 1 year post transplant was enhanced by the presence of pre-transplant IFI compared with controls [RR 1.35, 95%CI (1.16 – 1.58)]. Other risk factors associated with increased risk of fungal infection at 1 year post-transplant include older age, receipt of alemtuzumab [RR 1.51, 95%CI (1.24 – 1.85)] or ATG exposure [RR 1.16, 95%CI (1.04 – 1.29], advanced malignancy [RR 1.45, 95%CI (1.29–1.63)] and cord blood [RR 1.48, 95%CI (1.24 – 1.75)] or mismatched related donor [RR 1.43, 95%CI (1.13 – 1.79)). For the cases, there was no impact on OS, PFS, NRM, and GVHD based on the type of pre-transplant IFI, whether yeast or mold (data not shown).
Cause of Death
There were no differences between cause of death between patients with pre-transplant IFI’s and controls (see Table 4). Recurrent disease was the most likely cause of death in both cohorts. Organ failure, GVHD, and infections comprised the majority of other etiologies, with rare events including graft failure, secondary malignancy, bleeding, and idiopathic pneumonitis syndrome.
Table 4.
Cause of death of patients who underwent allogeneic transplants for AML, ALL, CML, MDS and had IFI within 12 months prior to HSCT vs. those who had no documented IFI within 12 months prior to HSCT, reported to the CIBMTR, from 2001 to 2009
| Variable | Pre-tx invasive fungal infection N(%) |
All others N(%) |
|---|---|---|
| Cause of death | ||
| Graft rejection | 2 (<1) | 63 (1) |
| Infection | 107 (21) | 919 (18) |
| IPN | 7 (1) | 93 (2) |
| Organ Failure | 76 (15) | 733 (14) |
| GVHD | 46 (9) | 585 (11) |
| Recurrent/Persistent Disease | 242 (47) | 2173 (42) |
| Secondary malignancy | 6 (1) | 52 (1) |
| Hemorrhage | 13 (3) | 131 (3) |
| other cause | 15 (3) | 288 (6) |
| Unknown | 5 (<1) | 77 (2) |
Secondary analysis
A secondary analysis was performed to determine the influence of era of diagnosis of IFI, acknowledging the impact of advances in antifungal therapy and supportive care in more recent years. This analysis was restricted to patients receiving myeloablative allogeneic transplantation for acute myeloid or lymphoid leukemia, and reflected the interval between 1995 and 2009 (suppl Table 1).
For subjects with pre-transplant IFI, the median age was lower in the earlier compared to the later time period, but again patients were more likely to have advanced disease and myeloid leukemia. Amphotericin products were most commonly used as the antifungal prophylaxis agent of choice, either solely or in conjunction with other agents, in the earlier years. No differences were seen in GVHD prophylaxis regimens containing steroids, ATG or alemtuzumab.
Similar to observations made from the primary analysis, within the secondary analysis, univariate and adjusted probabilities for overall mortality, NRM, and IFI developing within one year post-transplant were higher for patients with pre-transplant IFI versus the control cohort. PFS was also worse among patients with diagnosed pre-HSCT IFI. Interestingly, when outcomes of study subjects with pre-transplant IFI within the time frame 1995–2000 were compared to a more recent patient population who underwent myeloablative conditioning for AML and ALL performed between 2001 and 2009, improved OS (Figure 3) was noted in the modern era, which appear attributable to decreases in NRM (Figure 4).
Figure 3.
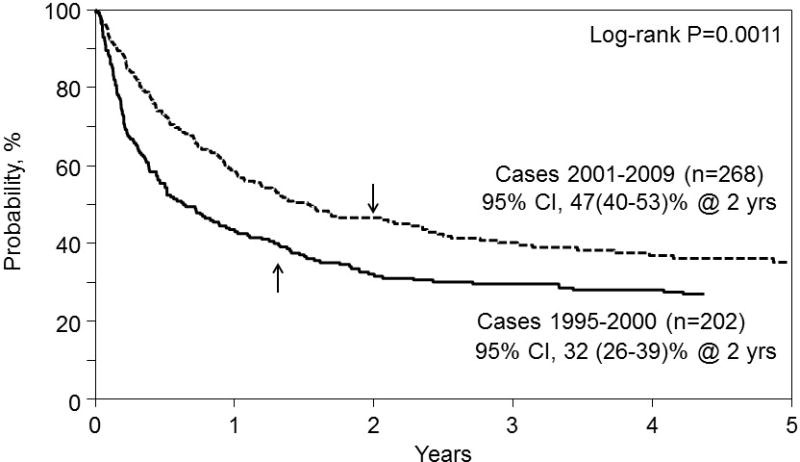
Overall survival from the time of transplant for AML and ALL patients receiving myeloablative conditioning with IFI transplanted between 1995 – 2000 (solid line) and between 2001 – 2009 (dashed line). The point-wise comparison at 5 years is shown.
Figure 4.
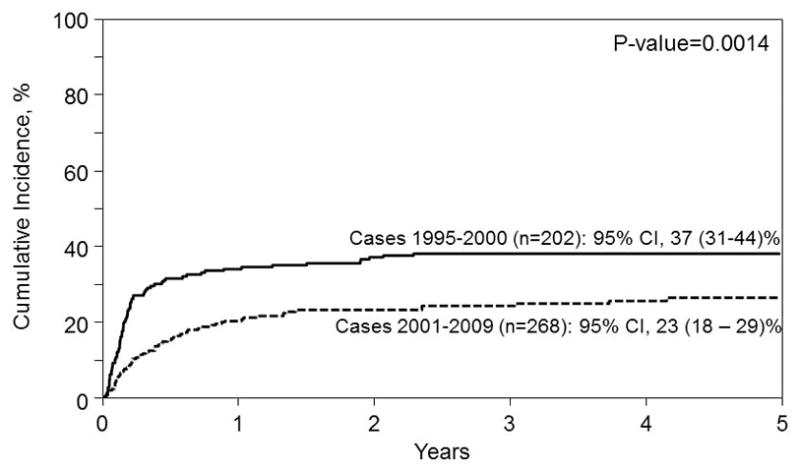
Non-relapse mortality from the time of transplant for AML and ALL patients receiving myeloablative conditioning with IFI transplanted between 1995 – 2000 (solid line) and between 2001 – 2009 (dashed line). The point-wise comparison at 5 years is shown.
Discussion
This retrospective CIBMTR analysis is performed on a large cohort of transplant patients reported to have documented or suspected pre-transplant IFI. The vast majority of these IFI were a consequence of infections with Candida spp. and Aspergillus spp., although other fungal infections were represented, including Fusarium and Mucormycosis. A similar analysis has recently been reported by the EBMT of a cohort of HSCT patients with acute leukemia, transplanted between 2005 and 2010, specifically with preexisting invasive Aspergillus spp. (IA) infections. Their analysis identified that excellent outcomes can be achieved despite the preexisting IA. Our data substantiate these observations13. Historically, before the emergence of mold-active echinocandins and azoles, many centers would not consider HSCT in patients with pre-existing IFI, particularly for those patients with antecedent mold infections. This current study demonstrates that positive outcomes can be obtained in these patients and that suspected or documented pre-transplant IFI by themselves, whether they are IA, candida or other identified mycoses, should not preclude patients from pursuing potentially curative transplant procedures for underlying malignant disease. Lower PFS and OS are seen in these patients; however, this augmented mortality was not due to the identified higher rates of recurrent fungal infection, but rather, influenced by disease status. This finding is not unexpected, as patients with fungal infections would be predicted to be more likely be transplanted later, to assure ample time for antifungal therapy to achieve response to treatment prior to transplantation (often defined by radiographic remissions11), to receive reduced intensity conditioning or to be electively transplanted only if disease is recurrent. The data reported within this analysis would suggest that delaying HSCT for patients with effectively treated pre-HSCT IFI may not be necessary and could actually contribute to worse outcomes due to disease recurrence.
The study also demonstrated that progress has been made in treating IFI with improved outcomes in the more modern era20. Our secondary analysis could not demonstrate any major change in any outcome in the amphotericin era between patients with pre-transplant IFI’s and those without when compared to the more recent expanded-spectrum azole/echinocandin era. This secondary analysis was performed as a surrogate for changes in antifungal prophylaxis since the data reported to the CIBMTR for antifungal prophylaxis collects antifungal drug received but not dose, sequence of agents or length of schedule. Notwithstanding these limitations, as a whole, patients transplanted in more recent years were more likely to survive the HSCT experience 21,22. A higher risk of death for patients with pre-transplant IFI was noted in all years, but this has recently diminished due to a more global reduction in NRM. Interestingly, in the primary analysis we found that 83% (n = 681) of the subjects with documented or suspected pre-transplant IFI were not reported to develop post-transplant IFI’s. One hundred forty-three (17%) patients were diagnosed with post-transplant IFI, similar to other reported fungal infection incidence for patients without prior history1. Interestingly, only one third of those patients who developed a post-transplant IFI actually reactivated their prior IFI, a lower incidence than reported previously 11 and actually could suggest that these individuals are possibly predisposed to developing IFI 23.
We do not have information about how many and which patients with pre-transplant IFI from the selected centers did not proceed to transplant. When one reviews the patient, disease and transplant variables, there are clues that indicate that these patients were indeed a selected population. They were more likely to have been treated with more aggressive antifungal prophylaxis and more likely to have been treated with reduced intensity/non-myeloablative transplant procedures. Interestingly, in the more recent patient population, there were less extra pulmonary fungal infections reported prior to transplant, possibly suggesting that new diagnostic approaches and improvements of radiologic detection may have contributed to better pre-transplant fungal detection followed by early therapy 24.
Unfortunately, we do not have documentation of the extent of the pre-transplant infection or the degree of infection control of the reported pre-transplant IFI. The patients who proceeded to transplant were more likely to have more advanced disease and lower performance status, but these observations might suggest that they were more deeply treated with chemotherapy before taking them to transplant. Additionally, it is curious to see that after transplant, there were lower rates of GVHD reported in the cases. There was a higher likelihood of utilizing RIT in the cases. One could also hypothesize that awareness of the pre-existing IFI influenced the treatment team to less aggressively taper calcineurin inhibitor immune suppression to avoid GVHD and subsequent use of high dose corticosteroid exposure, an approach that may not have been taken otherwise in patients with advanced disease. Alternatively, more frequent use of voriconazole in the cases may have led to higher levels of calcineurin inhibitors owing to drug-drug interactions, which may explain the lower rates of GVHD. Another possibility relates to recent observations that treatment of resistant candida has been augmented by adjunct therapy with calcineurin inhibitors 25,26. These hypotheses cannot be tested, as information regarding the decision-making of the transplant teams on management in these cases is not available, as is often the case in retrospective registry studies.
There are limitations to this study. Collected CIBMTR data do not provide pre-or post-HSCT detailed antifungal prophylaxis schedules. We are limited by the data included within the CIBMTR forms; we do not have information regarding dose, duration, possible sequence of treatment nor do we have availability of drug levels. There are ongoing updates of the data collection forms, but the forms’ evolution will not impact the data previously collected. Additionally, there is a dearth of immune reconstitution data that accompanies these analyses. Also, there are variations in how centers may report “suspected” IFI. We would anticipate that many of the academic centers contributing data to the CIBMTR would report “suspected” IFI for patients with “probable” IFI as defined according to established EORTC or other similar guidelines27. Reporting for this study is entered within the category defined as “suspected” IFI within the CIBMTR data collection fields but the congruence between the two definitions is not confirmed. Reporting is up to the transplant leaders at the transplant centers. Hopefully, as more detailed tools evolve to dissect mild, moderate and severe infections with their impact on the host, such as defined within the Manual of Procedures of the Blood & Marrow Transplant Clinical Trials Network, a greater understanding will emerge which should positively impact the transplant recipient28, 29. Ultimately, definitive conclusions of the impact on pre-existing IFI can only be drawn from prospective studies, but with the emergence of better antifungal prophylaxis agents, the patient numbers to perform such studies may not be present.
Overall, 30% long-term overall survival was achieved in patients with perceived life-threatening pre-transplant IFI and most commonly with advanced disease at time of transplant. Additionally, the risk of post-transplant fungal disease re-emergence was low, only recorded at 6% in this retrospective study. Forewarned is forearmed, and with more active primary or secondary fungal prophylaxis for mold and resistant Candida species, transplantation should remain a high priority consideration, rather than a contraindication, for patients with hematologic malignancies and prior IFI. However, these data still demonstrate that lower outcomes are seen in these patients with pre-transplant IFIs and further analysis is required to determine if we can identify those factors that impact survival such as the presence of yeast versus mold infections, the impact of complete or partial radiographic resolution at the time of transplantation, and whether or not, longer-term utilization of post-transplant antifungal therapies could improve survival.
Supplementary Material
Key points.
Documented pre-transplant IFI is associated with lower PFS and OS after allogeneic HSCT. However, mortality post-transplant is more influenced by advanced disease status than previous IFI. Pre-transplant IFI does not appear to be a contraindication to allogeneic HSCT
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from * Actinium Pharmaceuticals; Allos Therapeutics, Inc.; * Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; * Blue Cross and Blue Shield Association; * Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; * Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;* Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; * Milliman USA, Inc.; * Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; * Remedy Informatics; * Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; * Tarix Pharmaceuticals; * TerumoBCT; * Teva Neuroscience, Inc.; * THERAKOS, Inc.; University of Minnesota; University of Utah; and * Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
Conflict of Interest: The authors have no conflicts of interest to declare.
Bibliography
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant associated infection surveillance network (TRANSNET) database. Clinical Infectious Diseases. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2001 May;139:519–531. doi: 10.1111/j.1365-2141.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayes-Lattin B, Maziarz RT. Update in the Epidemiology, Prophylaxis, and Treatment of Fungal Infections in Patients with Hematologic Disorders. Leukemia & Lymphoma. 2004 Apr;45(4):669–80. doi: 10.1080/10428190310001625719. [DOI] [PubMed] [Google Scholar]
- 4.Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007 Sep 18;147(6):400–11. doi: 10.7326/0003-4819-147-6-200709180-00010. [DOI] [PubMed] [Google Scholar]
- 5.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood and Marrow Transplant Clinical Trials Network. Blood. 2010 Dec 9;116(24):5111–8. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002 Aug;347(6):408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 7.Marr KA, Bow E, Chiller T, Maschmeyer G, Ribaud P, Segal B, et al. Fungal infection prevention after hematopoietic cell transplantation. Bone Marrow Transplantation. 2009;44:483–487. doi: 10.1038/bmt.2009.259. [DOI] [PubMed] [Google Scholar]
- 8.Hermann S, Klein SA, Jacobi V, Thalhammer A, Bialleck H, Duchscherer M, et al. Older patients with high-risk fungal infections can be successfully allografted using non-myeloablative conditioning in combination with intensified supportive care regimens. Br J Haematol. 2001 May;113(2):446–54. doi: 10.1046/j.1365-2141.2001.02747.x. [DOI] [PubMed] [Google Scholar]
- 9.Bachanova V, Brunstein CG, Burns LJ, Miller JS, Luo X, Defor T, et al. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant. 2009 Feb;43(3):237–44. doi: 10.1038/bmt.2008.313. [DOI] [PubMed] [Google Scholar]
- 10.Aki ZS, Sucak GT, Yeğin ZA, Guzel O, Erbas G, Senol E. Hematopoietic stem cell transplantation in patients with active fungal infection: not a contraindication for transplantation. Transplant Proc. 2008 Jun;40(5):1579–85. doi: 10.1016/j.transproceed.2008.03.149. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda T, Boeckh M, Guthrie KA, Mattson DK, Owens S, Wald A, et al. Invasive aspergillosis before allogeneic hematopoietic stem cell transplantation: 10-year experience at a single transplant center. Biol Blood Marrow Transplant. 2004 Jul;10(7):494–503. doi: 10.1016/j.bbmt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Martino R, Parody R, Fukuda T, Maertens J, Theunissen K, Ho A, et al. Impact of the intensity of the pretransplantation conditioning regimen in patients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: A retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2006 Nov 1;108(9):2928–36. doi: 10.1182/blood-2006-03-008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penack O, Tridello G, Hoek J, Socie G, Blaise D, Passweg J, et al. Influence of pre-existing invasive aspergillosis on allo-HSCT outcome: a retrospective EBMT analysis by the Infectious Diseases and Acute Leukemia Working Parties. Bone Marrow Transplantation. doi: 10.1038/bmt.2015.237. advance online publication: 26 October 2015. [DOI] [PubMed] [Google Scholar]
- 14.Vaidya SJ, Ortín M, López-Duarte M, Sirsohi B, Powles R, Treleaven J, et al. Haemopoietic progenitor cell transplantation in patients with previous history of invasive fungal infection. Leuk Lymphoma. 2005 Aug;46(8):1143–50. doi: 10.1080/10428190500097052. [DOI] [PubMed] [Google Scholar]
- 15.Girmenia C, Raiola AM, Piciocchi A, Stanzani M, Cudillo L, Pecoraro C, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) BBMT. 2014;20:872–880. doi: 10.1016/j.bbmt.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Girmenia D, Barosi G, Piciocchi A, Arcese W, Aversa F, Bacigalupo A, et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by the Gruppo Italiano Trapianto Midollo Osseo (GITMO) BBMT. 2014;20:1080–1088. doi: 10.1016/j.bbmt.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PK, Klein JP, Rosthøj S. Generalized linear models for correlated pseudo-observations with applications to multi-state models. Biometrika. 2003;90:15–27. [Google Scholar]
- 18.Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudo-values of the cumulative incidence function. Biometrics. 2005;61:223–229. doi: 10.1111/j.0006-341X.2005.031209.x. [DOI] [PubMed] [Google Scholar]
- 19.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;12:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007 Jan 5;356(4):335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 21.Hahn T, McCarthy PL, Jr, Hassebroek A, Bredeson C, Gajewski J, Hale G, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013 Jul 1;31(19):2437–49. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010 Nov 25;363(22):2091–101.23. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak CC, Fisher BT, Sung L, Steinbach WJ, Nieder M, Alexander S, et al. Antifungal prophylaxis in pediatric hematology/oncology: new choices & new data. Pediat Blood Cancer. 2012;59:21–26. doi: 10.1002/pbc.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norkin M, Wingard JR. Diagnostic strategies for invasive fungal infections in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. JNCCN. 2013;11:941–949. doi: 10.6004/jnccn.2013.0115. [DOI] [PubMed] [Google Scholar]
- 25.de Cordeiro RA, de Macedo RB, Teixeira CE, Marques FJ, de Bandeira TJ, Moreira JL, et al. The calcineurin inhibitor cyclosporin A exhibits synergism with antifungals against Candida parapsilosis species complex. J Med Microbiol. 2014 Jul;63(Pt 7):936–44. doi: 10.1099/jmm.0.073478-0. Epub 2014 Apr 10. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Chen Z, Zhang C, Gao Y, Zhang X, Sun S. Resistance reversal induced by a combination of fluconazole and tacrolimus (FK506) in Candida glabrata. J Med Microbiol. 2015 Jan;64(Pt 1):44–52. doi: 10.1099/jmm.0.081760-0. Epub 2014 Oct 29. [DOI] [PubMed] [Google Scholar]
- 27.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) consensus group. Clin Inf Diseases. 2008 Jun 15;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J Center for International Blood and Marrow Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009 Oct;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://web.emmes.com/study/bmt2/public/MOP/BMT_CTN_Technical_MOP_ver_2.0.pdf,
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


