Significance
Activity and structural integrity of membrane proteins can be regulated by physical properties of the bilayer or specific lipid–protein interactions. This work shows that key properties of the Na,K-ATPase are modulated independently by specific binding of 18:0/18:1 phosphatidylserine (PS) and 18:0/20:4 phosphatidylcholine (PC) in the absence of a bilayer. PS stabilizes the protein, and PC/ phosphatidylethanolamine (PE) stimulates Na,K-ATPase activity. We characterized effects of both types of phospholipids by kinetic approaches, mutant analyses, and native MS. Modulation of Na,K-ATPase function by PS and PC/PE is defined by the phospholipid structural specificity, binding stoichiometry within two specific binding sites, and the kinetic mechanism. We provide detailed mechanistic insights, potentially with important implications for physiological regulation of active Na and K transport.
Keywords: Na,K-ATPase; specific protein–lipid interaction; protein stability; Na,K-ATPase activity; native mass spectrometry
Abstract
Membrane protein function can be affected by the physical state of the lipid bilayer and specific lipid–protein interactions. For Na,K-ATPase, bilayer properties can modulate pump activity, and, as observed in crystal structures, several lipids are bound within the transmembrane domain. Furthermore, Na,K-ATPase activity depends on phosphatidylserine (PS) and cholesterol, which stabilize the protein, and polyunsaturated phosphatidylcholine (PC) or phosphatidylethanolamine (PE), known to stimulate Na,K-ATPase activity. Based on lipid structural specificity and kinetic mechanisms, specific interactions of both PS and PC/PE have been inferred. Nevertheless, specific binding sites have not been identified definitively. We address this question with native mass spectrometry (MS) and site-directed mutagenesis. Native MS shows directly that one molecule each of 18:0/18:1 PS and 18:0/20:4 PC can bind specifically to purified human Na,K-ATPase (α1β1). By replacing lysine residues at proposed phospholipid-binding sites with glutamines, the two sites have been identified. Mutations in the cytoplasmic αL8–9 loop destabilize the protein but do not affect Na,K-ATPase activity, whereas mutations in transmembrane helices (TM), αTM2 and αTM4, abolish the stimulation of activity by 18:0/20:4 PC but do not affect stability. When these data are linked to crystal structures, the underlying mechanism of PS and PC/PE effects emerges. PS (and cholesterol) bind between αTM 8, 9, 10, near the FXYD subunit, and maintain topological integrity of the labile C terminus of the α subunit (site A). PC/PE binds between αTM2, 4, 6, and 9 and accelerates the rate-limiting E1P–E2P conformational transition (site B). We discuss the potential physiological implications.
The biological membrane is a dynamic unit of proteins and lipids. The lipid part is composed of sterols, sphingolipids, and glycerophospholipids; variations in head group structure, acyl chain length, and degree of unsaturation lead to the formation of hundreds of types of lipids (1). This complexity is not required for bilayer formation but provides second messengers, allows recognition processes, and maintains the activity of membrane proteins, which may depend on specific lipids (2). The lipid composition of membranes determines the hydrophobic thickness, fluidity, curvature, and lateral pressure profiles (3). These bulk properties may affect membrane protein function in a nonstoichiometric manner and are referred to as “nonspecific protein–lipid interactions.” Lipids associated weakly with protein transmembrane segments are termed “annular lipids” (4), and although they have restricted motion, they can exchange quickly with bulk lipids. Site-specific lipid binding is characterized by longer residence times at sites formed between transmembrane helices or protein subunits. These lipids are stabilized by polar interactions between head groups and charged amino acids at the water–lipid interface and hydrophobic interactions between acyl chains and aromatic or hydrophobic side chains of amino acids in transmembrane segments (2). Lipids observed in membrane protein structures are often copurified from the membrane, having withstood detergent solubilization, thus indicating tight binding. Because the functional roles of these lipids, such as modulation of kinetic properties, usually cannot be inferred, they are often considered relevant only for the structural integrity of membrane proteins (5).
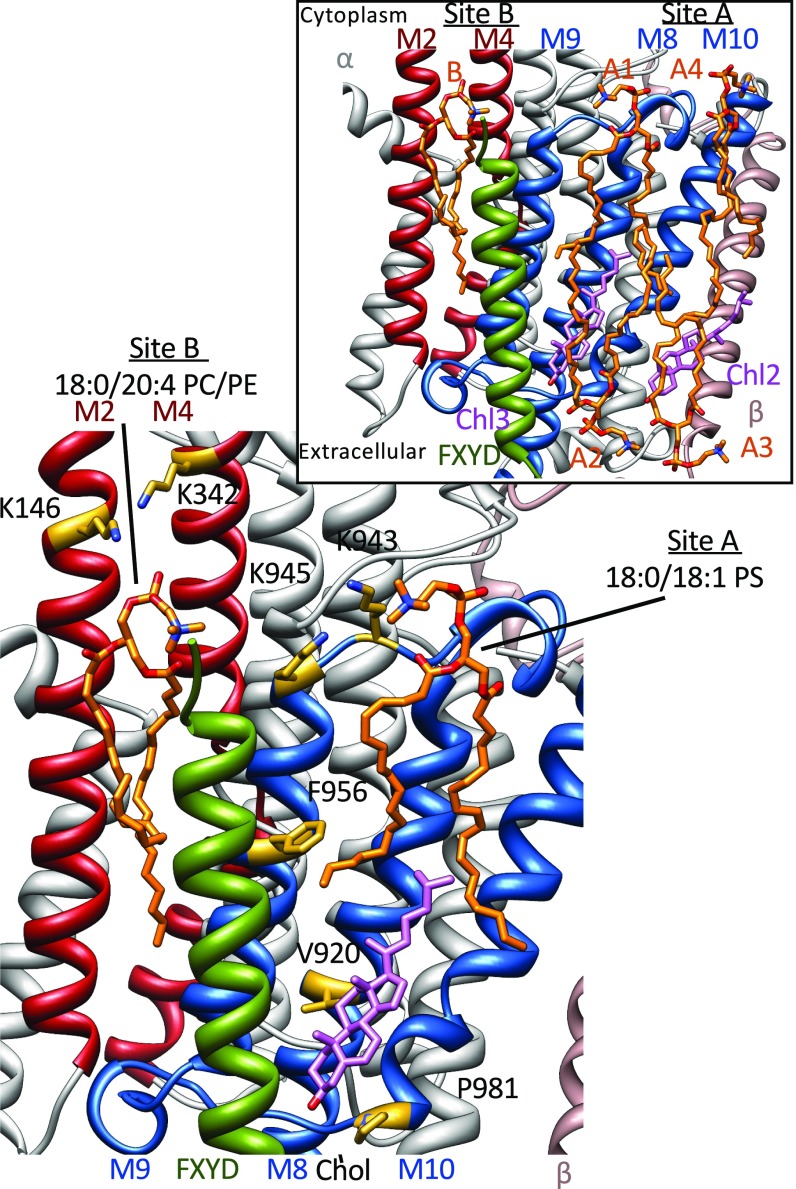
Na,K-ATPase is a primary active transporter of the P-type ATPase family that maintains the electrochemical gradients for Na and K in animal cells by exchanging three intracellular Na ions for two extracellular K ions (6). It has long been known that Na,K-ATPase activity depends on phosphatidylserine (PS) and cholesterol (7, 8), and recent biochemical studies suggest that both lipids interact specifically with Na,K-ATPase (9, 10). Furthermore, seven phospholipids and three cholesterol molecules have been identified in crystal structures at three different sites (referred to hereafter as sites “A,” “B,” and “C”) (Fig. 1, Inset) (11, 12), but their functional importance has remained elusive. Analysis of the effects of phospholipids and cholesterol on recombinant Na,K-ATPase purified in mixed detergent–lipid–protein micelles in the absence of a lipid bilayer allows the detection of specific lipid–protein interactions. For example, PS (optimally 18:0/18:1 PS) and cholesterol stabilize the Na,K-ATPase against thermal- and detergent-mediated inactivation (9). By contrast, polyunsaturated phosphatidylcholine (PC) and phosphatidylethanolamine (PE) (optimally 18:0/20:4 or 18:0/22:6 PC/PE) stimulate Na,K-ATPase activity but do not stabilize the protein (13). The structural specificity of the phospholipids is indicative of specific Na,K-ATPase–PS and Na,K-ATPase–PC/PE interactions. Mutation of three residues in transmembrane (TM) helices αTM8, 9, and 10 of the thermolabile α2 isoform to the corresponding α1 residues (V918, F955, and P979) (Fig. 1) significantly stabilized α2 by increasing the apparent affinity for 18:0/18:1 PS (1-stearoyl-2-oleoyl-sn-glycero-3-phospho-L-serine, SOPS) and FXYD1 and increasing the stabilizing effect of cholesterol (10, 14). This finding indicated that both 18:0/18:1 PS and cholesterol are bound between helices αTM8–10 and the FXYD subunit, all mutually interacting to stabilize the protein. The 18:0/18:1 PS in site A does not affect Na,K-ATPase activity per se. In crystal structures two cholesterol and four phospholipid molecules have been identified near the C terminus of the α-subunit in site A (Fig. 1, Inset). All the phospholipids were modeled as PC and are located near the FXYD subunit and along αM10 facing either the extracellular or intracellular surface of the membrane (11, 12). This modeling raises the question whether all or only a subset of these lipids in site A are relevant for stabilization or whether they are bound adventitiously from phospholipid added to the crystallization medium.
Fig. 1.
Na,K-ATPase lipid-binding sites: the location of functionally relevant phospholipids and cholesterol in sites A and B, as detected in this study. Lysine residues at the membrane–water interface mutated in this study are shown in stick representation. V920, F956, and P981, previously shown to stabilize Na,K-ATPase, are indicated also. (Inset) Lipids identified in the renal Na,K-ATPase structure (PDB ID code: 3WGV). The α-subunit is shown in white with transmembrane helices αM2 and αM4 highlighted in red and αM8–10 in blue; the β-subunit is shown in brown; and the FXYD subunit is shown in green. Phospholipids are depicted in orange, and cholesterol molecules are shown in pink. Site A, formed by αM8–10 and the FXYD transmembrane helix, harbors four phospholipids and two cholesterol molecules; site B is occupied by one phospholipid.
Stimulation of Na,K-ATPase activity by 18:0/20:4 PC/PE involves acceleration of the conformational transition E1P–E2P (14). The lipid is assumed to bind at site B, located between αTM2, 4, and 9, which undergoes structural rearrangements during the conformational transition (14). Site B was shown in the crystal structure to harbor a phospholipid (Fig. 1, Inset) (11).
A third effect of saturated PC or sphingomyelin together with cholesterol—inhibition of Na,K-ATPase—may be mediated by lipids bound between βTM and αM3, 5, and 7 (site C) (14). This site is not discussed further here.
Biochemical and structural studies provide independent evidence for specific Na,K-ATPase–phospholipid interactions. However, functionally relevant sites have not been clearly identified. The current study addresses this gap in our knowledge by combining kinetic approaches with mutant analyses and native MS and by linking structural and biochemical data.
Results
Detection of specific effects of lipids on Na,K-ATPase from Pichia pastoris is based on the replacement of endogenous yeast lipids by defined lipids solubilized in nondenaturing detergents. Solubilization is achieved by extensive washing of the His-tagged Na,K-ATPase bound to Co beads with buffers supplemented with lipids and detergents before elution of the enzyme. For this study Na,K-ATPase α1β1 was purified in a mixture of 18:0/18:1 PS, cholesterol, and octaethylene glycol monododecyl ether (C12E8), undecyl-maltopyranoside (UDM), or dodecyl-maltopyranoside (DDM). The specific Na,K-ATPase activity was not significantly different when using C12E8 or maltosides.
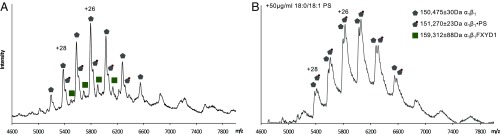
PS and cholesterol are essential for preserving Na,K-ATPase activity, but PS has not been resolved in crystal structures. To obtain direct proof of specific binding of PS and, in particular, to address the question of lipid-binding stoichiometry, we have applied native MS. Native MS analysis of membrane proteins is based on the transfer of protein–detergent micelles in the gas phase and the removal of the detergent molecules by collisional activation while specific phospholipid interactions are maintained (15, 16). Spectra were obtained following the purification of the protein in DDM or UDM and a size-exclusion chromatography step, which isolated the Na,K-ATPase monomer peak from aggregated proteins, a small fraction of higher oligomers, and excess lipid-detergent micelles (SI Appendix, Fig. S1A). The running buffer was supplemented with 10 µg/mL 18:0/18:1 PS in 0.25 mg/mL DDM, which preserved 70% of Na,K-ATPase activity. The mass spectra indicated a major charge series with an apparent mass of 150,475 ± 30 Da and a minor charge series with apparent mass of 159,312 ± 88 Da (Fig. 2A). These masses are in good agreement with the theoretical mass of the α1β1 (150,443 Da) and α1β1FXYD1 (159,126 Da) complexes. [Alkyl maltosides tend to dissociate the FXYD proteins from the αβ complex (17). C12E8 does not have this effect (18) but is incompatible with MS, probably because of sample inhomogeneity caused by the presence of higher oligomers in addition to αβ monomers (19). Thus, the present work on lipid binding is limited to the UDM- or DDM-soluble FXYD-free αβ complexes.] An adduct peak was observed at the major charge series with a 795-Da mass shift, suggesting binding of a PS molecule. After 18:0/18:1 PS was added to the sample and allowed to equilibrate overnight, a new peak emerged with an apparent mass of 151,270 ± 23 Da (Fig. 2B), in agreement with the expected mass of an α1β1•18:0/18:1 PS complex (151,232Da). We added 10, 30, and 50 μg/mL of 18:0/18:1 PS. The 50 µg/mL addition sufficed to produce a maximal effect. The K0.5 for 18:0/18:1 PS is in the range 20–30 μg/mL (25–38 μM), in line with that required to stabilize the enzyme in biochemical experiments (9, 10, 18). Overnight incubation with 18:0/18:1PS did not affect activity. MS did not detect cholesterol, presumably because it dissociated from the αβ complex on the size-exclusion column. Dissociation of DDM micelles required relatively high activation/collision energy, which might lead to the dissociation of additional specifically associated lipids, thus precluding their detection. Thus, we replaced DDM with the shorter UDM, which has a threefold higher critical micelle concentration and allows the acquisition of spectra at lower activation energy (Fig. 3A). The concentration of UDM (0.6 mg/mL) plus SOPS (30 µg/mL) was optimized to give maximal resolution of MS spectra and to avoid an excess of UDM, which inactivates the Na,K-ATPase and precludes the observation of well-defined spectra. Under these conditions six molecules of PS were observed (Fig. 3B; masses are given in SI Appendix, Table S2). However, all but one of these lipids was easily dissociated upon increase of collision energy, strengthening the notion of specific binding of only one molecule of PS (Fig. 3C). The calculated mass of bound 18:0/18:1PS was 787 ± 5 Da (n = 7), compared with the theoretical mass of 789 Da. Other SOPS molecules observed initially may represent annular or weakly associated lipids.
Fig. 2.
Na,K-ATPase specifically binds one molecule of 18:0/18:1 PS as indicated by native MS. (A) Mass spectrum of Na,K-ATPase. Pentagons represent apo α1β1 complexes, and squares represent apo α1β1FXYD1. (B) The increase in the intensity of the peaks corresponding in mass to α1β1•18:0/18:1 PS indicates the specific binding of this lipid.
Fig. 3.
One molecule of PS is specifically bound to Na,K-ATPase. (A) Mass spectra of Na,K-ATPase purified in 0.6 mg/mL UDM and 0.03 mg/mL 18:0/18:1 PS at increasing collision energy (112–150 V). The fine structure of the charge states is a result of the combination of peaks that correspond in mass to different numbers of bound 18:0/18:1 PS molecules. (B) Enlarged view of the 26+ charge state spectrum with deconvolution of the measured spectrum into a series of Gaussian functions (red dotted line) assuming the binding of one to six molecules of 18:0/18:1 PS to the apo protein. (C) Normalized intensity of peaks that correspond to Na,K-ATPase with one to three PS molecules bound, depending on collision energy.
To define the location of the specifically bound PS molecule, we mutated lysine residues to neutral glutamines, singly or together, at the cytoplasmic side of membrane crevices expected to harbor functionally relevant phospholipids [Lys945 and Lys943 in the αL8–9 loop (site A), Lys146 in αTM2, and Lys342 in αTM4 (site B); Fig. 1]. Single and double mutations (α1K943Q, α1K945Q, α1K943Q/K945Q, α1K146Q, α1K342Q, and α1K146Q/K342Q) were introduced by site-directed mutagenesis and transformed into P. pastoris as α1MutHis10-β1. As indicated in SI Appendix, Table S1, which documents the ouabain-binding capacity of the membranes, three single mutants—α1K943Q, α1K945Q, and α1K342Q—were expressed only weakly and could not be purified. By contrast, expression levels of mutants α1K943Q/K945Q, α1K146Q, and α1K146Q/K342Q reached ∼50% of WT levels. These three mutants and WT Na,K-ATPase were purified in a mixture of C12E8, 18:0/18:1 PS and cholesterol and were used either for stability assays (Fig. 4) or with added 18:0/20:4 PC to determine the stimulatory effect on Na,K-ATPase activity and rates of conformational transitions (Fig. 5). Specific ATPase activities of batch-purified enzyme were 15.3 ± 0.7 µmol Pi⋅mg−1⋅min−1 for WT α1 and 10.7 ± 0.8, 11.8 ± 0.7, and 10.9 ± 0.8 µmol Pi⋅mg−1⋅min−1for α1K943Q/K945Q, α1K146Q, and α1K146Q/K342Q.
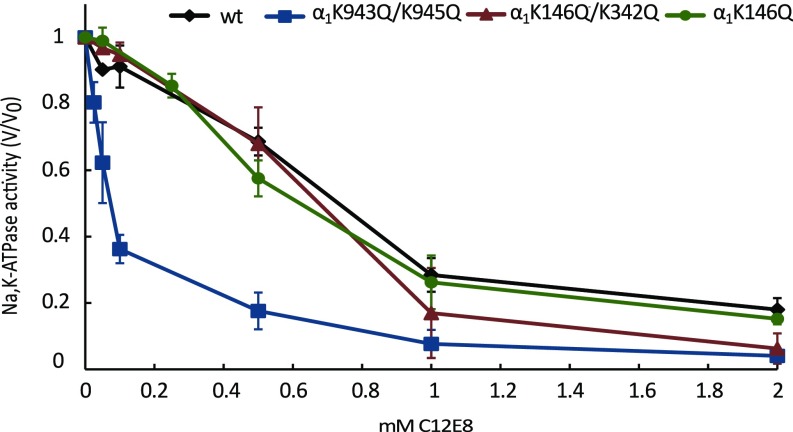
Fig. 4.
K943Q/K945Q substitutions increase sensitivity to detergent inactivation. WT Na,K-ATPase and its mutational variants α1K146Q, α1K146Q/K342Q, and α1K943Q/K945Q were incubated in the presence of the indicated concentrations of C12E8 for 10 min at 37 °C before residual activity was measured. Error bars represent the SEM. n = 3.
Fig. 5.
Lysine substitutions in M2 and M4 prevent stimulation by 18:0/20:4 PC. (A) 18:0/20:4 PC but not 18:0/18:1 PC stimulates pump activity. Activity was normalized to the reference sample purified in the presence of 18:0/18:1 PS and cholesterol (SOPS). (B) Mutation of lysines in M2 and M4 (K146Q and K146Q/K342Q) but not in the L8–9 loop (K943/K945Q) abolish stimulation by 18:0/20:4 PC. (C) Stopped-flow traces of the E1P–E2P conformational transition for α1K146Q/K342Q purified with and without 18:0/20:4 PC. Error bars represent the SEM. n ≥ 3; *P < 0.05, ***P < 0.0005.
Fig. 4 shows the effect of mutations on the inactivation of Na,K-ATPase activity by excess C12E8, which is thought to displace 18:0/18:1PS (see figure 11 in ref. 18) but is unable to mimic the stabilizing function, thus leading to inactivation. By comparison with the WT, the mutant K943Q/K945Q showed a large increase in sensitivity to C12E8 with apparent K0.5 of inactivation of 0.09 mM C12E8 compared with 0.65 mM for the WT. By contrast, both α1K146Q and α1K146Q/K342Q mutants show sensitivity similar to that of the WT with a K0.5 of about 0.75 mM C12E8, thus serving as negative controls for the selective effect of the K943Q/K945Q mutant on stability.
Cholesterol and SOPS are the only lipids essential for maintaining Na,K-ATPase function, but they are not sufficient to sustain maximal activity. As shown recently, PC or PE with saturated fatty acyl chains in sn-1 and polyunsaturated fatty acyl chains in sn-2 stimulate Na,K-ATPase activity by 50–60% (14). Fig. 5 compares the Na,K-ATPase activity of the WT and mutant proteins purified in the presence of 18:0/18:1 PS and cholesterol without or with 18:0/20:4 PC or 18:0/18:1 PC. In agreement with previous findings, 18:0/20:4 PC stimulated the activity of WT α1β1 by 44 ± 3% compared with the control, whereas 18:0/18:1 PC did not stimulate activity. We investigated the effect of 18:0/20:4 PC on the Na,K-ATPase activity of the lysine mutants. As shown in Fig. 5B, stimulation of α1K943Q/K945Q by 18:0/20:4 PC was comparable to stimulation of the WT (37 ±7 %). By contrast, the double mutation K146Q/K342Q completely abolished stimulation by 18:0/20:4 PC, and K146Q showed only about 10% stimulation by 18:0/20:4 PC. Na,K-ATPase transports ions by cycling between two principle conformations, E1 and E2 (SI Appendix, Fig. S2), and the rate-limiting steps are the conformational transitions E1P–E2P and E2(2K)–E1. Rates of conformational transitions can be measured by stopped-flow fluorescence using the electrochromic shift dye RH421 (14). The E1P–E2P transition is triggered by mixing Na,K-ATPase in the presence of Na with ATP (E13Na+ATP→E2P+3Na+ADP). We have shown previously that the stimulatory lipid brain PE accelerates the rate of the E1P–E2P transition (14). For stopped-flow experiments the mutant α1K146Q/K342Q was purified in the absence (control) and presence of 18:0/20:4 PC (Fig. 5C). In agreement with the lack of stimulatory effect of 18:0/20:4 PC on Na,K-ATPase activity, the traces are virtually overlapping, with rates determined as 99 ± 9/s for the control and 87 ± 7/s for the mutant purified with 18:0/20:4 PC.
In short, Figs. 4 and 5 show that mutations of lysine residues in site A (α1K943Q/K945Q) increase sensitivity to destabilization by C12E8 but do not affect the stimulation of activity by 18:0/20:4 PC, whereas mutations in site B (α1K146Q, α1K146Q/K342Q) prevent the stimulation of activity by 18:0/20:4 PC but do not affect stability. These findings confirm that the effects are separate and selective, resulting from specific lipid binding in the distinct sites A and B (Fig. 1).
Next, we used native MS to demonstrate directly the specific binding of 18:0/20:4 PC to the Na,K-ATPase. [We used 18:0/20:4 PC in preference to 18:0/20:4 PE because of its higher mass (810 versus 768 Da, respectively), allowing a readier distinction from bound 18:0/18:1 PS (789 Da).] The enzyme was purified by size-exclusion chromatography in the presence of 0.6 mg/mL UDM and 30 µg/mL 18:0/18:1 PS before increasing amounts of 18:0/20:4 PC were added. Samples then were allowed to equilibrate for 8 h on ice. As shown in ref. 13, overnight incubation with the unsaturated phospholipid increased Na,K-ATPase activity. Spectra were acquired with activation conditions tuned to dissociate most of the annular bound PS but allowing the binding of PC (Fig. 6A). The addition of 18:0/20:4 PC led to the concentration-dependent appearance of a peak with a mass corresponding to the mass of two phospholipids bound to the apo–α1β1 complex (152,078 ± 24 Da; the theoretical mass α1β1•18:0/18:1 PS•18:0/20:4 PC is 152,042 Da). The calculated mass of the bound 18:0/20:4 PC was 808 ± 7 Da (n = 4) compared with the theoretical mass of 810 Da. The increase in relative peak intensity was fitted to a Hill function with apparent K0.5 of 11.9 µg/mL (15 µM) and a Hill coefficient of 1.05, indicating, again, that only one molecule of 18:0/20:4 PC binds specifically to the Na,K-ATPase (Fig. 6B). As a control for specificity of its binding, we replaced 18:0/20:4 PC with 18:0/18:1 PC, which does not stimulate pump activity (Fig. 5A). Indeed, no significant binding of 18:0/18:1 PC was observed upon the addition of 30 µg/mL (Fig. 6C), as seen by comparing the relative peak heights with those of the control spectrum without added 18:0/20:4 PC (Fig. 6A).
Fig. 6.
One molecule of 18:0/20:4 PC binds specifically to the Na,K-ATPase. (A) Mass spectra of WT Na,K-ATPase in the presence of 0, 10, and 40 µg 18:0/20:4 PC, 0.6 mg/mL UDM, and 0.03 mg/mL 18:0/18:1 PS. (B) Hill-fit of peak intensity as a function of the 18:0/20:4 PC concentration (nHill = 1.05). Normalization was done by calculating the ratio of α1β1•2lipids/α1β1 for all added PC concentrations. The maximum ratio then was normalized to 1. Error bars represent the SEM. n = 3. (C) Mass spectrum of Na,K-ATPase in the presence of 30 µg/mL 18:0/18:1 PC showing no binding of the 18:0/18:1PC.
Attempts to look at mutants by MS were unsuccessful. An unexpected effect explains this observation (SI Appendix, Fig. S1B): Size-exclusion chromatography shows that all the mutants tended to aggregate or form higher oligomers. The same pattern was observed when WT Na,K-ATPase was purified in detergent alone without PS and cholesterol. Thus, these observations also indicate that the bound phospholipids maintain the functional monomeric state of the Na,K-ATPase.
Discussion
When a membrane protein is purified in its native membrane or reconstituted into a model bilayer, as is often necessary in order to maintain activity, it is difficult to distinguish whether functional effects of different lipids result from specific protein–lipid interactions or from physical forces associated with nonspecific bilayer interactions. Thus, the different types of protein–lipid interaction have been a matter of extensive debate for channels (20, 21), transporters (22), G-protein–coupled receptors (23), and P-type ATPases (SI Appendix, Supplementary Discussion). By contrast, when the membrane protein is purified in soluble mixed protein–lipid–detergent micelles in the absence of a lipid bilayer, the analysis is greatly simplified. Indeed, the ability to measure the Na,K-ATPase activity of the soluble Na,K-ATPase–lipid micelles allowed us to show that stability and activity are independently modulated by three different classes of lipids (10, 14). However, to understand the mechanism by which specifically bound lipids modulate Na,K-ATPase, it is essential to identify the sites and determine the stoichiometry of lipid binding. The present study provides biochemical and structural insights and unequivocally defines two lipid-mediated effects. The effects were inferred previously by their structural specificity for different lipids and the kinetic mechanisms (9, 14). Although those data were convincing, they were still indirect. By combining native MS and biochemical analysis of mutants, we could demonstrate directly both the stoichiometry of lipid binding and the location of sites A and B (Fig. 1).
As shown in previous work, optimal stabilization of the enzyme depends on specific binding of 18:0/18:1 PS, cholesterol, and the FXYD subunit (10, 14). These observations provided clear evidence for a specific SOPS-binding pocket between αTM8, 9, 10 and TMFXYD in the C-terminal domain of the α-subunit (site A). They imply that SOPS, cholesterol, and FXYD all interact with each other to stabilize the protein. The molecular structure of Na,K-ATPase with bound lipids in site A (Fig. 1, Inset) suggested that a particular phospholipid facing the cytoplasmic surface could be the relevant one. Accordingly, we mutated Lys943 and Lys945 in the cytoplasmic-facing L8–9 loop to neutral glutamines. The two lysines residues are conserved among Na,K-ATPase isoforms (except that Lys943 is an arginine in α4) and are the only two charged residues at the likely site of interaction with the phosphate and carboxyl groups of the SOPS. As predicted, these mutations significantly destabilized Na,K-ATPase against detergent inactivation, whereas the other mutations in TM2 and TM4 had no effect on stability. We conclude that SOPS is indeed bound to Lys943 and Lys945 in L8–9. To determine the number of specifically bound SOPS molecules, we performed native MS experiments. The optimal conditions required for structural MS are somewhat suboptimal for the preservation of Na,K-ATPase activity; namely, size-exclusion chromatography in the presence of high detergent–lipid ratios leads to the dissociation of FXYD1 and cholesterol and to some loss of activity (∼30%). Nevertheless, we observed specific binding of one molecule of 18:0/18:1 PS. Thus, we conclude that the one 18:0/18:1 PS molecule binds to the C-terminal domain of the Na,K-ATPase α-subunit near the FXYD subunit and a cholesterol (see site A in Fig. 1). [In the Na,K-ATPase structure, Protein Data Bank (PDB) ID code: 3WGV (11), three cholesterol molecules were identified, including two located between αTM8–10 (Chl3 and Chl2 in Fig. 1). Chl3 is inferred to stabilize the protein via interactions with TMFXYD, αV918, and αP979, known to stabilize α1 selectively over α2, and proximity to bound SOPS, as discussed previously (10, 14, 18).] Proteolytic digestion studies identified the αTM8–10 structure as a thermolabile domain or “hotspot” (24, 25). Heating alters the topological organization and ejects αTM8, 9, 10 from the membrane. Because Na,K-ATPase is more thermolabile in the absence of lipids or when lipid binding is impeded, we suggest that SOPS, cholesterol, and the FXYD protein together stabilize the topology of the C-terminal domain. This stabilization is essential, because residues in TM8 contribute ligands of the third Na-binding site (11).
As described earlier, the neutral phospholipids PC/PE, optimally 18:0/20:4 or 18:0/22:6 PC/PE, stimulate Na,K-ATPase activity but do not affect stability. Stimulation results from the acceleration of the E1P–E2P conformational transition which induces a population shift of the E1/E2 equilibrium toward E2 (14). Mutation of Lys146 alone or in combination with Lys342 abolished the effect of 18:20:4 PC. We suggest that the 18:0/20:4 or 18:0/22:6 PC/PE accelerates the transition by facilitating new conformations. The repeated pattern of double bonds in the sn-2 20:4 or 22:6 fatty acyl chains flanked by two saturated bonds allows free rotation and a high degree of conformational flexibility (26). The αTM2, 4, 6, 9 crevice that harbors 18:0/20:4 PC/PE changes its structure during the E1P–E2P transition because of the movement of TM2 away from TM9 (12); the phospholipids quickly adapt to and facilitate this movement. Specific binding of 18:0/20:4 PC could be observed by native MS in the presence of SOPS. Under these conditions three populations of the Na,K-ATPase were observed: lipid free and bound by either one or two phospholipids. The increase in peak intensity upon the addition of PC could be fitted with a one-side binding model (nHill = 1.05; Fig. 6B); given the specific binding of one molecule of 18:0/18:1 PS, the lipid-bound states represent Na,K-ATPase•18:0/18:1PS or Na,K-ATPase•18:0/20:4 PC for Na,K-ATPase with one lipid and Na,K-ATPase•18:0/18:1PS•18:0/20:4 PC for Na,K-ATPase with two lipids. Thus, we conclude that one molecule of 18:0/20:4 PC binds specifically in addition to one molecule of 18:0/18:1 PS.
Although our data show that 18:0/20:4 or 18:0/22:6 PC/PE stimulates Na,K-ATPase activity by specific interactions, which are likely to have important physiological functions, they do not exclude modulation by bulk bilayer properties in intact membranes. Polyunsaturated lipids have been shown to reduce Na,K-ATPase turnover in liposomes enriched in 22:6 PC (27). Conversely, Na,K-ATPase turnover rates in avian and mammalian cardiac membranes were positively correlated with the fraction of docosahexaenoic acid (22:6) in phospholipids (28), an effect attributed to an increase in the area of the phospholipids as the polyunsaturated acyl chain composition increased (29). However, given the inhibitory effect of 22:6 PC in reconstituted liposomes, it is more likely that the increased Na,K-ATPase activity was caused by the specific stimulatory interactions of the 22:6-containing phospholipid rather than by an effect of molecular packing. The activity of Na,K-ATPase purified in a mixture of 18:0/18:1 PS, cholesterol, and 18:0/20:4 or 18:0/22:6 PC /PE is comparable to the activity of purified renal Na,K-ATPase (30). Nevertheless, the physiological importance of specific protein–lipid interactions is not well established, in part because of the difficulty in determining changes in the lipid composition within cells. In one interesting case imaging MS has shown that arachidonic acid-PC and docosahexaenoic acid-PC are enriched across a proximal-to-distal gradient of cultured neurons (31). Na,K-ATPase activity is vital for neuronal function, particularly at nerve endings where it maintains the Na gradients required for neurotransmitter reuptake. It is intriguing to speculate that the gradient of fatty acyl chain unsaturation may have increased Na,K-ATPase activity along the axon by the specific interaction described here.
An unexpected finding in this study was that the lysine substitutions caused aggregation of Na,K-ATPase. The mutations did not significantly affect Na,K-ATPase activity and allowed biochemical studies, but the mutated proteins did not allow analysis by MS. These finding indicate that the specific binding of lipids protects against nonspecific interactions of transmembrane helices and protein aggregation.
In conclusion, by combining information from mutational analysis, biochemical data, and native MS data from WT Na,K-ATPase with available crystal structures, a rather detailed picture of specific lipid-binding sites of the Na,K-ATPase has emerged (Fig. 1). Two independent binding sites have been definitely identified. Site A is formed by αTM8–10 and the FXYD transmembrane helix and harbors one molecule of 18:0/18:1 PS and one cholesterol molecule, which together stabilize but do not per se affect Na,K-ATPase activity. At a second site, B, located between αM2, 4, 6, and 9, 18:0/20:4 or 18:0/22:6 PC/PE bind and stimulate activity by accelerating the E1P–E2P conformational transition but do not affect stability. The different effects of lipid classes at the separate sites are independent and modulate distinct properties of Na,K-ATPase. A further point of interest is that the phenomenon of selective lipid binding may not be restricted to Na,K-ATPase. Crystal structures of the closely related sarcoplasmic reticulum Ca-ATPase reveal bound lipids between the same transmembrane segments (analogous to sites A and B) (32). These lipids may have effects on the stability and activity of Ca-ATPase similar to those proposed for Na,K-ATPase (see SI Appendix, Supplementary Discussion for a discussion of lipid binding in Ca-ATPase and other P-type ATPases).
Since the description of the fluid-mosaic model by Singer and Nicolson (33), the effects of the physical state of the bilayer on membrane proteins have been studied in detail (3), but the understanding of how specifically bound lipids modulate enzymatic properties is still lacking. Identifying lipid-binding sites and their mechanism of modulation of Na,K-ATPase shows that specific interactions play a key role compared with bulk bilayer interactions. This work may provide a framework for the study of specific protein–lipid interactions for other P-type ATPases, such as P4-ATPases that flip PS and PE across the membrane to maintain bilayer asymmetry (34).
Materials and Methods
Reagents.
Phospholipids were purchased from Avanti Polar Lipids, cholesterol was purchased from Sigma Aldrich, and all other reagents were used at the highest available purity.
Mutagenesis.
Mutations were introduced into the PhilD2 vector harboring human α1 and His10-β1 by overlap-extension PCR (SI Appendix, SI Materials and Methods) (35). P. pastoris SMD1165 was transformed, and clones were selected as described (36).
Enzyme Purification.
Na,K-ATPase and FXYD1 were purified by metal affinity chromatography using a batch protocol or gravity columns in a mixture of 18:0/18:1 PS, cholesterol, and C12E8, DDM, or UDM as detergent (see SI Appendix, SI Materials and Methods for details) (14).
Biochemical Assays.
ATPase activity was measured in the presence of 120 mM NaCl, 20 mM KCl, and 1 mM ATP at 37 °C using the malachite green assay for phosphate detection (PiColorLock; Innova Bioscience). Rates of the E13Na–E2P transition were measured using an Applied Photophysics SX20 stopped-flow device by mixing 10 µg/mL Na,K-ATPase noncovalently labeled with RH421 with 2 mM ATP in the presence of 120 mM NaCl (14).
Native MS.
For native MS experiments eluted Na,K-ATPase was dialyzed and deglycosylated by EndoH, and aggregated proteins were removed by ultracentrifugation. The sample was then concentrated to 5–8 mg/mL using Vivaspin 100-kDa centrifugal concentrators. The buffer was exchanged with 400 mM ammonium acetate (pH 7.8) or 200 mM ammonium acetate, 4 mM ethylenediamine supplemented with 0.25 mg/mL DDM or 0.6 mg/mL UDM plus 10–30 µg/mL 18:0/18:1 PS by size-exclusion chromatography (Superdex 200 10/300 column). Mass spectra were acquired on a Synapt high-definition MS (HDMS) instrument (Waters) under native-like conditions. For lipid-binding experiments concentrated stock solutions of 18:0/18:1 PS or 18:0/20:4 PC were added to Na,K-ATPase with the buffer exchanged as described above and were equilibrated overnight. Typical experimental parameters for Na,K-ATPase in UDM were capillary voltage 1.6 kV, cone voltage 80–120 V, extractor 4 V, transfer and trap voltage 120–150 V, and backing pressure 4.5 mbar.
Note that ammonium acetate, used as a volatile buffer, stabilizes Na,K-ATPase in an E2(2NH4+) conformation because NH4+ replaces K+. In later experiments, ethylene diamine (4 mM) was found to reduce the average charge state of the complexes and to improve resolution. Buffers such as ethylene diammonium diacetate, which does not contain ammonium, have been introduced recently for use in structural MS but did not allow the release of Na,K-ATPase from the detergent micelle (37).
Data Analysis.
Stopped-flow fluorescence traces were fitted with a double exponential function: F = A1·e-k1·t + A2·e-k2·t+c, where A is the amplitude, k is the rate, and c the equilibrium fluorescence level after completion.
Peak intensities of apo-Na,K-ATPase and lipid-bound Na,K-ATPase were obtained by fitting the peaks to a series of Gaussian functions.
Supplementary Material
Acknowledgments
We thank the Minerva Foundation for providing a fellowship (to M.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620799114/-/DCSupplemental.
References
- 1.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunte C. Specific protein-lipid interactions in membrane proteins. Biochem Soc Trans. 2005;33(Pt 5):938–942. doi: 10.1042/BST20050938. [DOI] [PubMed] [Google Scholar]
- 3.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666(1-2):62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 4.East JM, Melville D, Lee AG. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosinetriphosphatase. Biochemistry. 1985;24(11):2615–2623. doi: 10.1021/bi00332a005. [DOI] [PubMed] [Google Scholar]
- 5.Hunte C, Richers S. Lipids and membrane protein structures. Curr Opin Struct Biol. 2008;18(4):406–411. doi: 10.1016/j.sbi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen PL, Hakansson KO, Karlish SJD. Structure and mechanism of Na,K-ATPase: Functional sites and their interactions. Annu Rev Physiol. 2003;65:817–849. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler KP, Whittam R. The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol. 1970;207(2):303–328. doi: 10.1113/jphysiol.1970.sp009063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius F. Cholesterol modulation of molecular activity of reconstituted shark Na+,K(+)-ATPase. Biochim Biophys Acta. 1995;1235(2):205–212. doi: 10.1016/0005-2736(95)80006-2. [DOI] [PubMed] [Google Scholar]
- 9.Haviv H, et al. Stabilization of Na(+),K(+)-ATPase purified from Pichia pastoris membranes by specific interactions with lipids. Biochemistry. 2007;46(44):12855–12867. doi: 10.1021/bi701248y. [DOI] [PubMed] [Google Scholar]
- 10.Kapri-Pardes E, et al. Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket. J Biol Chem. 2011;286(50):42888–42899. doi: 10.1074/jbc.M111.293852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai R, Ogawa H, Vilsen B, Cornelius F, Toyoshima C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 2013;502(7470):201–206. doi: 10.1038/nature12578. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius F, Habeck M, Kanai R, Toyoshima C, Karlish SJD. General and specific lipid-protein interactions in Na,K-ATPase. Biochim Biophys Acta. 2015;1848(9):1729–1743. doi: 10.1016/j.bbamem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Haviv H, Habeck M, Kanai R, Toyoshima C, Karlish SJD. Neutral phospholipids stimulate Na,K-ATPase activity: A specific lipid-protein interaction. J Biol Chem. 2013;288(14):10073–10081. doi: 10.1074/jbc.M112.446997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habeck M, et al. Stimulation, inhibition, or stabilization of Na,K-ATPase caused by specific lipid interactions at distinct sites. J Biol Chem. 2015;290(8):4829–4842. doi: 10.1074/jbc.M114.611384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laganowsky A, Reading E, Hopper JTS, Robinson CV. Mass spectrometry of intact membrane protein complexes. Nat Protoc. 2013;8(4):639–651. doi: 10.1038/nprot.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechara C, Robinson CV. Different modes of lipid binding to membrane proteins probed by mass spectrometry. J Am Chem Soc. 2015;137(16):5240–5247. doi: 10.1021/jacs.5b00420. [DOI] [PubMed] [Google Scholar]
- 17.Lifshitz Y, Lindzen M, Garty H, Karlish SJD. Functional interactions of phospholemman (PLM) (FXYD1) with Na+,K+-ATPase. Purification of alpha1/beta1/PLM complexes expressed in Pichia pastoris. J Biol Chem. 2006;281(23):15790–15799. doi: 10.1074/jbc.M601993200. [DOI] [PubMed] [Google Scholar]
- 18.Mishra NK, et al. FXYD proteins stabilize Na,K-ATPase: Amplification of specific phosphatidylserine-protein interactions. J Biol Chem. 2011;286(11):9699–9712. doi: 10.1074/jbc.M110.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen E, et al. Purification of Na+,K+-ATPase expressed in Pichia pastoris reveals an essential role of phospholipid-protein interactions. J Biol Chem. 2005;280(17):16610–16618. doi: 10.1074/jbc.M414290200. [DOI] [PubMed] [Google Scholar]
- 20.Baenziger JE, daCosta CJB. Molecular mechanisms of acetylcholine receptor–lipid interactions: From model membranes to human biology. Biophys Rev. 2013;5(1):1–9. doi: 10.1007/s12551-012-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.daCosta CJB, Dey L, Therien JPD, Baenziger JE. A distinct mechanism for activating uncoupled nicotinic acetylcholine receptors. Nat Chem Biol. 2013;9(11):701–707. doi: 10.1038/nchembio.1338. [DOI] [PubMed] [Google Scholar]
- 22.Koshy C, Ziegler C. Structural insights into functional lipid-protein interactions in secondary transporters. Biochim Biophys Acta. 2015;1850(3):476–487. doi: 10.1016/j.bbagen.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Soubias O, Teague WE, Gawrisch K. Evidence for specificity in lipid-rhodopsin interactions. J Biol Chem. 2006;281(44):33233–33241. doi: 10.1074/jbc.M603059200. [DOI] [PubMed] [Google Scholar]
- 24.Goldshleger R, Tal DM, Karlish SJ. Topology of the alpha-subunit of Na,K-ATPase based on proteolysis. Lability of the topological organization. Biochemistry. 1995;34(27):8668–8679. doi: 10.1021/bi00027a016. [DOI] [PubMed] [Google Scholar]
- 25.Arystarkhova E, Gibbons DL, Sweadner KJ. Topology of the Na,K-ATPase. Evidence for externalization of a labile transmembrane structure during heating. J Biol Chem. 1995;270(15):8785–8796. doi: 10.1074/jbc.270.15.8785. [DOI] [PubMed] [Google Scholar]
- 26.Feller SE, Gawrisch K, MacKerell AD., Jr Polyunsaturated fatty acids in lipid bilayers: Intrinsic and environmental contributions to their unique physical properties. J Am Chem Soc. 2002;124(2):318–326. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- 27.Cornelius F. Cholesterol-dependent interaction of polyunsaturated phospholipids with Na,K-ATPase. Biochemistry. 2008;47(6):1652–1658. doi: 10.1021/bi702128x. [DOI] [PubMed] [Google Scholar]
- 28.Turner N, Haga KL, Else PL, Hulbert AJ. Scaling of Na+,K+-ATPase molecular activity and membrane fatty acid composition in mammalian and avian hearts. Physiol Biochem Zool. 2006;79(3):522–533. doi: 10.1086/502815. [DOI] [PubMed] [Google Scholar]
- 29.Else PL, Wu BJ, Storlien LH, Hulbert AJ. Molecular activity of Na+,K+-ATPase relates to the packing of membrane lipids. Ann N Y Acad Sci. 2003;986:525–526. doi: 10.1111/j.1749-6632.2003.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen PL. Purification of Na+,K+-ATPase: Enzyme sources, preparative problems, and preparation from mammalian kidney. Methods Enzymol. 1988;156:29–43. doi: 10.1016/0076-6879(88)56005-6. [DOI] [PubMed] [Google Scholar]
- 31.Yang H-J, Sugiura Y, Ikegami K, Konishi Y, Setou M. Axonal gradient of arachidonic acid-containing phosphatidylcholine and its dependence on actin dynamics. J Biol Chem. 2012;287(8):5290–5300. doi: 10.1074/jbc.M111.316877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drachmann ND, et al. Comparing crystal structures of Ca(2+) -ATPase in the presence of different lipids. FEBS J. 2014;281(18):4249–4262. doi: 10.1111/febs.12957. [DOI] [PubMed] [Google Scholar]
- 33.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 34.Montigny C, Lyons J, Champeil P, Nissen P, Lenoir G. On the molecular mechanism of flippase- and scramblase-mediated phospholipid transport. Biochim Biophys Acta. 2016;1861(8 Pt B):767–783. doi: 10.1016/j.bbalip.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 36.Strugatsky D, Gottschalk K-E, Goldshleger R, Bibi E, Karlish SJD. Expression of Na+,K+-ATPase in Pichia pastoris: Analysis of wild type and D369N mutant proteins by Fe2+-catalyzed oxidative cleavage and molecular modeling. J Biol Chem. 2003;278(46):46064–46073. doi: 10.1074/jbc.M308303200. [DOI] [PubMed] [Google Scholar]
- 37.Dyachenko A, Gruber R, Shimon L, Horovitz A, Sharon M. Allosteric mechanisms can be distinguished using structural mass spectrometry. Proc Natl Acad Sci USA. 2013;110(18):7235–7239. doi: 10.1073/pnas.1302395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.