Significance
Here we describe a role for the synaptic vesicle glycoprotein 2C (SV2C) in dopamine neurotransmission and Parkinson disease (PD). SV2C is expressed on the vesicles of dopamine-producing neurons, and genetic deletion of SV2C causes a reduction in synaptic release of dopamine. The reduced dopamine release is associated with a decrease in motor activity. SV2C is suspected of mediating the neuroprotective effects of nicotine, and we show an ablated neurochemical response to nicotine in SV2C-knockout mice. Last, we demonstrate that SV2C expression is specifically disrupted in mice that express mutated α-synuclein and in humans with PD. Together, these data establish SV2C as an important mediator of dopamine homeostasis and a potential contributor to PD pathogenesis.
Keywords: Parkinson disease, SV2C, synaptic vesicles, dopamine, α-synuclein
Abstract
Members of the synaptic vesicle glycoprotein 2 (SV2) family of proteins are involved in synaptic function throughout the brain. The ubiquitously expressed SV2A has been widely implicated in epilepsy, although SV2C with its restricted basal ganglia distribution is poorly characterized. SV2C is emerging as a potentially relevant protein in Parkinson disease (PD), because it is a genetic modifier of sensitivity to l-DOPA and of nicotine neuroprotection in PD. Here we identify SV2C as a mediator of dopamine homeostasis and report that disrupted expression of SV2C within the basal ganglia is a pathological feature of PD. Genetic deletion of SV2C leads to reduced dopamine release in the dorsal striatum as measured by fast-scan cyclic voltammetry, reduced striatal dopamine content, disrupted α-synuclein expression, deficits in motor function, and alterations in neurochemical effects of nicotine. Furthermore, SV2C expression is dramatically altered in postmortem brain tissue from PD cases but not in Alzheimer disease, progressive supranuclear palsy, or multiple system atrophy. This disruption was paralleled in mice overexpressing mutated α-synuclein. These data establish SV2C as a mediator of dopamine neuron function and suggest that SV2C disruption is a unique feature of PD that likely contributes to dopaminergic dysfunction.
Synaptic vesicles, particularly those within the dopaminergic nigrostriatal pathway, have two important roles: packaging transmitters for neurotransmission and sequestering compounds, such as cytosolic dopamine, that may have adverse effects on the cell (1–3). Ineffective sequestration of dopamine leads to progressive cell loss and heightened vulnerability to dopaminergic toxicants in mice (4–10). Mutations in the gene encoding the vesicular monoamine transporter 2 (VMAT2) lead to infantile parkinsonism (11), whereas increased expression of VMAT2 is associated with decreased risk for Parkinson disease (PD) (12, 13). Dopamine vesicle function is impaired in patients with PD, suggesting that dysfunctional dopamine handling is fundamental to the disease (14). Genetic mutations in several other vesicle-associated proteins, such as α-synuclein, leucine-rich repeat kinase 2 (LRRK2), and synaptojanin, can lead to disrupted presynaptic dopamine handling and are commonly linked to PD (15–21). Disrupted vesicle function may represent a common pathway to degeneration, and identifying novel mediators of vesicular function could provide insight regarding disease pathogenesis.
Proteins within the synaptic vesicle glycoprotein 2 (SV2) family are thought to modulate vesicular function positively in a variety of ways, possibly by aiding in vesicular trafficking and exocytosis, interacting with synaptotagmin-1, or stabilizing stored transmitters (22–35). SV2A has been strongly implicated in epilepsy and is the specific pharmacological target for the antiepileptic levetiracetam (36); however, despite extensive research, the molecular function of each of the SV2 proteins has not been fully described. SV2C is distinguished from SV2A and SV2B by its enriched expression in the basal ganglia and preferential localization to dopaminergic cells in mice (37, 38). Intriguingly, SV2C was recently identified as a genetic mediator of one of the most robust environmental modulators of PD risk: nicotine use, which is strongly protective against PD (39, 40). Variation within the SV2C gene was also found to predict PD patients’ sensitivity to l-DOPA (41). These data suggest that SV2C may play a particularly important role in the basal ganglia, although there has been no experimental evidence linking SV2C to dopaminergic function, to the neurochemical effects of nicotine, or to PD.
We first characterized SV2C expression in multiple mouse models of PD to establish potential alterations in SV2C following dopaminergic cell loss. To test directly the hypothesis that SV2C is involved in dopamine function within the basal ganglia, we then developed a line of mice lacking SV2C (SV2C-KO mice). We quantified striatal dopamine content and dopamine metabolites in SV2C-KO animals with HPLC. We then investigated alterations in dopamine- and PD-related motor behavior and protein expression following genetic deletion of SV2C and evaluated a possible interaction between SV2C and α-synuclein. Next, we performed fast-scan cyclic voltammetry (FSCV) to measure stimulated dopamine release in the dorsal striatum (dSTR) of SV2C-KO and WT mice at baseline and in the presence of nicotine. Finally, we analyzed SV2C expression in the basal ganglia of postmortem PD cases and other neurodegenerative diseases. The data described below establish SV2C as a mediator of dopamine dynamics and provide a functional basis for a role for SV2C in PD.
Results
Distribution of SV2C in Mouse Basal Ganglia.
To analyze the expression and localization of SV2C, we designed two polyclonal antibodies raised in rabbits against amino acids 97–114 of (i) mouse SV2C (mSV2C; antibody = mSV2CpAb) and (ii) human SV2C (hSV2C; antibody = hSV2CpAb). To confirm the specificity of the hSV2CpAb, we performed immunoblots on lysates of transfected HEK293 cells overexpressing SV2A, SV2B, or SV2C. The hSV2CpAb immunoreactivity was present in the lysate from SV2C-transfected cells and not in the lysate from SV2A- or SV2B-transfected cells. Preincubating the hSV2CpAb with the immunizing peptide (“antigen-blocking”) blot ablates the immunoreactivity of the antibody on the same blot (Fig. 1A). We performed shRNA knockdown of SV2C in Neuro-2a (N2a) cells, which are mouse derived and endogenously express SV2C but not SV2A or SV2B (42). Two sequences of shRNA targeting SV2C mRNA were designed, along with a scrambled shRNA sequence. SV2C shRNA robustly reduced normal SV2C immunoreactivity in N2a cell lysates (Fig. 1C).
Fig. 1.
Polyclonal SV2C antibody characterization. We generated polyclonal SV2C antibodies. (A) SV2C antibody recognizes SV2C but does not recognize SV2A or SV2B in transfected HEK293 cells. Preincubating the SV2C antibody with the immunizing peptide (antigen blocking) eliminates antibody reactivity. (B) SV2C is highly colocalized with the dopaminergic marker TH in the mouse SNpc and dSTR. (Scale bars, 20 μm.) (C) Knocking down endogenous SV2C in N2a cells (which have no endogenous SV2A or SV2B) using shRNA reduces mSV2C antibody reactivity. (D) Sagittal DAB-stained sections showing ubiquitous SV2A and SV2B expression compared with SV2C expression enriched in the basal ganglia using the mSV2C antibody. Black arrowheads indicate areas of highest SV2C staining in the mouse brain: midbrain, dSTR, and ventral pallidum. Each sagittal image was compiled from a series of eight micrographs taken across a single section of tissue.
Staining patterns observed with our mSV2CpAb were consistent with reports using other SV2C antibodies (37, 38). SV2A and SV2B are strongly expressed throughout the mouse brain, whereas SV2C is preferentially expressed in limited nuclei (Fig. 1D). We identified strong SV2C immunoreactivity in the ventral pallidum (VP), as well as in cell bodies in the midbrain (substantia nigra pars compacta, SNpc, and ventral tegmental area, VTA). SV2C-positive fibers were additionally observed in the substantia nigra pars reticulata (SNpr), dSTR, and nucleus accumbens. SV2C was highly colocalized with the dopaminergic marker tyrosine hydroxylase (TH) in the dSTR and substantia nigra (SN) (Fig. 1B).
SV2C Expression in Models of Dopamine Degeneration.
Next, we explored SV2C expression in mouse models of dopamine cell loss. Each model recapitulates a distinct pathogenic process of cell loss associated with PD, allowing us to evaluate the potential involvement of SV2C in various mechanisms of PD-related degeneration.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication in mice.
To investigate whether SV2C expression is altered following a lesion to the nigrostriatal tract, we administered three different doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to animals to model various stages of degeneration. A 15 mg/kg dose delivered two times/d, which typically induces a mild loss of striatal dopamine terminals, resulted in a 52% loss of striatal TH immunoreactivity, as measured by immunoblotting [saline: 10,700 ± 996 arbitrary units (AU); MPTP: 5,010 ± 330 AU; P < 0.01]. A 15 mg/kg dose delivered four times/d, which results in an intermediate loss of both dopamine terminals and SNpc cell bodies, induced a 68% loss of striatal TH. Intoxication by a 20 mg/kg delivered five times/d, a dose that typically results in significant SNpc cell loss, ablated 86% of striatal TH (saline: 6, 310 ± 104 AU; MPTP: 916 ± 91.3 AU; P < 0.0001). Concordant with dopamine terminal loss, striatal SV2C levels were reduced after MPTP (n = 6, P < 0.05 for the 4× 15 mg/kg paradigm) (Fig. 2A). SV2C expression patterns appear unaltered after MPTP at any dose.
Fig. 2.
SV2C expression in mouse models of PD. (A) Striatal TH is 68% reduced after a 4× 15 mg/kg dose of MPTP (n = 6, P < 0.01). Striatal SV2C is reduced after MPTP (P < 0.05). Representative immunohistochemical staining after MPTP reveals that striatal SV2C expression patterns are unchanged. (B) Striatal SV2C levels are slightly, but not significantly, reduced in aged VMAT2-LO animals. SV2C expression patterns remain unaltered, as shown by representative immunohistochemistry in the dSTR. (C) An increase of SV2C-positive punctate staining is observed in the striatum of A53T-OE mice as compared with WT mice, particularly in the periventricular mediodorsal striatum. Clusters of punctate SV2C are found distributed sparsely in the striatum of A53T-OE mice. (Insets) Micrographs were taken at 2.5× and 40× magnification.
VMAT2-LO model of PD.
To determine whether long-term dysregulation of dopamine storage results in altered expression of SV2C, we evaluated SV2C in animals with a 95% reduction in VMAT2 expression (VMAT2-LO). This genetic reduction in VMAT2 results in reduced vesicular storage of dopamine, progressive loss of dopamine cells, and motor and nonmotor impairments (4, 8, 10). As expected, in aged (20- to 24-mo-old; n = 3) VMAT2-LO mice, we observed a 53% loss of striatal dopamine transporter (DAT) (Fig. 2B). This terminal loss is consistent with an observed slight but statistically insignificant reduction in striatal SV2C, with no change in expression pattern.
A53T α-synuclein overexpression in mice.
To determine if α-synuclein disruption alters SV2C, we explored SV2C expression in mice overexpressing PD-associated A53T α-synuclein (A53T-OE mice) under control of the pituitary homeobox 3 (Pitx3) promoter, limiting overexpression to midbrain dopamine neurons. These mice display reduced dopamine release, progressive dopamine terminal loss, and subsequent motor impairment by 12 mo of age (43). Dopaminergic overexpression of A53T α-synuclein in aged (12-mo-old) animals (n = 4 mice) resulted in an increase in punctate SV2C staining observed in the striatum compared with WT littermates (Fig. 2C). This punctate staining was particularly strong in the periventricular region of the dorsomedial striatum and was sparse with dense clusters of puncta throughout the dorsolateral striatum.
Genetic Deletion of SV2C Reduces Striatal Dopamine Content Without Overt Changes in Related Proteins.
To determine a potential role of SV2C in dopamine-related neurochemistry and behavior, we generated a line of SV2C-KO mice (Fig. 3 A–C). We performed HPLC in dorsal striatal tissue of SV2C-KO and WT animals to determine if genetic deletion of SV2C altered dopamine tone or metabolism. SV2C-KO animals had a 33% reduction in striatal dopamine content (WT: 741.8 ± 53.6 ng/mL; KO: 496.8 ± 53.8 ng/mL; P < 0.01; n = 7) (Fig. 3D) and a 19% reduction in 3, 4-dihydroxyphenylacetic acid (DOPAC), a dopamine metabolite (WT: 113.5 ± 5.5 ng/mL; KO: 91.85 ± 6.5 ng/mL; n = 7; P < 0.05) (Fig. 3E). This reduction in dopamine content does not appear to be the result of reduced TH, the rate-limiting enzyme in dopamine synthesis, and it does not result in up- or down-regulated DAT as assessed by immunoblot. Further, SV2C deletion does not appear to induce compensatory changes in the levels of either SV2A or SV2B or in total dopamine synapse number in the dSTR as measured by synaptophysin expression (Fig. 3B) and synapse density analysis (Fig. 3F).
Fig. 3.
Neurochemical characterization of SV2C-KO mice. (A) PCR genotyping of SV2C-KO mice generated using the EUCOMM knockout-first allele. This construct allowed the generation of several useful lines of mice: a preliminary knockout animal with a cassette creating an frt-flanked gene trap inserted into the second exon (KOF), a line with a floxed exon following a cross of KOF animals with a Flp-recombinase+ line, and finally SV2C-KO mice following a cross with a nestin-driven Cre-recombinase+ line. Animals used for experiments are denoted by a superscript 1 (WT control) and 2 (SV2C-KO). (B) SV2C knockout does not result in altered expression of SV2A, SV2B, TH, DAT, or synaptophysin in the striatum. (C) Genetic deletion of SV2C ablates mSV2C antibody reactivity in the dSTR and midbrain. The dotted line delineates the dorsolateral striatum where electrochemical recordings were taken (Figs. 6 and 7). (D and E) SV2C knockout results in a 33% reduction of dopamine content (D) and a 19% reduction in the dopamine metabolite DOPAC (E) in the dSTR (n = 7, **P < 0. 01). (F) SV2C knockout does not result in a reduction in dopaminergic synaptic density in the dSTR. ns, not significant. (Scale bars, 500 µm in C and 20 μm in F.)
An Interaction Between SV2C and α-Synuclein.
Following the observation of disrupted SV2C in A53T-OE mice, we performed immunoblotting and immunoprecipitations to investigate further interaction between SV2C and α-synuclein. SV2C-KO animals had slightly reduced monomeric (∼15 kD) α-synuclein (69.1 ± 6.17% of WT; n = 4–6; P = 0.06) and significantly increased high molecular weight (multimeric, ∼90 kD) α-synuclein expression (3,020 ± 561% of WT; n = 4–6; P < 0.01) (Fig. 4A). In striatal preparations from tissue of WT C57BL/6J mice, α-synuclein coimmunoprecipitated with SV2C. As expected, neither TH nor DAT coimmunoprecipitated with SV2C (negative control). Synaptotagmin-1, which binds to SV2C (31, 34), did coimmunoprecipitate (positive control). VMAT2 did not coimmunoprecipitate with SV2C, indicating that we immunoprecipitated protein complexes rather than intact vesicles. Further, using a control immunoprecipitation column with no bound antibody, we confirmed the specificity of the interaction between α-synuclein and mSV2CpAb-bounds resin (Fig. 4C). We did not detect SV2C immunoreactivity in an α-synuclein immunoprecipitation. Finally, to address the specificity of SV2C disruption in A53T-OE mice, we performed additional immunohistochemistry of related synaptic proteins. SV2C-positive puncta did not reflect similar patterns of α-synuclein aggregation (Fig. 4D). SV2A expression was not similarly disrupted (Fig. 4E).
Fig. 4.
An association between SV2C and α-synuclein. (A) Midbrain homogenates from SV2C-KO animals have a 31% decrease in monomeric (15-kD) α-synuclein (n = 4–6, P = 0.06) and a 30-fold increase in multimeric (90-kD) α-synuclein (n = 4–6, P < 0.01). (B) α-Synuclein coimmunoprecipitates with SV2C in WT mouse striatal homogenates. TH, a cytosolic protein, and DAT, a membrane-associated protein, were included as negative controls. VMAT2 was included as a vesicular protein control. Synaptotagmin-1 (STG-1), which binds to SV2C, was used as a positive control. (C) Further confirmation of α-synuclein and SV2C coimmunoprecipitation. A non–antibody-bound immunoprecipitation (No AB) column served as negative control. Striatal homogenates from multiple animals were pooled and aliquoted to form the input for all three immunoprecipitations. (D and E) By immunohistochemistry, the patterns of the expression of SV2C-positive puncta are not similar to those of SV2A (D) or α-synuclein (E).
SV2C-KO Animals Have Mild Motor Deficits.
To determine if SV2C knockout results in altered motor behavior consistent with their decreased dopamine content, we assessed motor function in a variety of tests. We performed a 24-h locomotor activity assay, gait analysis, and a rotorod test and found that SV2C knockout was associated with mild motor deficits. SV2C-KO animals exhibited reduced locomotor activity, with a 23% reduction in total ambulations over 24 h (WT: 38,900 ± 2,770 ambulations; KO: 30,100 ± 2,200 ambulations; n = 7 or 8; P < 0.05). Additionally, SV2C-KO mice displayed a shorter stride length (WT: 7.42 ± 0.17 cm; KO: 6.87 ± 0.22 cm; n = 22–24; P < 0.05). SV2C-KO animals showed no differences on latency to fall during the rotorod test (WT: 18.3 ± 3.01 s; KO: 20.4 ± 3.36 s; n = 7 or 8; P = 0.52) (Fig. 5). Additionally, SV2C-KO animals had a lower average body size (WT: 19.06 ± 0.91 g; KO: 17.24 ± 0.99 g; n = 53; P < 0.05).
Fig. 5.
Altered motor behavior of SV2C-KO mice. (A) SV2C-KO animals have an ∼10% reduction in stride length as measured by a gait analysis assay (*P < 0.05). (B) Genetic deletion of SV2C does not result in impairment on the rotorod test. (C) SV2C-KO animals display a 23% reduction in total ambulations in 24-h locomotor activity monitoring (n = 7 or 8; *P < 0.05). (D) The reduction in locomotor activity apparently is driven by an ablation of a circadian peak in activity at the end of the active period. The shaded region in D indicates the dark phase.
Genetic Deletion of SV2C Results in Reduced Striatal Dopamine Release.
To determine if the observed behavioral deficits of SV2C-KO mice were associated with reduced striatal dopamine release, we performed ex vivo FSCV in the dorsolateral striatum of SV2C-KO animals (see Fig. 3B for the delineation of the dorsolateral striatum). Genetic deletion of SV2C led to a 32% decrease in dopamine release compared with WT animals (WT: 5.47 ± 0.35 µM; KO: 3.71 ± 0.57 µM; P < 0.05; n = 9) (Fig. 6). The DAT-mediated dopamine clearance rate was enhanced in SV2C-KO animals as evidenced by reduced tau (WT: 0.39 ± 0.03 s; KO: 0.29 ± 0.02 s; P < 0.05).
Fig. 6.
Electrochemical measurement of stimulated dopamine release in SV2C-KO mice. Using FSCV, we measured dopamine release stimulated by a single electrical pulse in the dSTR. Genetically ablating SV2C reduces dopamine release by 32% as shown by a representative color blot (A), as quantified over all recording sites in each animal (n = 9; *P < 0.05) (B), and as represented by respective current traces (C).
SV2C Mediates a Neurochemical Effect of Nicotine.
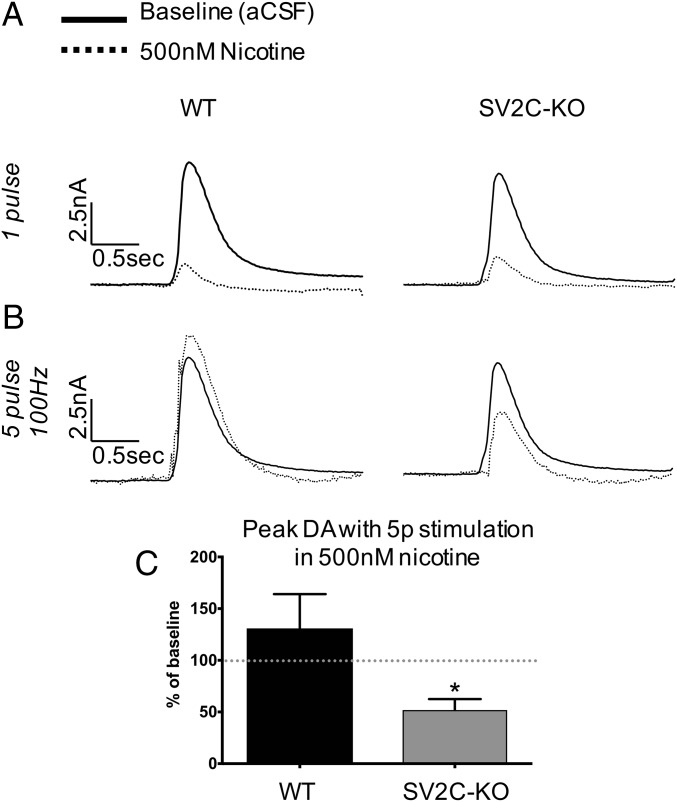
To assess a potential functional interaction between SV2C and nicotine, we performed FSCV in the presence of 500 nM nicotine. This concentration of nicotine typically dampens dopamine release elicited by single-pulse stimulation while enhancing dopamine release elicited by high-intensity stimulation (e.g., five pulses at 100 Hz) (44, 45). Genetic deletion of SV2C ablated this effect. In WT tissue, 500 nM nicotine enhanced the dopamine release elicited by five pulses at 100 Hz to 131% of baseline; in SV2C-KO tissue, 500 nM nicotine reduced the dopamine release elicited by five pulses at 100 Hz to 48% of baseline (P < 0.01). These data are presented as current traces (Fig. 7 A and B) and as peak dopamine release as a percentage of baseline release (Fig. 7C).
Fig. 7.
Altered neurochemical effect of nicotine after genetic ablation of SV2C as measured by FSCV. (A) Current traces showing dopamine release at baseline and in the presence of 500 nM nicotine. Nicotine normally reduces dopamine release elicited by a one-pulse stimulation to about 10% of baseline in both WT and SV2C-KO striatum. (B) Five-pulse stimulations in the presence of nicotine normally increase dopamine release over the one-pulse baseline, but this effect is not seen in SV2C-KO animals. (C) Maximal dopamine release elicited by a five-pulse stimulation in the presence of nicotine represented as percent of baseline (*P < 0.05).
SV2C Expression in Human Controls and Disease Cases.
To explore a potential involvement of SV2C in human PD, we obtained human striatum and midbrain samples from tissue banks at Emory University and the University of Washington. Control and disease cases were matched for age (72.4 ± 3.9 and 71.7 ± 2.3 y, respectively) and sex (71% and 68% male, respectively). Four PD cases, three comorbid dementia with Lewy bodies (DLB)/PD cases, seven Alzheimer disease (AD) cases, three cases of multiple system atrophy (MSA), including one with comorbid DLB and one with comorbid olivopontocerebellar atrophy (OPC), two progressive supranuclear palsy (PSP) cases, and seven age-matched controls were examined (Table 1). MSA and PSP were chosen for their clinical and pathological similarities to PD, and AD was chosen as a non-basal ganglia neurodegenerative disease.
Table 1.
Descriptions of human control and disease cases
| Disease and case no. | Neuropathologic diagnosis | Sex | Age, y | Illness duration, y | SV2C pathology* |
| Controls | |||||
| 1 | – | M | 72 | – | None |
| 2 | – | F | 80 | – | None |
| 3 | – | M | 78 | – | None |
| 4 | – | F | 88 | – | None |
| 5 | – | M | 61 | – | None |
| 6 | – | M | 69 | – | None |
| 7† | – | M | 59 | – | STR: ++ |
| PD | |||||
| 8 | PD | M | 78 | 32 | STR: +++ |
| 9 | PD | M | 64 | 12 | STR: +++ |
| 10 | PD | M | 81 | 10 | STR: ++/ |
| SN: +++ | |||||
| 11 | PD | M | 67 | 7 | STR: ++/SN: ++ |
| 12 | DLB/PD | M | 68 | 21 | STR: ++ |
| 13 | DLB/PD | F | 62 | 4 | STR: + |
| 14 | DLB/PD | M | 72 | 11 | STR: +/SN: + |
| AD | |||||
| 15 | AD | M | 63 | Unknown | None |
| 16† | AD | M | 75 | Unknown | STR: ++ |
| 17 | AD | F | 81 | Unknown | None |
| 18 | AD | M | 50 | Unknown | None |
| 19 | AD | F | 87 | Unknown | None |
| 20 | AD | F | 59 | Unknown | None |
| 21 | AD | M | 79 | Unknown | None |
| MSA/PSP | |||||
| 22 | MSA | M | 61 | Unknown | None |
| 23 | MSA/OPC | F | 80 | Unknown | None |
| 24 | MSA/DLB | M | 78 | Unknown | None |
| 25 | PSP | M | 72 | Unknown | None |
| 26 | PSP | F | 86 | Unknown | None |
SV2C disruption classification: +, SV2C-positive cell bodies plus sparse SV2C-positive puncta; ++, abundant SV2C-positive puncta with some SV2C-positive cell bodies;.+++, SV2C immunoreactivity almost entirely punctate.
Cases 7 and 16 had moderate SV2C disruption, suggesting that the disruption of SV2C expression may not be exclusive to PD; however, comorbidity with subclinical PD may be a possibility in these cases. SN, substantia nigra; STR, striatum.
We performed SV2C immunohistochemistry in the SN and striatum (Fig. 8). We observed SV2C-positive staining in the SNpc and in the SNpr as well as in terminal regions and cell bodies in the caudate nucleus and putamen. In PD, an unexpected and striking alteration in SV2C expression was revealed. We observed abnormal punctate SV2C-positive staining in the SNpc and a similar pathology throughout the dSTR, including the putamen (Fig. 8A). This disruption in SV2C expression was found in each PD case, although it was less severe in the three cases with neuropathology consistent with comorbid DLB. Striatal SV2C staining was largely unaltered in cases of AD, PSP, and MSA (Fig. 8B), suggesting that a disruption in SV2C may be a unique feature of PD. Finally, SV2A, α-synuclein, and synaptophysin do not show a similar pattern of expression in PD (Fig. 8 C–E). SV2C puncta are ubiquitin-positive (Fig. 8F).
Fig. 8.
SV2C expression is specifically disrupted in PD basal ganglia. Representative micrographs demonstrate SV2C staining in human SNpc and putamen. (A) In PD, SV2C expression is significantly disrupted in the SNpc and putamen. SV2C-positive puncta are distributed throughout the SNpc and putamen in PD but not in age-matched controls. (B) Representative micrographs indicate that SV2C staining is relatively normal in the putamen of representative DLB/PD, MSA, PSP, and AD cases. (C) SV2A is not disrupted in PD putamen. (D) SV2C puncta do not reflect putamen α-synuclein disruption. (E) SV2C puncta also do not represent a more general disruption of synaptic vesicle proteins, because synaptophysin expression is preserved in PD. (F) SV2C puncta also are ubiquitin positive. (Scale bars, 20 µm.)
Discussion
The present data reveal that SV2C mediates dopamine dynamics and is disrupted in PD. We demonstrate that SV2C and α-synuclein interact, suggesting an important involvement in disease pathogenesis. Further, we show that SV2C regulates dopamine release and dopamine content, as well as a neurochemical effect of nicotine, providing a molecular link to data from recent genome-wide association studies (GWAS) identifying SV2C as a mediator of nicotine neuroprotection (40). Results from these experiments establish a previously unrecognized role for SV2C and a basis for the potential functional consequence of the disruption of SV2C and nicotine neuroprotection in PD.
SV2C Expression in Models of PD.
As expected, striatal SV2C expression was slightly decreased following significant loss of dopaminergic terminals in mouse models of PD (Fig. 2). Unexpectedly, SV2C-positive puncta were observed in the striatum of A53T-OE animals. We did not observe similar punctate staining after MPTP lesion or in control animals. We modeled varying degrees of dopamine cell loss with multiple MPTP paradigms, including a moderate dose (4× 15 mg/kg) that closely matches the dopaminergic loss observed in A53T-OE mice. This finding indicates that the observed disruption in SV2C is related to a distinct pathogenic mechanism that is not recapitulated by mere nigrostriatal dopaminergic degeneration. Unlike the A53T-OE model, MPTP administration in mice does not typically induce α-synuclein disruption (46), thus further indicating that a link between α-synuclein and SV2C dysregulation may be particularly important. It is possible that SV2C pathology is not observed after MPTP intoxication because of the relatively acute nature of the lesion; however, we did not observe SV2C deposition in a genetic model, VMAT2-LO mice (4), suggesting that even a life-long pathogenic mechanism leading to dopamine cell loss is not sufficient to spur similar SV2C disruption.
Genetic Deletion of SV2C Results in Reduced Dopamine and Motor Deficits.
To explore further any functional consequences of disrupted SV2C on the dopamine system in humans or animal models, we first needed to characterize the involvement of the protein in basal ganglia functioning. SV2 proteins appear to promote vesicular function in various ways: by coordinating vesicular mobilization (26, 33), by interacting with synaptotagmin-1 to facilitate calcium-stimulated exocytosis (22, 27, 28, 31, 32, 34), or perhaps by stabilizing intravesicular transmitters (30, 35) [although functional evidence has not supported this last hypothesis (23, 24, 27)]. Although only SV2A has been strongly implicated in epilepsy, recent evidence has hinted at a potential link between SV2C, dopamine, and PD (40, 41, 47). Our neurochemical data directly indicate that SV2C plays a particularly important role in the dopaminergic nigrostriatal pathway. Here, we demonstrate that genetic deletion of SV2C results in a significant reduction in total dopamine content in the dSTR; this dampened dopamine tone does not appear to result from aberrant dopamine metabolism, because the ratio of dopamine to its metabolites is unaltered (Fig. 3). Pursuant to the observed reduction in total dopamine, genetic deletion of SV2C results in impaired release of dopamine as measured by FSCV (Fig. 6). The mechanism underlying this observed reduction in dopamine content and release is unclear. It does not appear to be caused by reduced dopamine synthesis or reuptake, because levels of TH (the rate-limiting enzyme of dopamine synthesis) and DAT (the protein that removes dopamine from the synaptic cleft and returns it to the presynaptic terminal) remain intact (Fig. 3). In fact, DAT function appears to be increased as measured by the uptake constant tau, perhaps as a mechanism to compensate in part for reduced vesicular dopamine release. As noted above, SV2 proteins likely have multiple functions within the synapse, and striatal SV2A and SV2B do not appear to increase in response to SV2C knockout (Fig. 3). Accordingly, an absence of SV2C could have significant effects on dopamine synapse function through a diminished readily releasable pool of vesicles, impaired calcium-induced endocytosis of vesicles, disrupted vesicular trafficking, or perhaps impaired storage or retention of vesicular dopamine. Future studies will elucidate the mechanisms responsible for the observed reduction in dopamine tone and release following genetic deletion of SV2C.
Following the observation of reduced nigrostriatal dopamine, we accordingly demonstrate that SV2C-KO animals have deficits in dopamine-related motor behavior, including reduced stride length and reduced locomotor activity (Fig. 5). The reduction in locomotor activity observed in the SV2C-KO animals is driven primarily by apparent disruptions in circadian-mediated spikes of activity at the end of the wakeful period. This phenotype is consistent with other models of reduced dopamine vesicle function, such as VMAT2-LO mice that exhibit motor impairment, reduced locomotor activity, altered circadian rhythm, and smaller body size (4, 8). Notably, SV2C-KO animals are not impaired on the rotorod test, a test that the G.W.M. laboratory has previously identified as insensitive in detecting motor deficits after moderate dopamine loss (48). Importantly, these deficits do not appear to be reflective of deficits in other dopaminergic proteins or reduced synapse density. Indeed, the only significant alteration in protein expression was α-synuclein (Fig. 4). Together, these behavioral and neurochemical data underscore that SV2C is involved in basal ganglia function and provide support for a potential disease relevance for SV2C in PD.
SV2C Mediates a Neurochemical Effect of Nicotine.
Nicotine application normally results in a “high-pass filter” effect in which dopamine release in the presence of 500 nM nicotine is reduced upon low-intensity stimulations but enhanced upon high-intensity stimulations (44, 45). Nicotine thus is thought to increase the effects of salient inputs while dampening the effects of transient dopamine release. Genetic deletion of SV2C ablates this effect of acute nicotine so that high-intensity stimulations elicit less dopamine than baseline release (Fig. 7). This finding may indicate a functional interaction between SV2C and nicotine. The precise relationship between SV2C and nicotine neuroprotection remains unknown, but these data indicate that SV2C-KO animals may have altered neurochemical, behavioral, or neuroprotective response to nicotine. These data may hold relevance for recent GWAS data identifying SV2C as a genetic mediator of the neuroprotective effects of smoking (40).
A Link Between SV2C Expression and α-Synuclein Disruption.
Another link between SV2C and PD highlighted by our data is an association between SV2C and α-synuclein (Fig. 4). We demonstrate that α-synuclein coimmunoprecipitates with SV2C from striatal homogenates, indicating a physical and perhaps a functional interaction. We were unable to detect SV2C in an α-synuclein pull-down, perhaps because of the relative abundance of α-synuclein compared with SV2C, the difficulty in immunoprecipitating untagged native proteins from animal tissues, the inability of our α-synuclein antibody to immunoprecipitate α-synuclein oligomers, or the particular conditions of our immunoprecipitation conditions. Nonetheless, an interaction between SV2C and α-synuclein may be the basis for the mutual disruption of the two proteins we observed in our mouse models. SV2C-KO animals have increased expression of high molecular weight α-synuclein with a commensurate decreased in low molecular weight α-synuclein. This disruption is notable, because the ratio of monomeric to multimeric α-synuclein is altered in PD and is likely important in the induction of α-synuclein aggregation and toxicity (49–51). Monomeric α-synuclein is thought to be the least toxic α-synuclein species, whereas aberrant soluble oligomers cause cellular toxicity by a variety of mechanisms (52–56). Our findings are supported by previous evidence indicating that SV2C and α-synuclein gene transcripts are normally highly correlated but that this relationship is abolished in PD (57). These data suggest an association between SV2C and α-synuclein and that disruptions in α-synuclein or SV2C modify this interaction.
SV2C Disruption in PD Basal Ganglia.
Finally, we directly connect SV2C to human PD by demonstrating altered SV2C expression in PD basal ganglia (Fig. 8). SV2C is normally distributed throughout the basal ganglia in dopaminergic regions of the SNpc and striatum in humans. This pattern of expression was largely unaltered in control cases and in other neurodegenerative diseases affecting the basal ganglia, such as MSA, PSP, and DLB, as well as in non-basal ganglia–related AD. However, SV2C expression alone was consistently altered in PD: SV2C staining was almost entirely punctate in the nigrostriatal tract, revealing intra- and extracellular inclusions of SV2C. These data expose a pathologic feature not previously identified in PD. Deposition of SV2C does not appear to reflect more general synaptic vesicle perturbation, because neither SV2A nor synaptophysin shows a similar pattern of expression. SV2C inclusions also do not show a pattern of deposition similar to that of α-synuclein–positive Lewy bodies, because Lewy bodies are not typically found in the striatum of PD cases. Furthermore, SV2C puncta are also ubiquitin-positive, possibly indicating a pathological accumulation of protein. It is important to note that this pathology appears to be restricted chiefly to PD. Basal ganglia pathologies are characteristic of PD, MSA, PSP, and DLB, but SV2C disruption was consistently evident in PD and was less severe in PD with neuropathology consistent with comorbid DLB. Although MSA, PSP, and DLB are all classified as Parkinson Plus syndromes, they differ clinically, etiologically, and histopathologically from PD and are not effectively treated by dopamine replacement therapeutics (reviewed in ref. 58). The lack of SV2C pathology in PSP, DLB, and MSA further distinguishes them from PD and suggests that SV2C disruption is related to the concurrent α-synuclein disruption and dopaminergic degeneration characteristic of PD. It is unknown if similar SV2C disruption is present in forms of PD lacking Lewy body pathology, such as cases stemming from certain LRRK2 mutations (59). SV2C expression was largely unaffected in AD, further emphasizing that SV2C disruption likely is not related merely to a neurodegenerative disease state. As with other neurodegeneration-related protein disruptions, more research is required to characterize fully the alteration in SV2C expression in PD; however, these data directly connect SV2C protein to PD and strengthen previous data implicating SV2C as a genetic mediator of PD risk in certain populations.
A Role for SV2C and PD Pathogenesis.
The cause and implications of SV2C deposition in the basal ganglia are unclear. As our mouse data suggest, SV2C is particularly important presynaptically in regulating dopamine release and in maintaining dopamine tone. Disruptions in SV2C may negatively affect dopaminergic vesicular function, as well as neuron integrity and neurotransmission, thereby contributing to human disease progression. As such, modulating the function of SV2C to enhance or restore its ability to preserve proper basal ganglia neurotransmission may be a promising avenue for therapeutics. Indeed, SV2C is a feasible target for pharmacotherapy: SV2C binds botulinum neurotoxin A (42, 60), and its close family member, SV2A, is the specific target for the antiepileptic drug levetiracetam (36). Because SV2C is also localized to striatal GABAergic cells, manipulating SV2C function may have functional and therapeutic implications for both the dopamine and GABA neurotransmitter systems.
Finally, the severity and specificity of SV2C disruption in PD is striking. It suggests a pathologic feature unique to PD that further distinguishes it from other basal ganglia neurodegenerative disorders. SV2C is localized to the basal ganglia, is associated with variable PD risk in smokers, promotes proper dopamine homeostasis and motor function, and is disrupted in PD. Taken together, these data establish a role for SV2C in nigrostriatal dopamine neurotransmission and identify it as a potential contributor to PD pathogenesis.
Materials and Methods
Antibodies.
Two polyclonal anti-SV2C sera were raised in rabbits against peptides in the N terminus region (amino acids 97–114) of SV2C: one against mouse SV2C (mSV2C; sequence STNQGKDSIVSVGQPKG), and one against human SV2C (hSV2C; sequence SMNQAKDSIVSVGQPKG). Peptides were conjugated to Imject Maleimide Activated mcKLH (Thermo Scientific), and sera were generated for the G.W.M. laboratory by Covance Custom Immunology Services. The following primary antibodies were purchased commercially: DAT, TH, and synaptophysin (Millipore), β-actin (Sigma), SV2A (E-15) (Santa Cruz Biotechnology), SV2B and synaptotagmin-1 (Synaptic Systems), α-synuclein (BD Transduction Laboratories), and ubiquitin (Fitzgerald Industries). Biotinylated, HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch, and fluorescent secondary antibodies were purchased from Abcam.
Tissue Culture.
HEK293 and N2a cells (ATCC) were cultured according to standard protocols. The growth medium was either DMEM with 10% (vol/vol) FBS + 1% penicillin/streptomycin (for HEK293 cells) or Eagles’ minimum essential medium (EMEM) + 10% (vol/vol) FBS + 1% penicillin/streptomycin (for N2a cells). Transfections of SV2C and SV2A in pcDNA3.1 vectors were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. SV2C shRNA constructs were transfected via electroporation (Amaxa Nucleofector) according to the manufacturer’s protocols. Cells were harvested 24 h posttransfection and were lysed in radioimmunoprecipitation assay (RIPA) buffer; total protein extraction was achieved through differential centrifugation.
SV2C shRNA.
shRNA against SV2C were custom-designed by OriGene for the G.W.M. laboratory. Two SV2C-specific sequences (GACAGCATCGTGTCTGTAG and ATCGTGTCTGTAGGACAGC) significantly reduced the expression of endogenous SV2C expression in N2a cells. One scrambled sequence not corresponding to any sequence in SV2C had no effect on SV2C expression.
Western Blotting.
Western blots were performed as previously described (61). Primary antibodies were used at the following dilutions: SV2C, 1:2,500; DAT, 1:5,000; TH, 1:1,000; synaptotagmin 1, 1:1,000; synaptophysin, 1:1,000; α-synuclein, 1:1,000; and β-actin, 1:5,000. HRP-conjugated secondary antibodies were diluted to 1:5,000. For antigen blocking, mSV2CpAb was diluted 1:2,500 in a solution containing 1 mg/mL immunizing peptide and 0.75% nonfat powdered milk for 1 h at room temperature and then was applied to the immunoblot.
Animals.
Twelve-month-old male and female A53T-OE mice and WT littermates and 22-mo-old male and female VMAT2-LO mice and WT littermates were used for studies involving genetic models of PD. Six- to twelve-month-old male WT C57BL/6J mice (Charles River Laboratories) were used for MPTP studies. Three to six animals were included in each group for all experiments. All procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (62) and were previously approved by the Institutional Animal Care and Use Committee at Emory University.
MPTP Treatments.
Male mice were injected s.c. with MPTP hydrochloride (Sigma) or saline. The “terminal” lesion consisted of two injections of 15 mg/kg MPTP with an interinjection interval of 12 h. The 4× 15 mg/kg lesion consisted of four injections of 15 mg/kg MPTP in 1 d with an interinjection interval of 2 h. The “cell body” lesion consisted of five injections of 20 mg/kg MPTP with an interinjection interval of 24 h.
Transgenic Mouse Generation.
SV2C-KO mice.
We obtained three lines of C57BL/6 ES cells from the European Conditional Mouse Mutagenesis Program containing the SV2C “knockout-first construct” (63). ES cells containing the construct were injected into blastocysts. Chimeric mice were bred with C57BL/6J mice. We achieved germline transmission of the construct in resulting offspring and chose these mice to found the SV2C-knockout first line. This line was subsequently crossed with mice containing Flp-recombinase followed by mice containing Cre under a NES (nestin) promoter to delete the intron and found the SV2C-KO line. Animals were genotyped using PCR, and loss of SV2C protein was confirmed by immunoblot following behavioral and electrochemical experiments. PCR genotyping primers are as follows:
Sv2c (Exon 2): (Fw) TCA TCT AGA AGG GTT AAG GTC TGG, (Rev) ACC ATC ATC CCG AGG TAC AC
LoxP: (Fw) GCC TCA ACC AGA CCT AAG AA, (Rev) TAG GAA CTT CGG AAT AGG
LacZ: (Fw) GTC GTT TGC CGT CTG AAT TT, (Rev) CAT TAA AGC GAG TGG CAA CA
Cre: (Fw) CCT GGA AAA TGC TTC TGT CCG TTT GCC, (Rev) GAG TTG ATA GCT GGC TGG TGG CAG ATG
Flp: (Fw) CAC TGA TAT TGT AAG TAG TTT GA, (Rev) CTA GTG CGA AGT AGT GAT CAG G
Back-crossed VMAT2-LO mice.
Mice expressing 5% of normal VMAT2 levels were generated as described previously (4, 8, 64) and were back-crossed to a C57BL/6J genetic background (65).
A53T-OE mice.
Mice overexpressing the human PD-associated α-synuclein A53T missense mutation in nigrostriatal dopamine cells under the control of a Pitx3 promoter were generated as described previously (43).
Immunohistochemistry.
Paraffin-embedded human tissue.
Sections were deparaffinized in xylene and were rehydrated in decreasing concentrations of ethanol. Endogenous peroxidase was quenched with 3% (vol/vol) H2O2, followed by antigen retrieval in citrate buffer (pH 6.0) at 95 °C. Nonspecific antibody binding was blocked with 3% (vol/vol) normal horse serum. The primary antibody was diluted to 1:2,500 (hSV2CpAb) or 1:1,000 (all others). The biotinylated secondary antibody (1:200) signal was enhanced with an avidin-biotin complex (Vector Laboratories) and developed with a 3-3′ diaminobenzidine (DAB) reaction for ∼45 s. Fluorescent secondary antibodies were diluted 1:800, and autofluorescence was quenched with a 7-min incubation in 0.1% Sudan Black solution before coverslipping.
Frozen sections.
Mice were killed by rapid decapitation or transcardial perfusion. Brains were removed and placed in 4% paraformaldehyde for fixation followed by storage in 30% (wt/vol) sucrose. Brains were sectioned to 40 µm. Staining was conducted as described previously (61), except that all washes and dilutions were conducted in PBS with 0.2% Triton X-100. All tissue was imaged using a Zeiss AX10 microscope (brightfield, fluorescent) or an Olympus FV1000-TIRF microscope with a 60× oil-immersion objective (confocal).
Quantification of Synapse Density.
Confocal micrographs (60×) were taken at a resolution of 3.7 pixels/μm. Images were analyzed with ImageJ software. Puncta with a minimum size of four pixels and an intensity of at least 20% of average brightness were identified and quantified. Three random sampling sites (212 μm2) from three sections of each animal were analyzed, and three animals per genotype were included.
HPLC in Striatal Tissue.
HPLC for striatal dopamine and DOPAC content was performed as described previously (61). Briefly, unilateral striatal dissections were sonicated in 10× their weight of 0.1 M perchloric acid and were pelleted at 10,000 × g for 10 min. Supernatants were filtered at 0.2 µm. Dopamine and DOPAC were detected using an MD-150 × 3.2 mm C18 column (ESA). The mobile phase consisted of 1.5 mM 1-octanesulfonic acid sodium, 75 mM NaH2PO4, 0.025% triethylamine, and 8% (vol/vol) acetonitrile at pH 2.9. A 20-µL sample was injected.
Immunoprecipitation of SV2C and α-Synuclein.
Coimmunoprecipitation experiments were performed using the Pierce Co-Immunoprecipitation Kit (Thermo Scientific) according to the manufacturer’s protocols. mSV2CpAb was cross-linked to agarose beads. WT animals were killed by rapid decapitation, and a bilateral striatal dissection was performed followed by homogenization and centrifugation to achieve a crude synaptosomal preparation. Samples were treated with immunoprecipitation lysis/wash buffer [25 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, and 5% glycerol] and were incubated with antibody-bound resin overnight at 4 °C. Protein complexes were eluted with low-pH elution buffer.
Locomotor Activity.
Animals were individually placed in locomotor activity monitoring boxes 2 h before the onset of the dark (waking) period. Beam-breaks detected by accompanying software were recorded as a measure of total ambulations for a 24-h observation period (61).
Gait Analysis.
Animals were trained to walk along a clean paper path to their home cage. On the testing day, forepaws were dipped in water-soluble ink, and animals were prompted to transverse the paper. Stride length was measured as the total toe-to-toe distance across an average of three steps (48).
Rotorod.
Animals were trained to balance on a slowly rotating rod. On the test day, animals were placed on the rod which accelerated to 12 rpm over 10 s. Latency to fall, speed of rotation at the time of fall, and total distance traveled was recorded and averaged over three testing trials (48).
FSCV in Striatal Slices.
FSCV was performed as described previously (61, 65, 66) in 300-μm striatal slices bathed in 30 °C oxygenated artificial cerebrospinal fluid (aCSF). Stimulations consisted of either a single 700-μA 4-ms monopolar pulse (baseline release) or five pulses at 100 Hz (high-intensity stimulation). Four to five single-pulse recordings were taken at each of four dorsolateral striatal sites with a 5-min interstimulus rest period followed by one five-pulse, 100-Hz stimulation recording. Electrode sensitivity was calibrated to known dopamine standards using a flow-cell injection system. Maximum dopamine release was averaged across sites, and kinetic constants were calculated using nonlinear regression analysis of dopamine release and uptake.
Acute Nicotine Application.
An aCSF bath was replaced with increasing concentrations of nicotine in aCSF: 10, 50, 100, and 500 nM for 30 min each. Five single-pulse recordings were taken in each concentration, followed by one five-pulse, 100-Hz stimulation recording taken in 500 nM nicotine.
Statistics.
All data were analyzed in GraphPad Prism. Differences between genotypes or treatments were compared by two-tailed t tests. Densitometric analyses were performed with Image Lab software. All errors shown are SEM, and significance was set to P < 0.05.
Data Management.
All primary data are available upon request.
Acknowledgments
We thank Dr. Marla Gearing for expert insight and Merry Chen for excellent technical assistance. This research project was supported by National Institute of Environmental Health Services (NIEHS) Grants R01ES023839 and P30ES019776 (to G.W.M.), the Lewis Dickey Memorial Fund (G.W.M.), National Institute of Neurological Disorders and Stroke (NINDS) Grant F31NS089242 (to A.R.D.), NIEHS Grant K99ES024570 (to A.I.B.), the Neuropathology Core of the Emory Neuroscience NINDS Core Facilities supported by NIH Grant P30NS055077, The Udall Parkinson’s Disease Center at Emory University supported by NINDS Grant P50NS071669, The University of Washington Aging and Disability Resource Center Brain Bank supported by NIH Grant P50AB005136, and National Institute on Aging Intramural Research Program Grants NIA AG000928 and AG000929 (to H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Goldstein DS, et al. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126(5):591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: A review. Neurotox Res. 2000;1(3):181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 4.Caudle WM, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fon EA, et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19(6):1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 6.Mooslehner KA, et al. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: Potential mouse model for parkinsonism. Mol Cell Biol. 2001;21(16):5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi N, et al. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94(18):9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor TN, et al. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29(25):8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillot TS, et al. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem. 2008;106(5):2205–2217. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76(Pt A):97–105. doi: 10.1016/j.neuropharm.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rilstone JJ, Alkhater RA, Minassian BA. Brain dopamine-serotonin vesicular transport disease and its treatment. N Engl J Med. 2013;368(6):543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- 12.Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15(2):299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brighina L, et al. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson's disease. Neurobiol Aging. 2013;34(6):1712.e9–1712.e13. doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pifl C, et al. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci. 2014;34(24):8210–8218. doi: 10.1523/JNEUROSCI.5456-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belluzzi E, Greggio E, Piccoli G. Presynaptic dysfunction in Parkinson’s disease: A focus on LRRK2. Biochem Soc Trans. 2012;40(5):1111–1116. doi: 10.1042/BST20120124. [DOI] [PubMed] [Google Scholar]
- 16.Krebs CE, et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat. 2013;34(9):1200–1207. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 18.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41(14):4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 19.Cirnaru MD, et al. LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front Mol Neurosci. 2014;7(49):49. doi: 10.3389/fnmol.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccoli G, et al. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31(6):2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang W-P, Südhof TC. SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J Neurosci. 2009;29(4):883–897. doi: 10.1523/JNEUROSCI.4521-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowder KM, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci USA. 1999;96(26):15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci. 2006;26(4):1303–1313. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow ML, et al. Alignment of synaptic vesicle macromolecules with the macromolecules in active zone material that direct vesicle docking. PLoS One. 2013;8(7):e69410. doi: 10.1371/journal.pone.0069410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci. 2005;118(Pt 23):5647–5660. doi: 10.1242/jcs.02658. [DOI] [PubMed] [Google Scholar]
- 27.Janz R, Goda Y, Geppert M, Missler M, Südhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24(4):1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 28.Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem. 2004;279(50):52124–52131. doi: 10.1074/jbc.M407502200. [DOI] [PubMed] [Google Scholar]
- 29.Nowack A, Yao J, Custer KL, Bajjalieh SM. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 2010;299(5):C960–C967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reigada D, et al. Control of neurotransmitter release by an internal gel matrix in synaptic vesicles. Proc Natl Acad Sci USA. 2003;100(6):3485–3490. doi: 10.1073/pnas.0336914100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci. 2005;29(1):56–64. doi: 10.1016/j.mcn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Wan QF, et al. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron. 2010;66(6):884–895. doi: 10.1016/j.neuron.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol. 2001;3(8):691–698. doi: 10.1038/35087000. [DOI] [PubMed] [Google Scholar]
- 34.Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci. 2010;30(16):5569–5578. doi: 10.1523/JNEUROSCI.4781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vautrin J. SV2 frustrating exocytosis at the semi-diffusor synapse. Synapse. 2009;63(4):319–338. doi: 10.1002/syn.20610. [DOI] [PubMed] [Google Scholar]
- 36.Lynch BA, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101(26):9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dardou D, et al. Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Res. 2011;1367:130–145. doi: 10.1016/j.brainres.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 38.Janz R, Südhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: Anatomy of a synaptic vesicle protein family. Neuroscience. 1999;94(4):1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- 39.Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 40.Hill-Burns EM, et al. A genetic basis for the variable effect of smoking/nicotine on Parkinson’s disease. Pharmacogenomics J. 2013;13(6):530–537. doi: 10.1038/tpj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altmann V, et al. Influence of genetic, biological and pharmacological factors on levodopa dose in Parkinson’s disease. Pharmacogenomics. 2016;17(5):481–488. doi: 10.2217/pgs.15.183. [DOI] [PubMed] [Google Scholar]
- 42.Dong M, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312(5773):592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 43.Lin X, et al. Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32(27):9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7(6):583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7(6):581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 46.Shimoji M, Zhang L, Mandir AS, Dawson VL, Dawson TM. Absence of inclusion body formation in the MPTP mouse model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134(1):103–108. doi: 10.1016/j.molbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Dardou D, et al. A role for Sv2c in basal ganglia functions. Brain Res. 2013;1507:61–73. doi: 10.1016/j.brainres.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 48.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178(1):80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 49.Brown DR. Oligomeric alpha-synuclein and its role in neuronal death. IUBMB Life. 2010;62(5):334–339. doi: 10.1002/iub.316. [DOI] [PubMed] [Google Scholar]
- 50.Dettmer U, et al. Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138(Pt 6):1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi B-K, et al. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110(10):4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pieri L, Madiona K, Bousset L, Melki R. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J. 2012;102(12):2894–2905. doi: 10.1016/j.bpj.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts HL, Brown DR. Seeking a mechanism for the toxicity of oligomeric α-synuclein. Biomolecules. 2015;5(2):282–305. doi: 10.3390/biom5020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waxman EA, Giasson BI. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta. 2009;1792(7):616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhinn H, et al. Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat Commun. 2012;3:1084. doi: 10.1038/ncomms2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitra K, Gangopadhaya PK, Das SK. Parkinsonism plus syndrome--a review. Neurol India. 2003;51(2):183–188. [PubMed] [Google Scholar]
- 59.Gaig C, et al. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. J Neurol Neurosurg Psychiatry. 2007;78(6):626–628. doi: 10.1136/jnnp.2006.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580(8):2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 61.Lohr KM, et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci USA. 2014;111(27):9977–9982. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 63.Skarnes WC, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474(7351):337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taylor TN, Caudle WM, Miller GW. VMAT2-deficient mice display nigral and extranigral pathology and motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis. 2011;2011:124165. doi: 10.4061/2011/124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lohr KM, et al. Vesicular monoamine transporter 2 (VMAT2) level determines MPTP vulnerability and clearance of excess dopamine in mouse striatal terminals. Toxicol Sci. 2016;153(1):79–88. doi: 10.1093/toxsci/kfw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stout KA, et al. Selective enhancement of dopamine release in the ventral pallidum of methamphetamine-sensitized mice. ACS Chem Neurosci. 2016;7(10):1364–1373. doi: 10.1021/acschemneuro.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]