Significance
In this study we report the discovery of a previously unrecognized chemical class, dihydroxymagnesium carboxylates, [(HO)2MgO2CR]−, gained from nonterrestrial meteoritic analyses. The existence of such low-coordination organomagnesium anionic compounds expands our knowledge and understanding of extreme environments from which the early solar system emerged and has evolved. The appearance this CHOMg chemical class extends the previously investigated vast diversity of CHNOS groups in meteoritic soluble organics. Experimental evidence is given for the connection between the evolution of organic compounds and minerals. These thermostable compounds might have contributed to the stabilization of organic molecules on a geological time scale, which emphasizes their potential astrobiological relevance.
Keywords: metalorganic chemistry, meteorites, astrochemistry, Fourier transform ion cyclotron resonance mass spectrometry, organic evolution
Abstract
The rich diversity and complexity of organic matter found in meteorites is rapidly expanding our knowledge and understanding of extreme environments from which the early solar system emerged and evolved. Here, we report the discovery of a hitherto unknown chemical class, dihydroxymagnesium carboxylates [(OH)2MgO2CR]−, in meteoritic soluble organic matter. High collision energies, which are required for fragmentation, suggest substantial thermal stability of these Mg-metalorganics (CHOMg compounds). This was corroborated by their higher abundance in thermally processed meteorites. CHOMg compounds were found to be present in a set of 61 meteorites of diverse petrological classes. The appearance of this CHOMg chemical class extends the previously investigated, diverse set of CHNOS molecules. A connection between the evolution of organic compounds and minerals is made, as Mg released from minerals gets trapped into organic compounds. These CHOMg metalorganic compounds and their relation to thermal processing in meteorites might shed new light on our understanding of carbon speciation at a molecular level in meteorite parent bodies.
The molecular diversity of extraterrestrial organic matter in carbonaceous chondrites has been studied by means of both targeted (1–4) and nontargeted (5–7) analytical methodologies, which are complementary to each other. The targeted approach focuses on molecules of biological/prebiotic interest in greater detail, such as amino acids, nucleobases, or carbohydrates (8), overlooking other analytes. In the nontargeted approach all analytes are globally profiled to gain comprehensive information. As such, holistic nontargeted analyses of meteoritic soluble organic matter revealed a much higher degree of molecular diversity than that found in any organic matter of terrestrial origin, as observed in Murchison (5, 6). The Murchison meteorite (CM2 type, where CM refers to Mighei-type carbonaceous chondrite) is the most investigated meteorite, typically seen as an example of abiotic organic complexity and a model of the processes that occurred inside its asteroid parent body (5).
Few metalorganic compounds have hitherto been described in the meteoritic context (9), despite the close proximity and intercalation of the mineral and organic phases in meteoritic materials. Fioroni predicted the identification of metalorganic species in measureable quantities; however, these have not been detected yet, either by spectroscopic techniques or upon meteorite analyses. Carbonaceous meteorites, such as Murchison (CM2) or Orgueil (CI1), are heterogeneous in organic molecular species and their abundances (10, 11). These organic materials, including carboxylic compounds, are known to be mixed with Mg-rich phyllosilicates (11). The interaction of organic matter and minerals, especially clay minerals, plays an important role in the evolution of meteoritic organic matter via catalytic effects (12).
Mg is one of the most abundant elements in the solar system (13) and is an important component in many common rock-forming minerals. Furthermore, relative to other elements in the first three groups of the periodic table, Mg offers the highest propensities of forming metalorganic compounds (14), for example chlorophyll or Grignard reagents. Classical metalorganic compounds with a covalent Mg–C bond exhibited high binding energies with a distinct thermal robustness and an appreciable photostability (15). Mg commonly occurs as a divalent cation that is coordinated to six water molecules or other oxygen-containing ligands (16). Meteorites contain Mg-rich minerals (Fig. S1) (17) and complex organic compounds (1, 5), which are thought to evolve chemically, not simultaneously, in the early solar system (18, 19). For example, Fischer–Tropsch-type (FTT) reactions are believed to play an important role in providing pathways to form (complex) organic molecules. The reacting molecules in FTT reactions are CO, H2, and inorganic minerals as catalysts (20). Another hypothesis for organic matter formation is the mineral alteration by aqueous alteration (18). In both cases, minerals, including those that bear Mg (21), have a potential consequence on the organic chemistry in space.
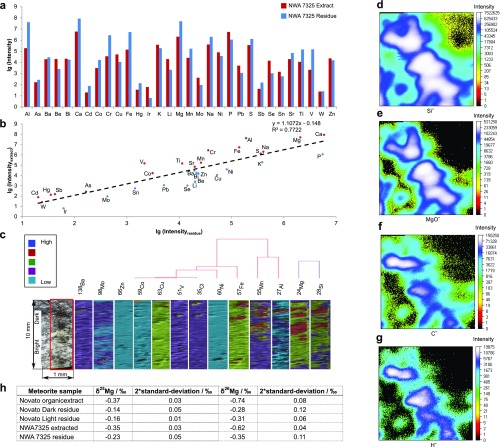
Fig. S1.
Elemental analysis. Element abundances in the methanolic extract and the residue sample of the NWA 7325 meteorite are shown, as obtained from ICP-MS data. (A) A bar graph with the decimal logarithm of the measured element’s intensities, provided for 31 elements in the extract (red) and in the residue (blue). (B) A linear regression between the extract’s intensity and the residue’s intensity, both expressed in the decimal logarithm, is presented to reflect the solubility potential of the measured elements. The red data points represent elements enriched in extracts, and the blue-labeled elements remain in residue. High abundances for Al, Ca, and Mg and volatile elements are enriched in the extract, with exceptions, mainly observed at trace levels. Spatially resolved elemental distribution of the Novato meteorite surface is depicted, as gained from LA-ICP-MS measurements, for the dark and the bright site of the specimen (C). HCA of 13 element profiles reveals extensive concordance of the abundant elements Mg and Si, which is in agreement with dominant magnesium silicates in the solid-state phase. SIMS analyses results of a glassy vein of the LL5 ordinary chondrite Chelyabinsk specimen are shown with the mapped intensities of Si−, MgO−, C−, and H− (D–G) This supports that the analyzed specimen is rich, both in C− and H− (likely organic matter), next to congruent areas of Si− and MgO− (magnesium silicates). (H) Mg isotope results of organic extract and whole-rock residues for Novato and NWA 7325.
Here, we demonstrate the occurrence and remarkable diversity of previously unrecognized CHOMg compounds within meteoritic soluble organic matter and present the chemical class of dihydroxymagnesium carboxylates, followed by a discussion of their chemical properties and reactivity. The exceptional molecular diversity makes meteorites ideal samples to elucidate fundamental chemical reactivity of organic compounds. Additionally, CHOMg signatures can be related to meteoritic thermal history and fractionation processes.
Results and Discussion
Evaluating the CHOMg Chemical Space.
Our methods and processes for conducting electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI-FT-ICR-MS) on soluble organics in meteorites are described in Materials and Methods and in SI Materials and Methods. To understand the nature of previously unassigned peaks, we studied 61 meteorites with different petrologic types, covering a wide range of meteorite classes (Table S1). The selected representative meteorites include achondrite Northwest Africa 7325 [NWA 7325, ungrouped (22)], ordinary chondrites Novato and Chelyabinsk (23, 24), and carbonaceous chondrite Murchison [CM2 (5)].
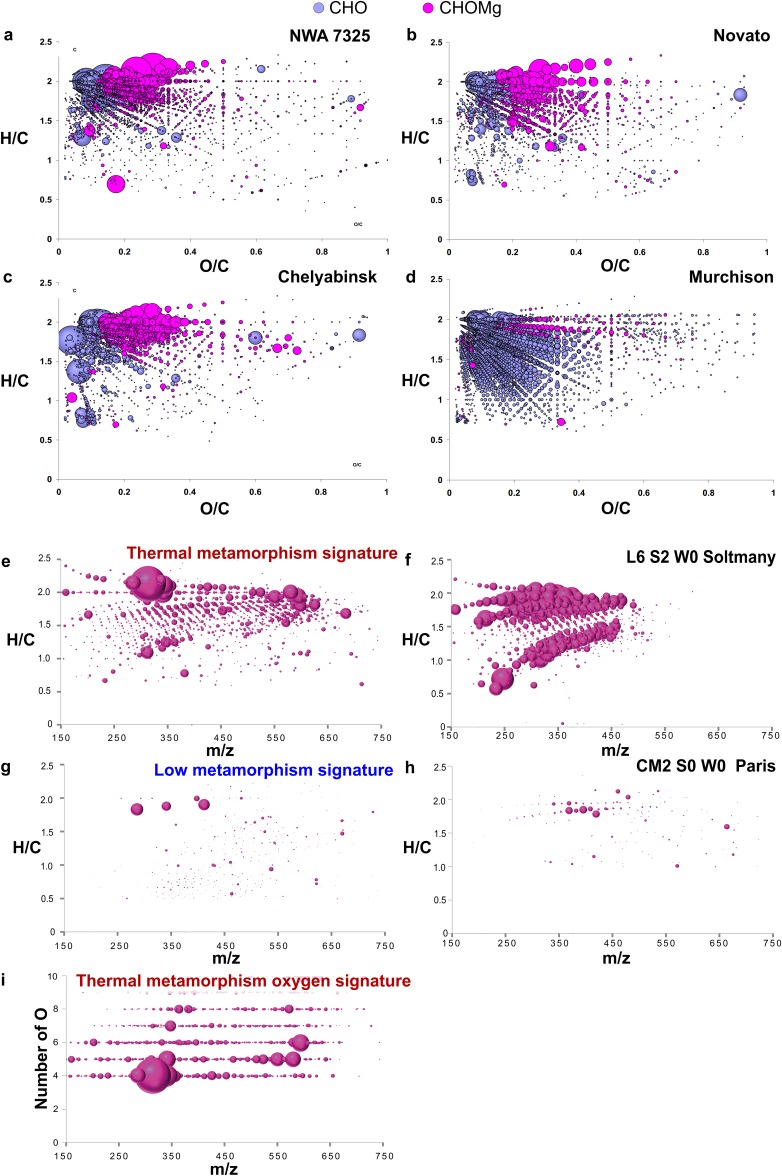
The mass spectra of the ungrouped achondrite NWA 7325 show a very dense CHNOS space of soluble organic compounds, comparable to ordinary chondrites (Fig. 1). Recurrent patterns of 876 unassigned mass peaks were discovered to which we assigned CHOMg formulas (Fig. 1). These mass peaks accounted for 22% of peaks in the soluble organic matter of NWA 7325, 26% in Novato, and 24% in Chelyabinsk, all of which underwent significant heating during petrogenesis, but only 2% in the comparatively primitive meteorite Murchison; absolute quantities are not directly accessible via ESI. Nevertheless, CHO and CHOMg compounds are observed in almost equal mass peak counts n for thermally stressed meteorites [n(CHO):n(CHOMg) ≈ 1:1]. CHO compounds represent the major soluble organic compounds in ordinary chondrites, ranging up to ∼300 ppm (1). Thus, CHOMg compounds are expected to be in a similar concentration range.
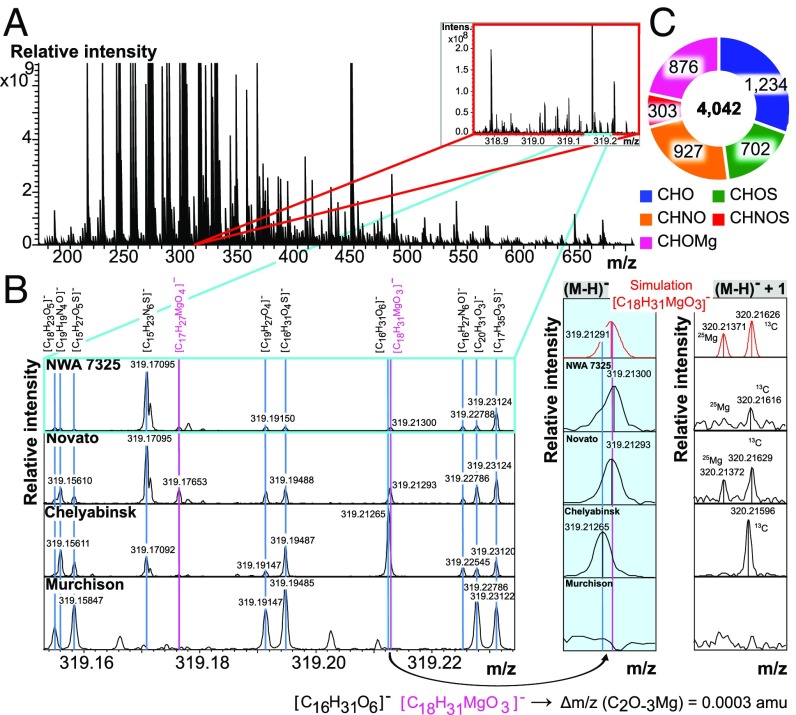
Fig. 1.
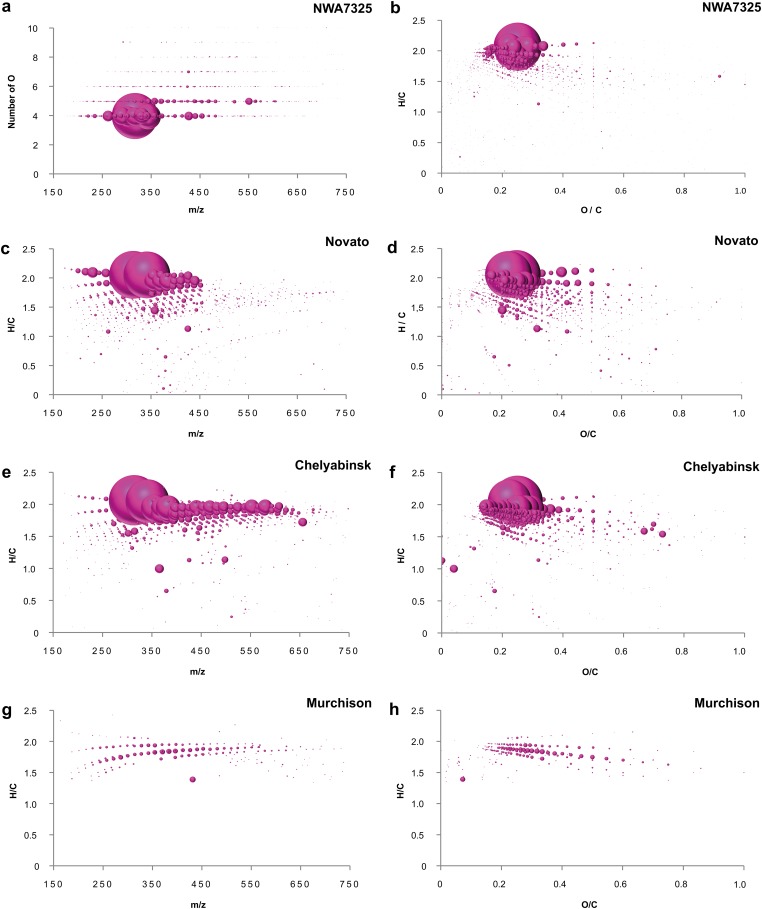
Detection of the CHOMg chemical space. Negative ionization mode ESI-FT-ICR mass spectrum of an ungrouped achondrite (NWA 7325) is shown (A). It is aligned together with two ordinary chondrites (Novato and Chelyabinsk) and a carbonaceous chondrite meteorite (Murchison) by CHNOS compounds (B). Some distinct mass peaks were detected that are nonaligned and represent CHOMg compounds (pink labels). Less than one electron mass difference (Δm/z = 0.0003 amu) between the isobaric molecule ions [C16H31O6]− and [C18H31MgO3]−, with the corresponding mass difference of C2O-3Mg, requires an ultrahigh mass resolving power and high mass accuracy to enable unambiguous differentiation between the CHNOS and the CHOMg chemical spaces. The second most abundant CHOMg isotopologue, here at m/z = 320, consists of two peaks (25Mg and 13C) of comparable amplitude. The specific presence of [C18H31MgO3]− is confirmed in NWA 7325 and Novato but excluded in Chelyabinsk and Murchison meteorites, which only display the single 13C-based peak and no second isotopologue mass peak at m/z = 320. (C) Relative abundances of NWA 7325 chemical species are depicted.
The unambiguous distinction between the CHNOS and the CHOMg chemical spaces requires an extremely high mass resolving power (R > 106) and mass accuracy (<200 ppb, Fig. 1) to differentiate mass differences less than the mass of an electron. At lower mass resolving power, CHOMg compositions would be largely occluded by merging with the CHNOS compositional space. To avoid any alignment error due to this m/z overlap, CHNOS compounds are shown to reveal the precise internal calibration (Fig. 1). The 24/25/26Mg isotopic fine structure analysis validated the existence of C-, H-, O-, Mg-based compositions (Fig. 1 and Fig. S2).
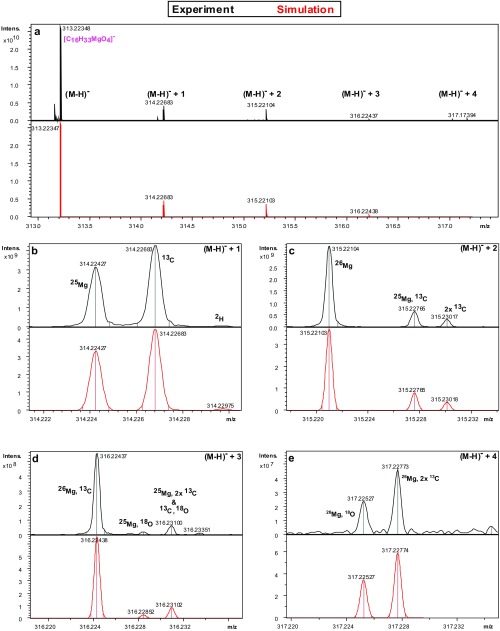
Fig. S2.
(A–E) Isotopic fine structure for the [C16H33MgO4]− molecule ion. [C16H33MgO4]− was identified via its isotopologues in the Novato methanolic extract, as seen in ESI-FT-ICR mass spectra. The black spectrum is the experimental mass spectrum from Novato extract, and the red profile represents the theoretically computed isotopic pattern at natural abundance of C, H, O and Mg. The observed isotopic distribution is primarily caused by the isotopes 24/25/26Mg, 12/13C, 16/18O and 1/2H. To separate each isotopologue at FWHM, a minimum mass resolving power of R ∼125,000 at m/z = 313 is required. Beyond CHOMg, the total combinatorial molecular complexity within the sample set includes N and S compositions. The mass resolving power of R ∼500,000 helps to exclude more complex combinatorial formula solutions, which contain, for example, nitrogen and sulfur and discriminates unambiguously the CHOMg from the CHNOS chemical compositions.
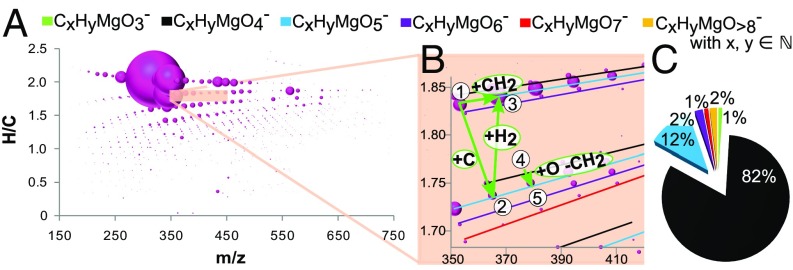
The diversity of CHOMg species within soluble organic matter of NWA 7325 highlights the complex chemical space that is occupied by these metalorganic compounds (Fig. 2 and Fig. S3). This van Krevelen-type representation shows several extended, methylene-based fairly complete homologous series (Fig. S3). The absence of odd–even preferences in alkyl chains testifies to a nonbiological origin of CHOMg compounds (25). Biological synthesis of fatty acids or general aliphatic chain molecules is usually a C2 unit propagation process (26). Therefore, the extraterrestrial origin (C1 step chemosynthesis) can be distinguished from a terrestrial synthesis environment. Compounds bearing four oxygen atoms (MgO4R−, with R = hydrocarbon CxHy and x, y ∈ ℕ) dominate the CHOMg chemical compositions (>80%, Fig. 2C and Fig. S3A) with a prevalence of nearly saturated aliphatics R, including long alkyl chains, which is uncommon for meteoritic soluble organic matter (6). The sequential traces of the CHOMg compositional space of the other three meteorites, Novato, Chelyabinsk, and Murchison, demonstrate their wide molecular ranges and diversity (Fig. S3).
Fig. 2.
Characteristics of the CHOMg chemical space. CHOMg chemical compositions of NWA 7325 soluble organic matter are depicted by mass-edited H/C ratio, for the complete (A) and zoomed-in compositional space (B), illustrating the density of fairly complete homologous series within the CHOMg compositional space. The bubble size represents the relative intensity of the mass peaks. The detailed arrangement of CHOMg compounds allows visualization of nominal elemental/molecular transformations, with examples provided (circled numbers: ①, C18H33MgO5−; ②, C19H33MgO5−; ③, C19H35MgO5−; ④, C21H37MgO4−; and ⑤, C20H35MgO5−). (C) Relative abundances of these Mg-metalorganics, shown in different colors (B and C), demonstrate the dominance of the MgO4R− molecular subspace, with R = hydrocarbon CxHy and x, y ∈ ℕ.
Fig. S3.
Complexity of the CHOMg chemical space. (A) The dominance of CHOMg molecules possessing four oxygen atoms compared with the whole CHOMg chemical space for the soluble organic matter of NWA 7325. H/C vs. O/C for NWA 7325 (B), H/C vs. O/C and H/C vs. m/z representations of CHOMg chemical compositions for Novato (C and D), Chelyabinsk (E and F), and Murchison (G and H) meteorite samples illustrate the chemical complexity of their organic extracts. The bubble size represents the relative intensity of the mass peaks. The mass-edited H/C ratio diagram of NWA 7325 is shown in Fig. 2A in the main text.
Dihydroxymagnesium Carboxylates: A Previously Unreported Chemical Class.
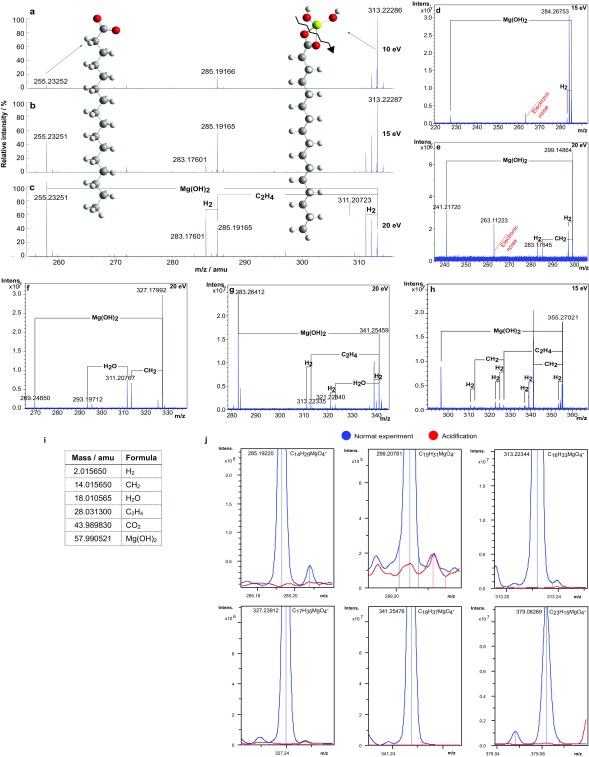
To establish the chemical structure responsible for those peaks, the most intense mass peaks of MgO4R− compounds (R = hydrocarbon CxHy and x, y ∈ ℕ) were subjected to collision-induced dissociation tandem mass spectrometry (CID-MS/MS) to initiate fragmentation. These CHOMg compounds were found to be highly thermostable. High collision energies (>10 eV, 965 kJ/mol) were necessary to observe Mg(OH)2 abstraction [∆m/z = 57.99052 atomic mass units (amu), Fig. S4] from the parent ions. Fragmentation patterns were characteristic of long-chain aliphatic compounds [∆m/z = 2.01565 amu for H2 loss and ∆m/z = 28.03130 amu for C2H4 elimination (27), Fig. S4]). Acidification of the samples caused Mg-metalorganics (organomagnesium complexes) to hydrolyze. The precipitation of Mg(OH)2 substantiates the idea that the observed CHOMg molecules are [(OH)2MgO2CR]− anionic complexes (Fig. S4), namely dihydroxymagnesium carboxylates, which have not been reported to date in chemical databases (e.g., ChemSpider, SciFinder, and PubChem).
Fig. S4.
Fragmentation experiments to characterize dihydroxymagnesium carboxylates. (A–C) CID mass spectra of NWA 7325 of C16-dihydroxymagnesium carboxylate [C16H33MgO4]− complex are shown at collision energies of 10 eV, 15 eV, and 20 eV to identify the MgO4R− structures with R = hydrocarbon CxHy and x, y ∈ . Additionally, five precursor molecular ions, m/z = 285 for [C14H29MgO4]− (D), m/z = 299 for [C15H31MgO4]− (E), m/z = 327 for [C17H35MgO4]− (F), m/z = 341 for [C18H37MgO4]− (G), and m/z = 355 for [C19H39MgO4]− (H) show fairly congruent fragmentation patterns of dihydroxymagnesium carboxylates with the release of Mg(OH)2 out of the fragmented parent molecule. The collision energies used in these CID MS/MS experiments were high (15–20 eV) to ensure fragmentation of the complexes. The computed coordinates of the structures of C16-dihydroxymagnesium carboxylate ([C16H33MgO4]−) and its product ion (hexadecanoic acid) are presented in Table S2. (I) Exact masses of the fragmented molecules. (J) Negative ionization mode ESI-FT-ICR mass spectra of selected dihydroxymagnesium carboxylates [(OH)2MgO2CR]− in the NWA 7325 methanolic extract are shown, where the standard extract is labeled blue and the red signal is the extract in presence of formic acid (HCOOH). The complex is hydrolyzed in the presence of formic acid and Mg(OH)2 is precipitated. This indicates that the CHOMg anionic complexes possess Mg(OH)2 functional groups. The peaks were smoothed via the Gauss smoothing algorithm, as implemented in Bruker Compass DataAnalysis 4.2 SR1, with a smoothing width of 0.001 amu (2.1 points).
Thermodynamic properties of dihydroxymagnesium carboxylates were elucidated both experimentally and theoretically. Mass spectrometric and computed fragmentation energies are in agreement, indicating a remarkable stability of [(OH)2MgO2CR]− as well as a strong (covalent) binding between Mg(OH)2 and the carboxyl group (Eqs. S1 and S3 and Table S2). The [(OH)2MgO2CC15H31]− anion approaches a tetrahedral coordination geometry with Mg as coordination center (Fig. 3C). Interestingly, Mg atoms seem to occur in a rarely observed fourfold coordination (28). The reactivity of dihydroxymagnesium carboxylates as a function of chain length was assessed by determining Gibbs free energies ΔG for the reaction, shown in Eq. 1; ΔG was computed both by means of density functional theory (B3LYP-DFT) and by second-order Møller–Plesset perturbation theory (MP2):
| [1] |
The measured equilibrium constant K′ [K′ ∼ K·c(Mg(OH)2, Eqs. S2 and S3] of the complex formation, following Eq. 1, relates to Gibbs free energy ΔG via Eq. 2:
| [2] |
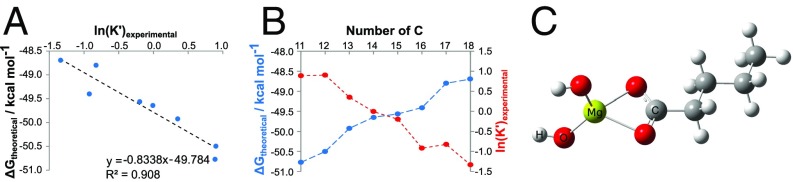
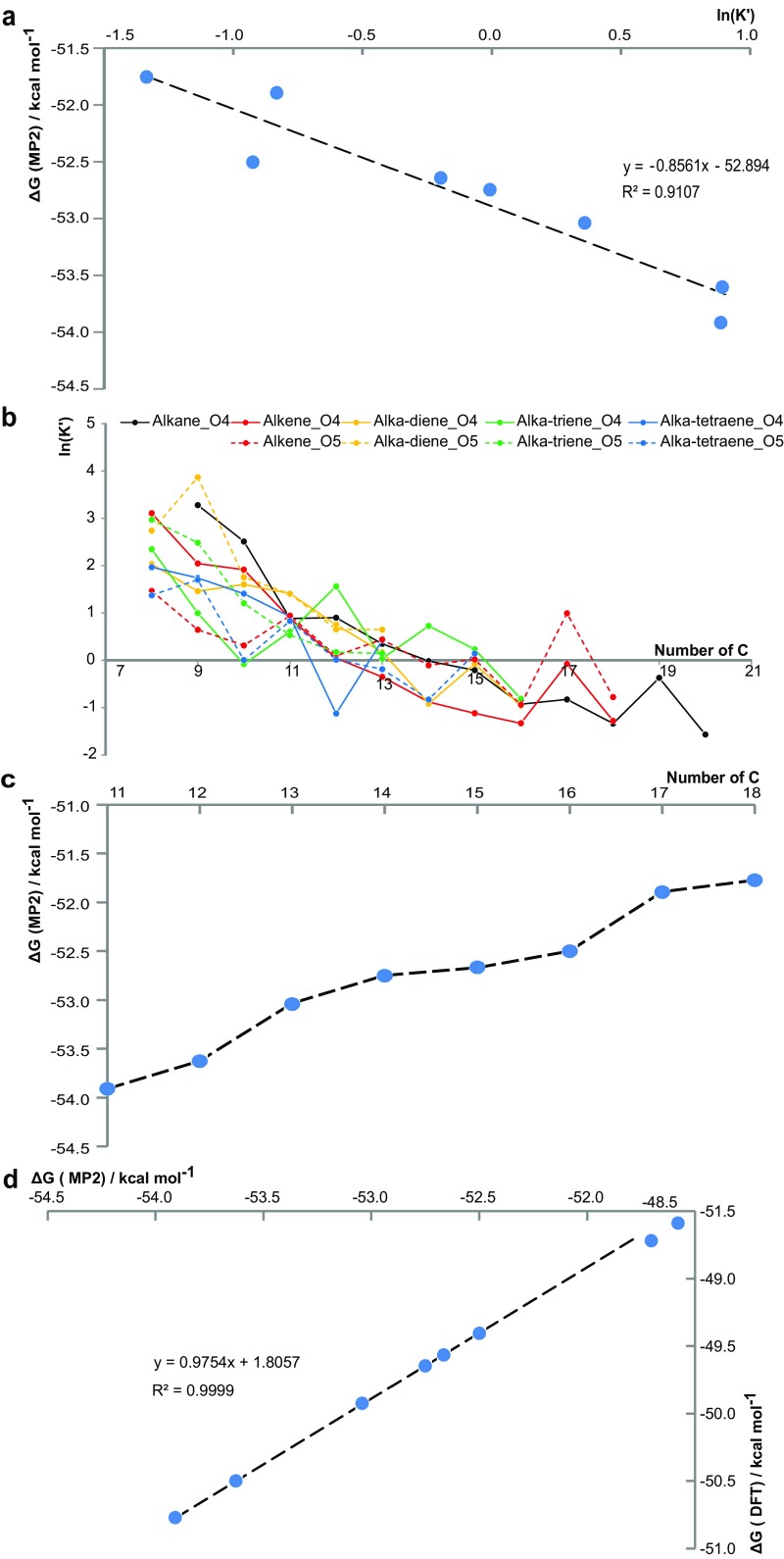
Eq. 2 provides a negative correlation of ΔG with K′ (Fig. 3A and Fig. S5). The tendency of carboxylate complex formation continually decreases with increasing alkyl chain lengths R, as a result of two opposite effects. The inductive, bond-polarizing +I effect increases with higher numbers of alkyl carbons, making the carboxyl groups better nucleophiles, thereby shifting the equilibrium toward complex formation. However, the inverse effect of chain length on the acidity or the deprotonation potential of the ligand dominates. Here, longer alkyl chain carboxylates have higher potential to remain in their protonated form (RCOOH), which makes them weaker nucleophiles. Consequently, a higher coordination tendency for short-chain organic acids results (Fig. 3B), which was verified for various homologous series (Fig. S5).
Fig. 3.
Characterization of dihydroxymagnesium carboxylates. (A) The negative correlation between the experimental equilibrium constant, expressed as ln(K′), and the computed Gibbs free energy ΔG of the [(OH)2MgO2CCnH2n+1]− complex formation with n ∈ ℕ for different linear alkyl chain lengths between C11 and C18, as computed with density functional theory (DFT). (B) The dependency of ln(K′) (experimentally via MS) and ΔG (theoretically via DFT) on different alkyl chain lengths is displayed to illustrate the reactivity of dihydroxymagnesium carboxylates, whereas the optimized computed geometry for the representative ion dihydroxymagnesium-n-pentanoate [(OH)2MgO2CC4H9]− is depicted in C (see Table S2 for the computed coordinates of relaxed geometry of C5-dihydroxymagnesium carboxylate complex anion).
Fig. S5.
General CHOMg reactivity and verification of DFT simulations with MP2 level of theory. (A) Negative correlation of the Gibbs free energy ΔG with ln(K′), following Eq. 2 for different linear alkyl chain lengths between C11 and C18, computed with MP2 extracts (discussed in the main text). (B) ln(K′) is plotted vs. the number of carbon atoms for several homologous series, varying by alkyl saturation and number of oxygen atoms in the aliphatic chain. A general decreasing trend with increasing numbers of C atoms is observed, indicating that smaller alkyl chain CHO molecules are more reactive to form CHOMg compounds, relatively longer aliphatic chain molecules. Additionally, local reactivity anomalies are highlighted by functional fluctuations. (C) ΔG is plotted vs. the number of carbon atoms in linear alkyl chain lengths of the [(OH)2MgO2CCn]− complex formation with n ∈ , as computed on MP2-level of theory. (D) Correlation between the DFT-B3LYP and MP2 methods, which illustrates the accuracy of DFT, describing this complex formation reaction properly.
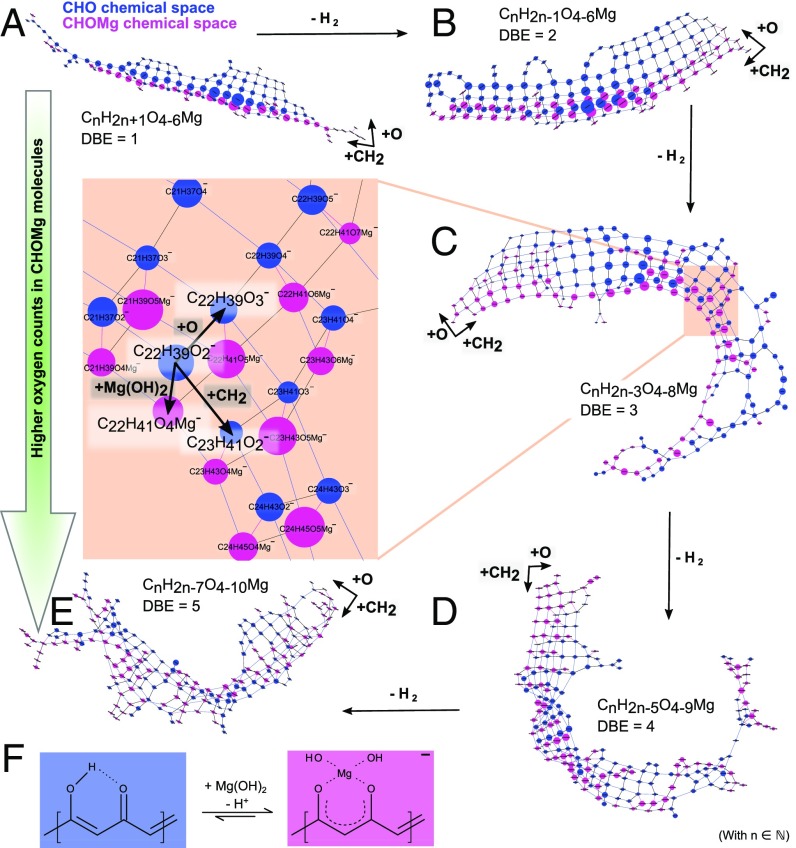
Mass difference network analysis visualizes holistic chemical diversity of CHOMg in detail. In this data-driven analytical approach, nodes represent experimental m/z values (here, FT-ICR-MS data of NWA 7325 soluble organic matter) and edges (connections within the network) represent exact mass differences, which are equivalent to a net molecular formula of a chemical reaction (29). The chemical complexity/reactivity of the CHOMg space (pink-coded nodes) and its regular connection to certain CHO compositions (blue-coded nodes, Fig. 4A and Fig. S6) is revealed. Here, CxHyOz+Mg(OH)2 reaction pairs (with x, y, z ∈ ℕ) were identified for various degrees of unsaturation and numbers of oxygen atoms. First, highly connected methylene-based homologous series can be observed (CH2 as an edge) for the CHO and CHOMg compositional spaces, respectively. Second, different subseries with varying oxygen numbers are present. This functional network is split into five disconnected subnetworks, differing in their saturation states and laid out in the CH2 vs. O directions. The degree of unsaturation (described via double-bond equivalent values, DBE) affects the reactivity of CHO compounds (CxHyOz + Mg(OH)2 → CxHy+2Oz+2Mg reaction, with x, y, z ∈ ℕ); the number of possible reactions increases with increasing DBE. Saturated CxHyO2 compounds (DBE = 1) almost exclusively react to MgO4R− compositions (like dihydroxymagnesium carboxylates, R = hydrocarbon CxHy and x, y ∈ ℕ). With increasing numbers of DBE, additional varieties of organomagnesium complex formation become available due to increased numbers of isomers of CHO compounds. On average, the transition from Fig. 4A to Fig. 4B doubles the number of organomagnesium compounds (pink chains), representing an increase in chemical CHOMg complexity.
Fig. 4.
Mass difference networks, presenting the chemical complexity/reactivity of the CHOMg space and its connection to CHO compositions. (A–E) Five subnetworks, each representing one distinct degree of unsaturation, as well as a gradual increase in the number of oxygen in CHOMg molecules, are shown for NWA 7325 soluble organic matter. The variance in unsaturation is expressed via DBE values. CHOMg nodes are pink and CHO nodes are blue. The nodal diameter is proportional to the natural logarithm of each mass peak’s intensity. Three types of edges are defined, the mass differences of CH2 and O illustrate the systematic connection within the CHO or CHOMg chemical space, and Δm/z(Mg(OH)2) addresses reaction pairs that connect CHO and CHOMg compositions. (F) Proposed alternative organomagnesium complex formation with unsaturated β-hydroxy ketones as chelate ligands.
Fig. S6.
Van Krevelen diagrams for the comparison of CHO and CHOMg compositions for several meteorites and modified van Krevelen diagrams representing the thermal metamorphism signature. Van Krevelen representations of soluble organic matter in NWA 7325 (A), Novato (B), Chelyabinsk (C), and Murchison (D) are shown. It is obvious that the less-altered CM2 chondrite Murchison is poor in CHOMg compositions, compared with the ungrouped achondrite NWA 7325 and the ordinary chondrites, Novato and Chelyabinsk. Modified van Krevelen diagrams of the overlapped CHOMg molecules (50th percentile of positive loading values on the x axis) reflect the highly aliphatic structure of the CHOMg compositional space (A) for relevant loadings, which represent high (E) and low (G) thermal metamorphism signatures seen in the 61 meteorites studied. These plotted m/z values reflect the 50th percentile of the variables, which are unique for the positive (E) and negative (G) values of the first component (x axis) of the OPLS score plot (Fig. 5). These compounds in E may represent thermal metamorphism markers. The bubble size represents the relative intensity of the mass peaks. (F and H) Mass-edited H/C ratios of two representative examples for low- (Paris) and high-degree thermally processed meteorites (Soltmany) (35). Paris is known to be one of the least-altered meteorites (36). The convergence of O = 4 in CHOMg molecular formulas for thermal stress loading values is presented by an oxygen number-m/z diagram. (I) Again, these plotted m/z values reflect the 50th percentile of the variables, which are unique for the positive values of the first component (x axis) of the OPLS score plot (Fig. 5).
The presence of carbonyl and hydroxyl groups in meteoritic soluble organic matter has previously been demonstrated (1, 6). We propose the additional presence of β-hydroxy carbonyl functionalities for unsaturated compounds that are isomeric and vinylogous to carboxylic compositions. Unsaturated β-hydroxy ketones are stabilized via conjugation effects, which enhance the likelihood for alternating σ and π bonds within the aliphatic chain (Fig. 4F). The enol form is preferred, relative to the keto form, due to the presence of a pseudo ring, driven by hydrogen bonding. Additionally, keto–enol tautomerism explains the acidic character of β-hydroxy ketones. They are able to form chelate complexes (30), similar to organomagnesium coordination compounds. This alternative Mg coordination motif, compared with carboxylate ligands, may explain why highly unsaturated oxygenated CHO molecules react to CHOMg compositions. Further, the presence of two organic ligands enhances the probability of forming organomagnesium complexes, compared with one single organic educt class.
Chemosynthesis of CHOMg Compounds and the Link to Thermal History.
One might ask about the origin of these organomagnesium compounds and whether the genesis of this compound class is coupled to the individual “history” of the various meteorites, meteoroids, and parent bodies. The effects of shock events, thermal metamorphism, and aqueous alteration play a major role in the classification of meteorites, which are commonly based on petrologic indicators (31). CM-type meteorites (5, 32) have shown the highest number of CHNOS compositions. The thermally altered Sutter’s Mill shows losses of these signatures with additional new polysulfidic patterns (33). The recent falls of the ordinary chondrites, such as Novato (L6), Chelyabinsk (LL5), or Vicência (LL3.2), show similar losses in nitrogen and sulfur compounds. Conversely, formation of compounds with high numbers of nitrogen at higher shock levels (23, 24, 34) are observed, suggesting that shock events/thermal metamorphism play a role in this context as well.
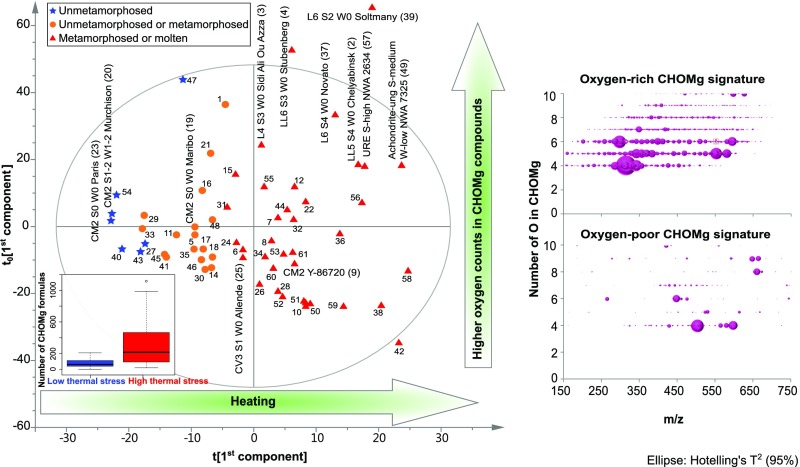
The CHOMg signatures relate to shock stage (with S0 being unshocked and S5 highly shocked meteorites, as assigned for examples in Fig. 5) and thermal processing among 61 meteorites of various classes, as demonstrated by orthogonal partial least square regression analysis (OPLS) (Fig. 5, x axis as first component). For example, the thermally altered meteorite Soltmany (35) contains >700 CHOMg compounds. In comparison, the less-altered meteorite Paris (CM) with only weak thermal alteration (36) shows merely 90 CHOMg compounds of low mass peak intensity. The information on the variation of oxygen numbers within CHOMg formulas is revealed by the y axis (orthogonal to the first component) of the OPLS analysis. Based on the mass difference network analysis (Fig. 4), we propose that the y axis potentially also represents a discrimination of the degree of unsaturation. High oxygen numbers of CHOMg molecular formulas correspond to a higher degree of unsaturation.
Fig. 5.
Relationship of CHOMg signatures with different thermal processing stages of meteorites. An OPLS score scatter plot for 61 meteorites (CI, CK, CM, CO, CR, CV, EUC, H, L, LL, URE, and achondrite classes) with different inter- and intrameteorite class metamorphism stages is shown, based on the abundance and molecular diversity of CHOMg compounds. Details on the OPLS analysis are given in SI Materials and Methods and meteorite assignments are listed in Table S1. The box plots represent the averaged numbers of CHOMg molecular formulas for low and highly thermally altered meteorites, respectively. Thermal processing states vary from low to high along the x axis (first component), proceeding from negative to positive values. This first component (x axis) is related to the number and intensity of CHOMg molecular formulas, as represented in the box plot. The y axis (orthogonal to the first component) represents the proportion of oxygen atoms within the molecular formulas, as illustrated by modified van Krevelen diagrams of the most relevant loading values. Independent of the specimen, CHOMg signatures were observed with increased Mg-metalorganic diversity for thermally stressed meteorites. The two CM2 chondrites, Y-793321 and Y-86720, reported to be thermally metamorphosed (39), well-described meteorites, with respect to their shock history [Chelyabinsk (24) and Novato (23)], as well as the recently classified fall Sidi Ali Ou Azza (L4) and a very new German fall Stubenberg (LL6) were also assigned to the thermally stressed region.
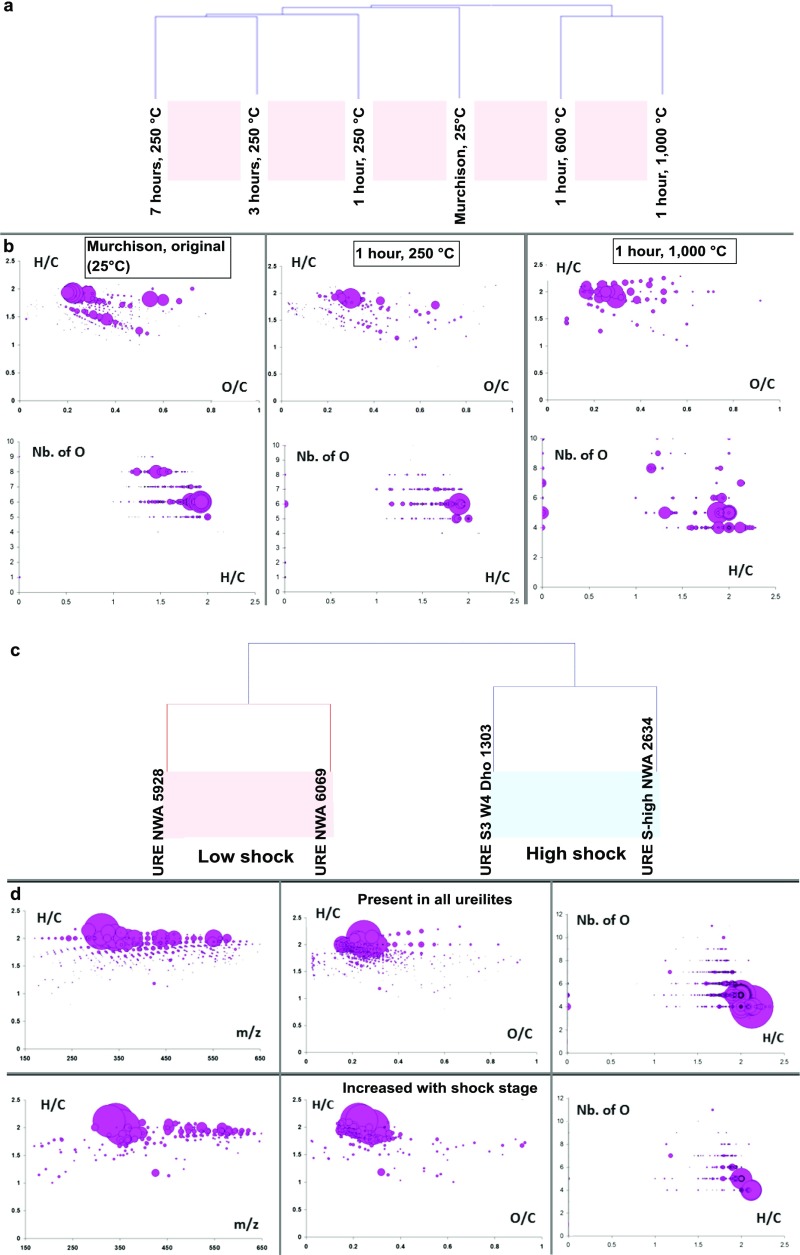
Parent body thermal metamorphism also imposes a compositional variance of CHOMg compounds. High thermal metamorphism is associated with an elevated saturation (high H/C ratio) and a convergence of oxygen numbers to 4 within the organomagnesium molecules at high thermal stress (Fig. S6). By heating Murchison, a meteorite with a low degree of metamorphism, we were able to simulate and follow the effect of short-duration thermal stress in a laboratory experiment. Here, CHOMg-based hierarchical cluster analysis revealed differentiation according to temperature regimes (Fig. S7). Similarly, the number of oxygen atoms in CHOMg molecules converges toward O = 4 at high temperatures, as expected (Fig. S7). A detailed comparison within highly shocked/thermally stressed ureilite meteorites also agrees with the above results (Fig. S7).
Fig. S7.
Simulated thermal metamorphism of Murchison and CHOMg-based shock stage differentiation. (A) Laboratory experiment, in which a Murchison meteorite sample was heated to selected temperatures for variable durations (25 °C, 250 °C, 600 °C, and 1,000 °C). Number and distribution of organomagnesium compounds enabled reconstruction of thermal exposure by means of HCA. The simulation of thermal metamorphism grades by increasing the temperature narrows the oxygen number in organomagnesium compounds to the MgO4R− class (R = hydrocarbon CxHy and x, y ∈ ), which dominates the entire CHOMg chemical space. This effect of convergence with increasing temperature is depicted in modified van Krevelen diagrams in B, resulting from negative ionization ESI-FT-ICR-MS data. The bubble size represents the relative intensity of the mass peaks. HCA organizes the samples, as a graphical output, into a dendrogram (cluster tree) whose branches are the desired clusters. Based on different similarity rules the clusters are defined. Similar samples are within a cluster. The samples clustered according their elevated temperatures (25 °C, 250 °C, 600 °C, and 1,000 °C). Sampling the effect of time-dependency on the variation of the CHOMg chemical space, three different time points were sampled for temperature 250 °C and clustered together. (C) Detailed differentiation of metamorphic states is illustrated, deduced from CHOMg-based HCA. Four ureilite meteorites were studied (NWA 5928, NWA 6069, Dhofor 1303, and NWA 2634), which had experienced high thermal and shock conditions (62). (D) (Top) CHOMg chemical spaces of four ureilite methanolic extracts (overlapped CHOMg compounds) are shown on top. (Bottom) CHOMg compositions, which increased in abundance in the higher-shocked ureilite meteorites Dhofor 1303 and NWA 2634.
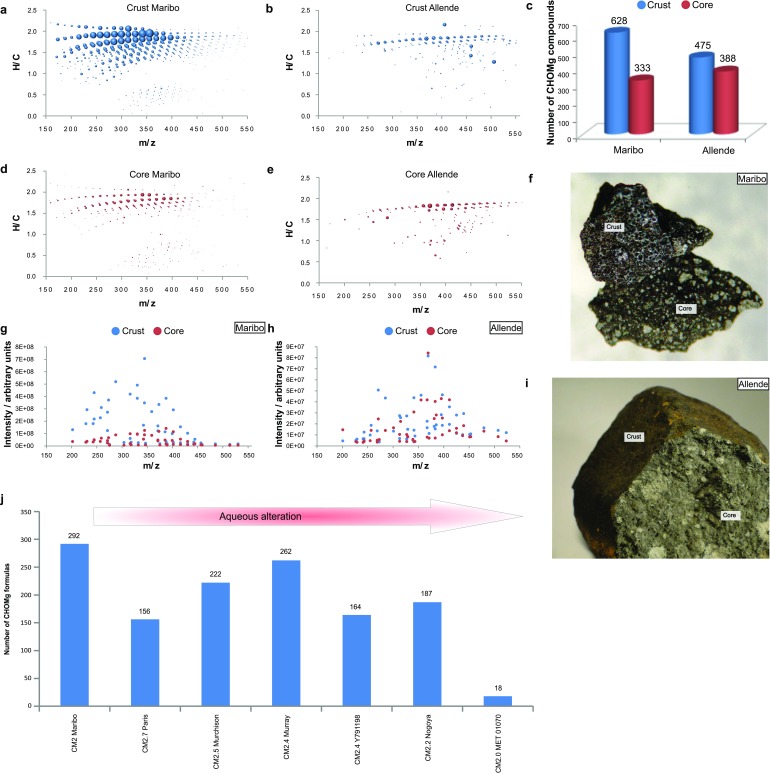
The production of CHOMg compounds by heating is further demonstrated by analyses of meteorite’s fusion crust. Freshly fallen meteorites are found with a glassy coating that formed at ∼1,400 °C surrounding their cold interior. The fusion crust is formed upon atmospheric entry by melting the meteoroid’s surface as it enters the Earth’s atmosphere at supersonic speed. During the brief melting, the liquid-like crust loses volatile elements and reacts with atmospheric matter faster, relative to the heterogeneous solid-state interior. ESI-FT-ICR mass spectra were acquired for Maribo (CM2) and Allende (CV3) by probing their outer crust and their inner core. Higher numbers and higher molecular diversity of CHOMg compounds were obtained from the crusted surfaces, relative to the core regions (Fig. S8). The different thermal conditions experienced by the outer and inner parts of a meteorite lead to different potential chemical activities, which promote the synthesis of these organomagnesium compounds at elevated temperatures within a short time scale. This observation agrees with the above experimental results, demonstrating that reaction energy, namely pressure and temperature, as substantiated by Eq. 2, relate with higher abundance of CHOMg molecules.
Fig. S8.
Crust-core comparison and their influences on the CHOMg synthesis and dependency of CHOMg formulas on the aqueous alteration. (A) Number of CHOMg compounds in crust and core sections of the Maribo and Allende meteorites. The mass-edited H/C ratios (B–E) illustrate the increased coverage within the CHOMg chemical space for the crust, compared with the core (interior) region. Among the specific organomagnesium molecules, some are unique for the crust and for the core regions, respectively, indicative of a preferential spatial accumulation for certain CHOMg (F and G). The bubble size represents the relative intensity of the mass peaks. Pictures of the Maribo and the Allende specimens are shown in H and I, to reflect the different morphology of their outer crusts and their interiors. (J) Number of CHOMg molecular formulas in methanolic extracts of several CM2 meteorites that had been subjected to variable extents of aqueous alteration [classified from CM2.7 (left) to CM2.0 (right)]. No significant correlation between the extent of aqueous alteration of a meteorite and the number of organomagnesium compounds was retrieved, suggesting that the CHOMg synthesis is not directly dependent on the aqueous alteration.
The role of alteration can also be evaluated from the isotopic signature of the Mg atoms in CHOMg compounds. Isotopic analyses of Mg were performed on both organic extracts and residual fractions of NWA 7325 and Novato (Fig. S1). The organic extract of Novato had a δ26Mg value of −0.74 ± 0.08 ‰, and the residue had a δ26Mg value of −0.29 ± 0.09 ‰. Details on the Mg isotopic analysis are given in SI Materials and Methods. Similarly, the organic extract of NWA 7325 had a δ26Mg value of −0.62 ± 0.04 ‰, and the residue had a δ26Mg value of −0.35 ± 0.11 ‰. Thus, for both Novato and NWA 7325, the organic extracts were relatively enriched in isotopically light Mg, compared with the isotopic composition of Mg in the bulk rock. This is consistent with the observation by Black et al. (37), who found chelation during intracellular processes enrich light Mg isotopes.
However, Mg isotopic fractionation occurs upon abiotic aqueous alteration as well. Aqueous alteration leads to clay-mineral formation and Mg-rich phases (11). We did not observe any significant direct correlation between the numbers of organomagnesium compounds and the extent of aqueous alteration within CM2 meteorites, ranging from CM2.7 to CM2.0 (Fig. S8). Studies from Wimpenny et al. (38) show that the removal of exchangeable magnesium from alteration phases preferentially liberates isotopically light Mg, compared with the bulk mineral. This suggests that aqueous alteration may have an indirect effect on the synthesis of organomagnesium compounds. If a released Mg educt, produced by aqueous alteration, is consecutively exposed to high temperatures, enhanced CHOMg formation would be expected to result by close spatial proximity and intercalation of the mineral and organic phases in CM2 meteoritic materials (11). Secondary ion mass spectrometric (SIMS) analyses of the Chelyabinsk meteorite indicated a spatial proximity of Mg and organic compounds (Fig. S1), which has not been reported previously by this method. Ordinary chondrites do not typically undergo aqueous alteration.
The composition of soluble CHOMg compounds is shown to be highly related to the thermal-processing states of meteorites. Molecular complexity of MgO4R− compositions (R = hydrocarbon CxHy and x, y ∈ ℕ) is increasingly diversified, because a meteorite experiences increasing degrees of thermal processing. The most abundant subclass of CHOMg compounds in meteorites is the four-oxygen-containing MgO4R− type and represents the previously unreported chemical class of dihydroxymagnesium carboxylates [(OH)2MgO2CR]−.
The use of CHOMg compound distributions as potential chemical markers, together with the CHNOS chemical space, may help to expand our knowledge of (i) astrochemistry of higher molecular masses and chemical complexity within the solar nebula and/or (ii) postaccretional processes in meteoritic parent body metamorphism. In the context of meteorite classification, CHOMg content and diversity may provide a useful estimate of the degree of thermal alteration reflecting their temporal evolution under high temperature.
Additionally, this work raises the questions of whether these CHOMg compositions are specific for extraterrestrial chemistry and what we can learn from these findings within ongoing studies on natural metalorganic compounds in terrestrial systems and deep carbon sequestration in the Earth interior under high temperature and pressures (17).
Metal ions are essential for the origin of living systems on Earth (40–42). Metal ions can either support reactions via catalytic effects or stabilize organic molecules, because life-relevant organics are often thermolabile and celestial bodies undergo high-energy gradients through time and space. Here, highly thermostable organomagnesium compounds might have contributed to the stabilization of organic molecules, such as fatty acids, on a geological time scale, being in contact with Mg-bearing minerals at high energetic conditions. These protecting metalorganic motifs might represent important intermediate molecules in the selection history of organic molecules of life. A concentration/fractionation of fatty acids can be accomplished via the stabilization in their organomagnesium motifs, which is highly relevant in the formation of protocells/cells due to compartmentization/vesicle formation in membranes.
Due to their high abundance (13) and known metalorganic chemistry, Fe, Ni, Al, Zn, and V (9, 43, 44) may also be present as astrobiologically relevant molecular building blocks in meteorites, next to Mg-bearing compounds. No other metalorganics could be experimentally detected yet.
Potential future detections of organometallic compounds (or organics in general) from sample return missions to Mars, asteroids, or the Moon would imply that meteoritic organic compounds might survive some of the high-temperature, early phases of planetary accretion processes. This may not necessarily mean that life existed at a certain point in the histories of these planetary bodies. Insights into potential amplification of abiogenesis probabilities among planetary systems with various chemistries and molecular complexities can be achieved.
SI Materials and Methods
ICP-MS Experiments.
Methanolic extracts and residues of the meteorites were analyzed for total elemental composition. Extracts were diluted appropriately 10- or 26-fold to achieve the necessary sample volume. Extract residues were pressure-digested. Samples were exactly weighed within quartz vessels, to which 1 mL HNO3, Suprapur, subboiling distilled (Merck), was added. The vessels were closed and introduced into a pressure digestion system (Seif) for 10 h at 170 °C. The resulting clear solutions were filled up exactly to the mark at 10 mL with Milli-Q H2O and were then ready for element determination.
An ELEMENT 2 Thermo-Electron inductively coupled plasma sector field mass spectrometry instrument was used for determination of the elements. A solution of 103Rh was added as an internal standard to each sample at a concentration of 1 µg/L. Sample introduction was carried out using a peristaltic pump connected to a Meinhard nebulizer with a cyclon spray chamber. The rf power was adjusted to 1,300 W, the plasma gas was 15 (L Ar)/min, whereas the nebulizer gas was ∼0.9 (L Ar)/min after daily optimization. Measured element isotopes were as follows: 9Be, 114Cd, 59Co, 52Cr, 56Fe, 202Hg, 193Ir, 55Mn, 98Mo, 60Ni, 208Pb, 121Sb, 78Se, 51V, and 184W.
Each determination method has been validated previously by regular laboratory intercomparison studies and by regular analyses of adequate certified reference materials, the latest directly before this study. Routinely, every 10 measurements, three blank determinations and a control determination of a certified standard for all mentioned elements were performed. Calculation of results was carried out on a computerized laboratory-data management system, relating the sample measurements to calibration curves, blank determinations, control standards, and the weight of the digested sample.
The solid-state meteorite sample was scanned using a commercial laser ablation system coupled to inductively coupled plasma mass spectrometry (LA-ICP-MS). The laser ablation system was a NWR 213 instrument from New Wave Research/ESI, equipped with a beam expander, yielding laser spot sizes between 4 and 250 µm. The laser system was coupled to a PerkinElmer NexIon 300 ICP-mass spectrometer (Sciex). The ICP-MS was synchronized with the LA unit in external triggering mode. The meteorite piece was mounted into the standard sample cell of the LA system with laboratory plasticine between the sample holder and the lower side in such a way that the flat upper side surface of the meteorite was placed on a level, where the laser would be focused. The exact position for ablation lines was defined by using the dual microscope of the LA system. The laser settings were as follows: energy, 30 J/cm2; power output, 100%; pulse repetition rate, 10 Hz; scan speed, 20 µm/s; spot size, 100 µm; and ablation pattern, lines. The Ar-gas flow to ICP-MS was 1.14 L/min. The ICP-MS settings were as follows: rf power, 1,200 W; plasma gas, 15 (L Ar) /min; and dwell time per isotope, 15 ms. The following isotopes have been selected for analysis: 24Mg, 27Al, 28Si, 35Cl, 51V, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 66Zn, 98Mo, and 138Ba. ICP-MS data were exported to Microsoft Excel, where signal intensities were color-coded.
SIMS Experiments.
Measurements were performed using an IMS-4f Cameca secondary ion mass spectrometer. Etching in oxygen plasma for 10 min was performed to remove carbon contamination on the sample surface after grinding and polishing. These conditions were used to study a glassy vein of Chelyabinsk meteorite as selected target.
The sample target is a good insulator and charged during analysis. A normal incidence electron gun was used to compensate the positive charge on the sample surface. This gun has been effectively applied to detect negative secondary ions. In this case, the field accelerating the secondary ions was decelerating for electrons and therefore a cloud of electrons was created above the sample surface. This cloud discharges the charging areas of the sample surface, which was coated additionally with thin a layer of gold (∼10 nm) to improve the discharge.
Because negatively charged secondary ions were selected for analysis, it was necessary to provide a high yield. This technique is well-known and consists of using Cs+ as the primary ions with impact energy of 14.5 keV to ablate analyte atoms/molecules. In contrast to the positive secondary ions, the detection of negative secondary ions has its own characteristics. Not all chemical elements have strong electron affinity. For example N, Ca, Mn, and Mg have electron affinity values close to zero. However, the analyzed sample was a mixture of oxides. To determine the elements of these oxides, molecular ions of MgO− as well as C−, H−, and Si– ions were detected and mapped.
Mass spectral resolution of 5,000 was used to overcome interference problems. The map of element distributions was performed using a dynamic transfer system. Lateral resolution was determined by the field-of-view aperture and equals 5 μm with a raster of 250 × 250 μm. Output data of the SIMS results were the signal intensities at the coordinates (x, y) analysis at a certain depth. The software, using the obtained coordinates and the intensities of the analyzed element signal, allows visualizing the data as a 2D distribution, where each point is assigned as a color depending intensity at that point.
ESI-FT-ICR-MS Experiments: Instrumental Details.
The experimental study was performed on a high-field FT-ICR mass spectrometer from Bruker Daltonics with a 12-T magnet from Magnex. A time-domain transient with 4 MWords was obtained and Fourier-transformed into a frequency domain spectrum. The frequency domain was afterward converted to a mass spectrum by the solariX control program of Bruker Daltonics. The ion excitations were generated in broadband mode (frequency sweep radial ion excitation) and 3,000 scans were accumulated for each mass spectrum in a mass range of 147–1,000 amu. Ions were accumulated for 300 ms before ICR ion detection. The pressure in the quadrupole/hexapole and ICR vacuum chamber was 3 × 10−6 mbar and 6 × 10−10 mbar, respectively. For CID-MS/MS, ions were accumulated for 3 s.
The ESI source (Apollo II; Bruker Daltonics) was used in negative ionization mode. The methanolic solutions were injected directly into the ionization source by means of a microliter pump at a flow rate of 120 μL⋅h−1. A source heating temperature of 200 °C was maintained and no nozzle-skimmer fragmentation was performed in the ionization source. The instrument was previously externally calibrated by using arginine negative cluster ions (5 mg·L−1 arginine in methanol).
FT-ICR mass spectra with m/z from 147 to 1,000 amu were calibrated externally and internally to preclude alignment errors. Subsequently, the mass spectra were exported to peak lists at a signal-to-noise ratio ≥3. Elemental formulas were calculated combinatorially within a mass accuracy window of ±0.2 ppm for each peak in batch mode by an in-house software tool and validated via the senior-rule approach/cyclomatic number (45), assuming valence 2 for S and valence 4 (coordination number) for Mg. Following senior’s rules, twofold-coordinated Mg(II) resulted in invalid molecular formulas, which were excluded for further data evaluation.
Computations.
The electronic structure simulations were performed on a stand-alone computer by ab initio quantum mechanical computations, based on density functional theory (DFT), as implemented in Gaussian 03 (46). The hybrid DFT-functional B3LYP was implemented with d-polarization functions for each heavy atom and 1p for each hydrogen atom in all single-point energy calculations. All geometry optimizations were performed with the 6–31+G(d,p) basis set. Frequency calculations were done for each optimized geometry with the same 6–31+G(d,p) basis set to obtain the zero-point vibrational energy. This value was multiplied by a scaling factor of 0.9804 to correct for vibrational anharmonicities (47). Another intention for performing the frequency analysis is the identification of transition states. Detecting negative frequencies (imaginary frequencies) implies that the optimized geometry is not fully relaxed as a stationary point on the potential energy surface. The single-point energy calculations were done at the 6–311+G(2d,p) level of theory. The use of diffuse functions was important to represent the correct geometry and thermodynamic properties of anionic species (48). Stability tests were performed in critical cases (significantly different energy values relative to the homologous series of treated attachment systems) to ensure that the used wave function represents the lowest energy solution of the self-consistent field equations.
For geometry optimization, the Berny analytical gradient optimization routines (49, 50) were used in combination with the GDIIS algorithm (51). The requested convergence value in the density matrix was 10−8, the threshold value for maximum displacement was 0.0018 Å, and the threshold value for the maximum force was 0.00045 Hartree Bohr−1. The nature of the stationary points was established by calculating and diagonalizing the Hessian matrix (force-constant matrix). All geometries of electronic structures calculated were viewed with the GaussView or Avogadro program.
The Gibbs free energy ΔG between each neutral Mg(OH)2 and the anionic carboxylate anion in the gas phase was calculated with Eq. S1, satisfying the reaction of Eq. 1:
| [S1] |
E(x) are the single-point energies of respective species x and R = hydrocarbon CxHy with x, y ∈ ℕ. Therefore, the two educts were first optimized in the absence of the reaction partner (step 1). Afterward, the charged complex was relaxed (step 2). The mentioned energies in Eq. S1 are the single-point energies of steps 1 and 2. All computations are referred to gas-phase conditions.
Correlation Between the Experimental and Computed Fragmentation Energies.
The minimum collision energy of 10 eV, required to initiate dissociation of the C16-dihydroxymagnesium carboxylate complex anion, had to be scaled because the magnitude of internal energy deposition from kinetic acceleration in an electric field in a quadrupole can be estimated to reach 20% (52). The corresponding energy of 193.3 kJ/mol is in the range of the DFT-computed binding energy (BE) for [(OH)2MgO2CR]− for the reaction of Mg(OH)2 with the carboxylate anion (BEcomputed = 206.7 kJ/mol).
Calculation of the Measured Equilibrium Constant K′.
The equilibrium constant K for the reaction in Eq. 1 is given as
| [S2] |
with R = hydrocarbon CxHy with x, y ∈ ℕ. Thermodynamic activities a(x) can be approximated by concentrations c(x) for different molecules x, when ideal behavior is assumed. c(x) is expressed as the compound’s mass spectrometric intensity I, which is proportional to the compound’s abundances (53). For K, a measured equilibrium constant K′ is used with the assumption that c(Mg(OH)2) >> c(RCOO−) because of the much higher amount of mineral compounds relative to the organic compounds (54). Therefore, the reaction is considered as a pseudo-first-order reaction, where d(c(Mg(OH)2))/dt ∼ 0. Thus, K′ is calculated with Eq. S3 instead:
| [S3] |
Mass Difference Network Reconstruction.
Theoretical ion masses of the above assigned molecular formulas were used for mass difference network reconstruction. Theoretical ion masses (nodes) were connected by edges if their mass differences were equal (±0 ppm) to the theoretical mass differences of ∆CH2 (14.015650 amu), ∆O (15.994915 amu), or ∆Mg(OH)2 (57.990521 amu). Mass difference networks were laid out using the Cytoscape software (Allegro version 3.2.1 layout) (55).
Statistical Evaluation of Meteorite Samples.
CHOMg mass lists were aligned with an in-house software (56). To stabilize the variance, only m/z values present in more than 5% of all samples were further considered in the statistical elaboration to remove unique compounds. The peak intensities were scaled to unit variance and logarithm-transformed.
To evaluate the data, several multivariate models were set up. Orthogonal partial least square discriminant analysis (OPLS-DA) and OPLS regression models were performed by the sevenfold cross-validation procedure, as implemented in the software SIMCA, and then verified with CV-ANOVA (cross-validation ANOVA), to exclude overfitting. Relevant indicators, such as P value, R2(Y), and Q2(cum), were subsequently reported to indicate the significance, the goodness of the fit, and the quality of the model prediction. Moreover, potential discriminating variables were chosen by examining the loading plot. The software tools, used for the statistical elaboration, were SIMCA 13.0.3.0 (Umetrics) and RStudio (version 0.98.1103; RStudio, Inc.).
First, a classification model was set up via OPLS-DA using a meteorite subset, formed by samples with assigned information about their thermal stress level. All samples were correctly classified with a Fisher probability of 5e−81 and the model was valid (P < 0.001). Based on this model, properties were predicted for additional samples without any available thermal stress information. Based on the classification, a gradient for the thermal stress was set up among all samples from low- to high-thermally stressed meteorites. This gradient was used as Y variable in an OPLS model to reveal a possible relation between the m/z variables and Y. The OPLS score scatter plot (Fig. 5) was robust to overfitting (P = 0.004) with R2(Y) = 0.91 and Q2(cum) = 0.27. The ellipse represents the Hotelling’s T2 confidence region (95%). The first axis (x axis) is the so-called first component with the new samples coordinate. It can be seen as the best-fit line that traverses the geometric origin of the dataset accounting for the greatest amount of variance of the data. The second greatest variance is explained by the second component, orthogonal to the first, and so on. All of the components have to be uncorrelated each other and cumulatively all together account for the 100% of the variance. The aforementioned coordinates of data points on the first component are the first principal component values, or component scores; they are computed as the product of (centered) data matrix and the eigenvector.
The most relevant masses, 50th percentile of loading values of the y axis (oxygen variation in CHOMg formulas), were visualized in modified van Krevelen diagrams, as depicted in Fig. 5. Concerning the thermal stress variation, the most relevant masses, 50th percentile of loading values of the x axis, were visualized in modified van Krevelen diagrams, as depicted in Fig. S6. The box plot was set up by counting CHOMg formulas for negative and positive loading values on the x axis, respectively.
Hierarchical cluster analysis (HCA) was performed using the average linkage method (UPGMA) for the distance between clusters and the Pearson correlation coefficient as criteria to cluster the variables. The HCA was done by the Hierarchical Clustering Explorer 3.0 (Human-Computer Interaction Lab, University of Maryland). Hierarchical cluster analysis organizes the samples, as a graphical output, into a dendrogram (cluster tree) whose branches are the desired clusters. Based on different similarity rules the clusters are defined. Similar samples are within a cluster.
Mg Isotope Measurements.
All samples (both the methanolic extracts and residues) were dissolved subsequently using a standard 3:1 HF:HNO3 dissolution technique in Savillex 3-mL vials (hexagonal cap, square body type) (57). After dissolution, ca. ∼1% aliquot of the solution was saved for 27Al/24Mg analyses. The remainder was centrifuged and processed through a cation exchange column to separate Mg from the sample matrix and potential isobaric interferences. The column procedure was first calibrated to separate Mg from matrix elements, such as Na, Al, K, and Ca. In particular, separation of Mg and Ca is important because this has been shown to cause fractionation of Mg isotope ratios by up to 1‰ (58). Standards and unknowns were dried down (∼20 µg of Mg) and redissolved in 0.5 mL of 1 N HNO3 before eluting through 0.75 mL of BioRad AG50W-X12 resin (200–400 mesh). We added a rinse step involving 3 mL of 0.15 M HF, which causes removal of Al and Ti from the column but leaves Mg unaffected. The Mg cut was collected in 12 mL of 1 N HNO3. Once eluted, the Mg fraction was collected and dried down and the residue was redissolved in 20 µL of concentrated HNO3 to oxidize any organic molecules derived from the resin. After repeating this column chemistry, the final Mg fraction was dried down and redissolved in 1 mL of 2% HNO3. After sample processing, the resin was cleaned by repeated elutions of 7 M double-distilled HNO3 and Milli-Q ultrapure H2O. Both Mg isotope compositions and 27Al/24Mg ratios were measured on a Thermo Neptune Plus HR-MC-ICP-MS in the Department of Earth and Planetary Sciences at the University of California, Davis.
Isotopic analyses of Mg were bracketed using the DSM-3 pure Mg standard (59) to account for instrumental mass bias and drift throughout the analysis period. Mg isotope ratios are expressed using delta notation as parts per thousand (‰) differences from DSM-3. Each sample was initially prescreened using 1% of the final Mg solution to ensure accurate dilution of the sample to match the signal intensity of the bracketing standard to within 10%. Samples were analyzed under dry plasma conditions using an ESI Apex IR desolvating nebulizer, which suppresses oxide interferences. The samples were analyzed in medium resolution to avoid the CN+ peak on 26Mg, which cannot be resolved at low resolution. Using a high-sensitivity “x” skimmer cone the typical intensities for a 500-ppb solution at medium resolution were between 20–25 V of 24Mg (amplified with a 1011-ohm register board). Blank intensities on 24Mg were typically 0.003–0.005 V.
To assess the accuracy of our measurements, the pure Mg standard CAM-1 was routinely measured throughout each analytical session. In total, CAM-1 was measured 78 times with an average δ26Mg value of −2.61 ± 0.08 ‰, which is within error of the accepted value (−2.59 ± 0.14 ‰) (59). To assess the accuracy of the column chemistry and our external reproducibility, the USGS basalt standard BCR-2 was processed with every batch of column chemistry. A total of 26 separate analyses from eight separate chemical separations were performed on this standard. On average, BCR2 had a δ26Mg value of −0.20 ± 0.07 ‰ and δ25Mg value of −0.10 ± 0.04 ‰, which is within error of the estimated average composition of the upper continental crust (δ26Mg = −0.22 ‰) (60) and other recent published values for basalts (61).
Materials and Methods
For ESI-FT-ICR-MS experiments, fragments of fresh interior samples were first washed by stirring for a few seconds within the extraction solvent (methanol, LC-MS grade; Fluka) before crushing in 1 mL solvent poured into the corresponding agate mortar. This procedure was shown to limit the number of peaks resulting from terrestrial and human contamination, for example fatty acids arising from sample handling. The mixture (suspension) was transferred into an Eppendorf vial and underwent ultrasonic cleaning for ≤10 min and then was centrifuged. The supernatant liquid was removed with a microsyringe, ready for flow injection into the ESI source. A solvent methanolic blank was measured in accordance to be able to detect indigenous meteoritic (metal)organic matter in each sample. Organomagnesium compounds were absent in blank spectra. Further details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Rainer Bartoschewitz, Maria Elizabeth Zucolotto, Ansgar Greshake, Herbert Raab, Michael Farmer, Greg Hupé, André Moutinho, Sonny Clary, Fabien Kuntz, Valery Bogdanovsky, Aid Mohamed, Ismailly Sidi Mohamed, Abdel Fattah Gharrad, and the National Institute of Polar Research for providing meteorite samples. We also thank the reviewers for their open-mindedness regarding our concept and their constructive comments, which substantially improved the quality of the manuscript, and Matthew Sanborn for proofreading the manuscript. This work was supported by NASA Cosmochemistry Grant NNX14AM62G and Emerging Worlds Grant NNX16AD34D (to Q.-Z.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616019114/-/DCSupplemental.
References
- 1.Sephton MA. Organic compounds in carbonaceous meteorites. Nat Prod Rep. 2002;19(3):292–311. doi: 10.1039/b103775g. [DOI] [PubMed] [Google Scholar]
- 2.Cronin JR, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275(5302):951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 3.Cooper G, et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature. 2001;414(6866):879–883. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 4.Meierhenrich UJ, Muñoz Caro GM, Bredehöft JH, Jessberger EK, Thiemann WH-P. Identification of diamino acids in the Murchison meteorite. Proc Natl Acad Sci USA. 2004;101(25):9182–9186. doi: 10.1073/pnas.0403043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt-Kopplin P, et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc Natl Acad Sci USA. 2010;107(7):2763–2768. doi: 10.1073/pnas.0912157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertkorn N, Harir M, Schmitt-Kopplin P. Nontarget analysis of Murchison soluble organic matter by high-field NMR spectroscopy and FTICR mass spectrometry. Magn Reson Chem. 2015;53(9):754–768. doi: 10.1002/mrc.4249. [DOI] [PubMed] [Google Scholar]
- 7.Cody GD, Alexander CO, Tera F. Solid-state (1H and 13C) nuclear magnetic resonance spectroscopy of insoluble organic residue in the Murchison meteorite: A self-consistent quantitative analysis. Geochim Cosmochim Acta. 2002;66(10):1851–1865. [Google Scholar]
- 8.Burton AS, Stern JC, Elsila JE, Glavin DP, Dworkin JP. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev. 2012;41(16):5459–5472. doi: 10.1039/c2cs35109a. [DOI] [PubMed] [Google Scholar]
- 9.Fioroni M. Astro-organometallics of Fe, Co, Ni: Stability, IR fingerprints and possible locations. Comput Theor Chem. 2016;1084:196–212. [Google Scholar]
- 10.Ehrenfreund P, Glavin DP, Botta O, Cooper G, Bada JL. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of CI type carbonaceous chondrites. Proc Natl Acad Sci USA. 2001;98(5):2138–2141. doi: 10.1073/pnas.051502898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Guillou C, Bernard S, Brearley AJ, Remusat L. Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim Cosmochim Acta. 2014;131:368–392. [Google Scholar]
- 12.Yesiltas M, Kebukawa Y. Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteorit Planet Sci. 2016;51(3):584–595. [Google Scholar]
- 13.Lodders K. Solar system abundances and condensation temperatures of the elements. Astrophys J. 2003;591(2):1220–1247. [Google Scholar]
- 14.Elschenbroich C, Salzer A. Organometallics. Wiley-VCH; New York: 1989. [Google Scholar]
- 15.Khairallah GN, Thum CC, Lesage D, Tabet J-C, O’Hair RA. Gas-phase formation and fragmentation reactions of the organomagnesates [RMgX2]−. Organometallics. 2013;32(8):2319–2328. [Google Scholar]
- 16.Bock CW, Kaufman A, Glusker JP. Coordination of water to magnesium cations. Inorg Chem. 1994;33(3):419–427. [Google Scholar]
- 17.Hazen RM, Jones AP, Baross JA. 2013 Carbon in earth. Available at minsocam.org/MSA/Rim/RiMG075/RiMG075_Title_Page.pdf. Accessed December 27, 2016.
- 18.Schulte M, Shock E. Coupled organic synthesis and mineral alteration on meteorite parent bodies. Meteorit Planet Sci. 2004;39(9):1577–1590. [Google Scholar]
- 19.Ferralis N, Matys ED, Knoll AH, Hallmann C, Summons RE. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon. 2016;108:440–449. [Google Scholar]
- 20.Johnson NM, Elsila JE, Kopstein M, Nuth JA. Carbon isotopic fractionation in Fischer-Tropsch-type reactions and relevance to meteorite organics. Meteorit Planet Sci. 2012;47(6):1029–1034. [Google Scholar]
- 21.Hill HG, Grady CA, Nuth JA, 3rd, Hallenbeck SL, Sitko ML. Constraints on nebular dynamics and chemistry based on observations of annealed magnesium silicate grains in comets and in disks surrounding Herbig Ae/Be stars. Proc Natl Acad Sci USA. 2001;98(5):2182–2187. doi: 10.1073/pnas.051530998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irving AJ, et al. 2013 Ungrouped mafic achondrite Northwest Africa 7325: A reduced, iron-poor cumulate olivine gabbro from a differentiated planetary parent body. Available at www.lpi.usra.edu/meetings/lpsc2013/pdf/2164.pdf.
- 23.Jenniskens P, et al. Fall, recovery, and characterization of the Novato L6 chondrite breccia. Meteorit Planet Sci. 2014;49(8):1388–1425. [Google Scholar]
- 24.Popova OP, et al. Chelyabinsk Airburst Consortium Chelyabinsk airburst, damage assessment, meteorite recovery, and characterization. Science. 2013;342(6162):1069–1073. doi: 10.1126/science.1242642. [DOI] [PubMed] [Google Scholar]
- 25.Summons RE, Albrecht P, McDonald G, Moldowan JM. Molecular biosignatures. Space Sci Rev. 2008;135(1-4):133–159. [Google Scholar]
- 26.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28(11):4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C, Gross ML. Applications and mechanisms of charge-remote fragmentation. Mass Spectrom Rev. 2000;19(6):398–420. doi: 10.1002/1098-2787(2000)19:6<398::AID-MAS3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Dove AP, et al. Low coordinate magnesium chemistry supported by a bulky β-diketiminate ligand. Dalton Trans. 2003;(15):3088–3097. [Google Scholar]
- 29.Tziotis D, Hertkorn N, Schmitt-Kopplin P. Kendrick-analogous network visualisation of ion cyclotron resonance Fourier transform mass spectra: Improved options for the assignment of elemental compositions and the classification of organic molecular complexity. Eur J Mass Spectrom (Chichester) 2011;17(4):415–421. doi: 10.1255/ejms.1135. [DOI] [PubMed] [Google Scholar]
- 30.Stary J, Liljenzin JO. Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates. Pure Appl Chem. 1982;54(12):2557–2592. [Google Scholar]
- 31.Huss GR, Rubin AE, Grossman JN. Thermal metamorphism in chondrites. Meteor Early Sol Syst. 2006;II:567–586. [Google Scholar]
- 32.Haack H, et al. Maribo-A new CM fall from Denmark. Meteorit Planet Sci. 2012;47(1):30–50. [Google Scholar]
- 33.Jenniskens P, et al. Sutter’s Mill Meteorite Consortium Radar-enabled recovery of the Sutter’s Mill meteorite, a carbonaceous chondrite regolith breccia. Science. 2012;338(6114):1583–1587. doi: 10.1126/science.1227163. [DOI] [PubMed] [Google Scholar]
- 34.Keil K, et al. The Vicência meteorite fall: A new unshocked (S1) weakly metamorphosed (3.2) LL chondrite. Meteorit Planet Sci. 2015;50(6):1089–1111. [Google Scholar]
- 35.Schmitt-Kopplin P, et al. Chemical footprint of the solvent soluble extraterrestrial organic matter occluded in Soltmany ordinary chondrite. Meteorites. 2012;2(1-2):71–92. [Google Scholar]
- 36.Martins Z, Modica P, Zanda B, d’Hendecourt LLS. The amino acid and hydrocarbon contents of the Paris meteorite: Insights into the most primitive CM chondrite. Meteorit Planet Sci. 2015;50(5):926–943. [Google Scholar]
- 37.Black JR, Yin Q, Casey WH. An experimental study of magnesium-isotope fractionation in chlorophyll-a photosynthesis. Geochim Cosmochim Acta. 2006;70(16):4072–4079. [Google Scholar]
- 38.Wimpenny J, Colla CA, Yin Q-Z, Rustad JR, Casey WH. Investigating the behaviour of Mg isotopes during the formation of clay minerals. Geochim Cosmochim Acta. 2014;128:178–194. [Google Scholar]
- 39.Tonui E, et al. Petrographic, chemical and spectroscopic evidence for thermal metamorphism in carbonaceous chondrites I: CI and CM chondrites. Geochim Cosmochim Acta. 2014;126:284–306. [Google Scholar]
- 40.Williams RJ. Systems biology of evolution: The involvement of metal ions. Biometals. 2007;20(2):107–112. doi: 10.1007/s10534-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 41.Martin W, Russell MJ. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc Lond B Biol Sci. 2003;358(1429):59–83, discussion 83–85. doi: 10.1098/rstb.2002.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saladino R, Botta G, Bizzarri BM, Di Mauro E, Garcia Ruiz JM. A global scale scenario for prebiotic chemistry: Silica-based self-assembled mineral structures and formamide. Biochemistry. 2016;55(19):2806–2811. doi: 10.1021/acs.biochem.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witt M, Roesky HW. Organoaluminum chemistry at the forefront of research and development. Curr Sci. 2000;78:410–430. [Google Scholar]
- 44.Rehder D. Is vanadium a more versatile target in the activity of primordial life forms than hitherto anticipated? Org Biomol Chem. 2008;6(6):957–964. doi: 10.1039/b717565p. [DOI] [PubMed] [Google Scholar]
- 45.Senior JK. Partitions and their representative graphs. Am J Math. 1951;73(3):663–689. [Google Scholar]
- 46.Frisch MJ, et al. 2004. Gaussian 03, revision c. 02 (Gaussian, Inc., Pittsburgh)
- 47.Foresman JB, Frisch Æ. Exploring Chemistry with Electronic Structure Methods: A Guide to Using Gaussian. Gaussian, Inc.; Pittsburgh: 1993. [Google Scholar]
- 48.Jensen F. Polarization consistent basis sets. III. The importance of diffuse functions. J Chem Phys. 2002;117(20):9234–9240. [Google Scholar]
- 49.Schlegel HB. Optimization of equilibrium geometries and transition structures. J Comput Chem. 1982;3(2):214–218. [Google Scholar]
- 50.Schlegel HB. Estimating the Hessian for gradient-type geometry optimizations. Theor Chim Acta. 1984;66(5):333–340. [Google Scholar]
- 51.Császár P, Pulay P. Geometry optimization by direct inversion in the iterative subspace. J Mol Struct. 1984;114:31–34. [Google Scholar]
- 52.Kanawati B, Joniec S, Winterhalter R, Moortgat GK. Mass spectrometric characterization of small oxocarboxylic acids and gas phase ion fragmentation mechanisms studied by electrospray triple quadrupole-MS/MS-TOF system and DFT theory. Int J Mass Spectrom. 2007;266(1):97–113. [Google Scholar]
- 53.Gabelica V, Galic N, Rosu F, Houssier C, De Pauw E. Influence of response factors on determining equilibrium association constants of non-covalent complexes by electrospray ionization mass spectrometry. J Mass Spectrom. 2003;38(5):491–501. doi: 10.1002/jms.459. [DOI] [PubMed] [Google Scholar]
- 54.Davis AM. Meteorites, Comets, and Planets: Treatise on Geochemistry. 2nd Ed Elsevier; Amsterdam: 2005. [Google Scholar]
- 55.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucio M, Fekete A, Frommberger M, Schmitt-Kopplin P. Metabolomics: High-resolution tools offer to follow bacterial growth on a molecular level. In: de Bruijn FJ, editor. Handbook of Molecular Microbial Ecology Metagenomics I: Metagenomics and Complementary Approaches. Wiley; New York: 2011. pp. 683–695. [Google Scholar]
- 57.Jacobsen B, et al. 26Al–26Mg and 207Pb–206Pb systematics of Allende CAIs: Canonical solar initial 26Al/27Al ratio reinstated. Earth Planet Sci Lett. 2008;272(1-2):353–364. [Google Scholar]
- 58.Young ED, Galy A. The isotope geochemistry and cosmochemistry of magnesium. Rev Mineral Geochem. 2004;55(1):197–230. [Google Scholar]
- 59.Galy A, et al. Magnesium isotope heterogeneity of the isotopic standard SRM980 and new reference materials for magnesium-isotope-ratio measurements. J Anal At Spectrom. 2003;18(11):1352–1356. [Google Scholar]
- 60.Li W-Y, et al. Heterogeneous magnesium isotopic composition of the upper continental crust. Geochim Cosmochim Acta. 2010;74(23):6867–6884. [Google Scholar]
- 61.Bourdon B, Tipper ET, Fitoussi C, Stracke A. Chondritic Mg isotope composition of the Earth. Geochim Cosmochim Acta. 2010;74(17):5069–5083. [Google Scholar]
- 62.Rubin AE. Shock, post-shock annealing, and post-annealing shock in ureilites. Meteorit Planet Sci. 2006;41(1):125–133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.