Abstract
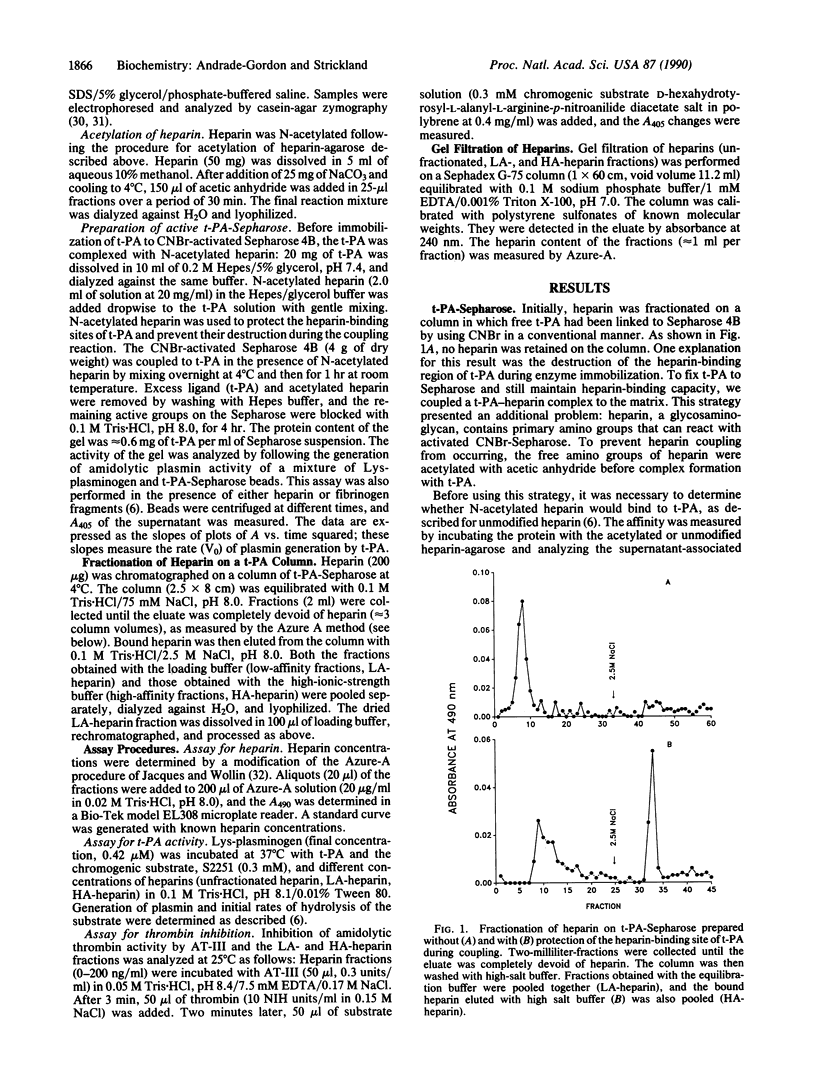
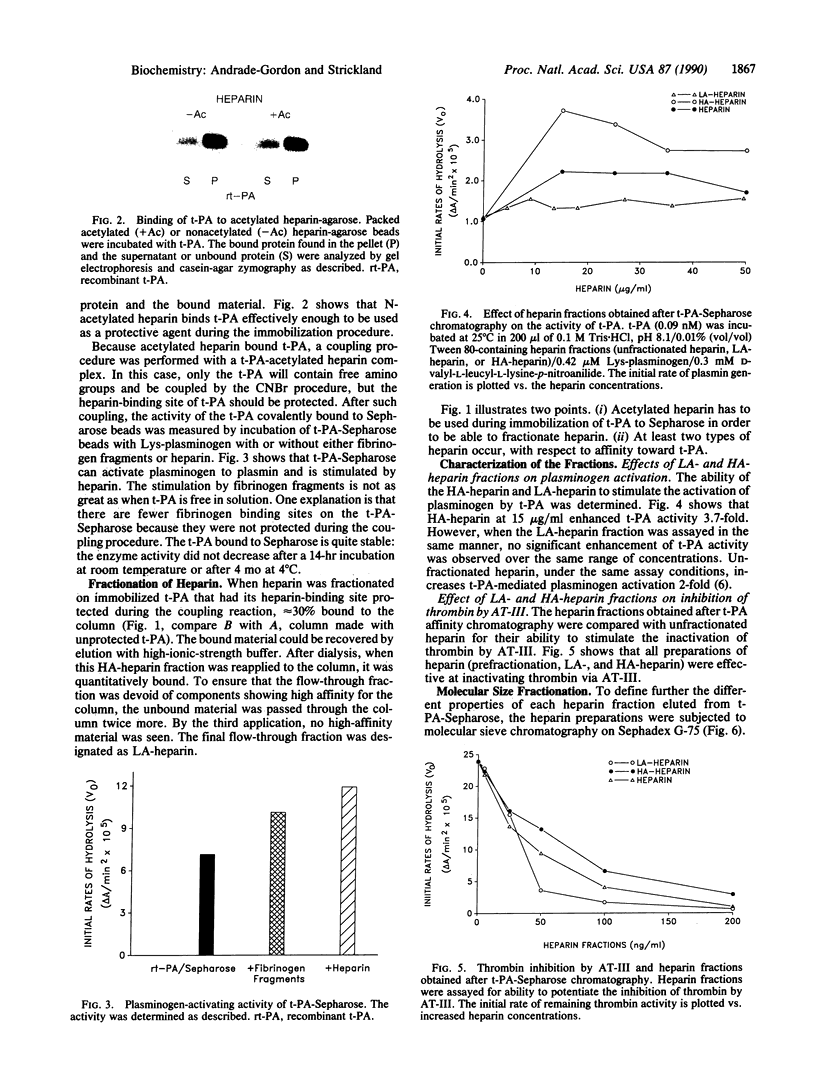
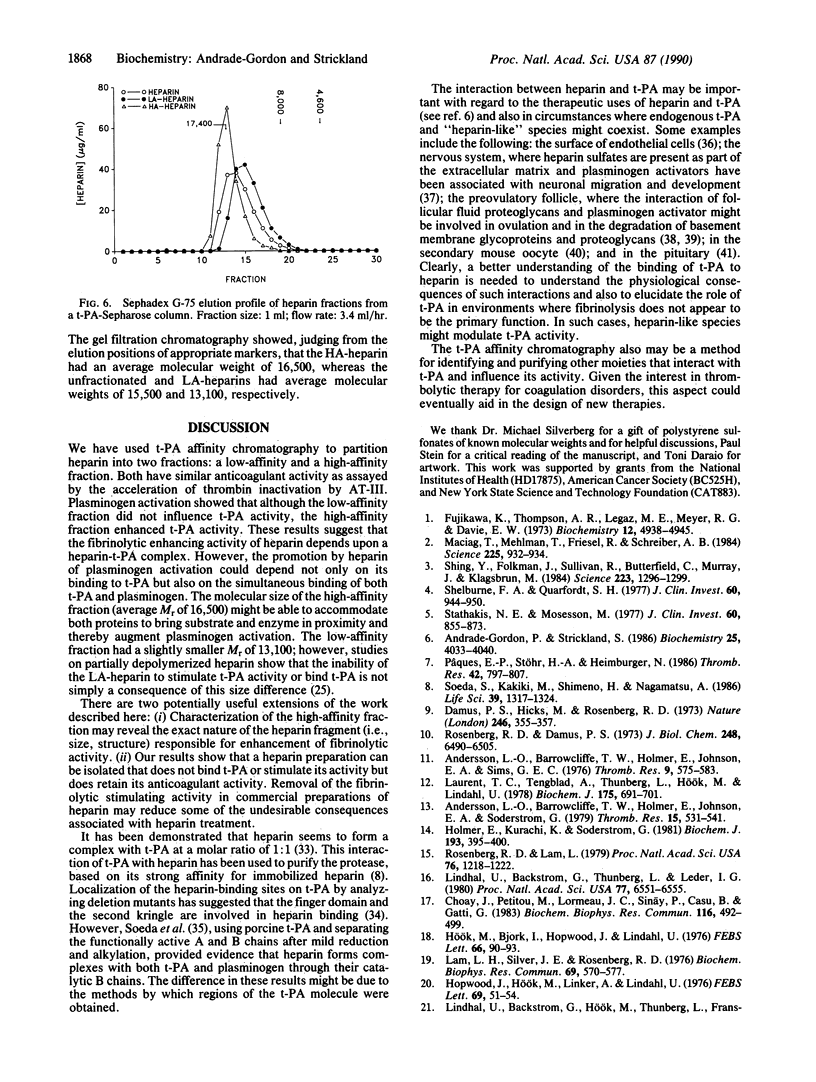
Heparin stimulates the activity of tissue plasminogen activator (t-PA) and binds to t-PA. To study this interaction, a complex between t-PA and N-acetylated heparin was formed and then linked to Sepharose. This procedure selectively links the t-PA to the column because the acetylated heparin has no free amino groups. The procedure also protects the heparin-binding site(s) on the enzyme during coupling to the matrix. The t-PA column separates heparin into two fractions, one with low affinity for t-PA and one with high affinity. Both fractions of heparin effectively accelerate inactivation of thrombin by antithrombin III. However, the fractions differ in their ability to stimulate t-PA: the low-affinity heparin has no effect on the activity of t-PA, whereas the high-affinity heparin enhances this activity. These heparin fractions will be useful in characterizing the biochemical basis and physiological consequences of the heparin--t-PA interaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Söderström G. Molecular weight dependency of the heparin potentiated inhibition of thrombin and activated factor X. Effect of heparin neutralization in plasma. Thromb Res. 1979;15(3-4):531–541. doi: 10.1016/0049-3848(79)90159-2. [DOI] [PubMed] [Google Scholar]
- Andrade-Gordon P., Strickland S. Anticoagulant low molecular weight heparin does not enhance the activation of plasminogen by tissue plasminogen activator. J Biol Chem. 1989 Sep 15;264(26):15177–15181. [PubMed] [Google Scholar]
- Andrade-Gordon P., Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986 Jul 15;25(14):4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Canipari R., O'Connell M. L., Meyer G., Strickland S. Mouse ovarian granulosa cells produce urokinase-type plasminogen activator, whereas the corresponding rat cells produce tissue-type plasminogen activator. J Cell Biol. 1987 Aug;105(2):977–981. doi: 10.1083/jcb.105.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choay J., Petitou M., Lormeau J. C., Sinaÿ P., Casu B., Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):492–499. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Hicks M., Rosenberg R. D. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355–357. doi: 10.1038/246355a0. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fears R. Kinetic studies on the effect of heparin and fibrin on plasminogen activators. Biochem J. 1988 Jan 1;249(1):77–81. doi: 10.1042/bj2490077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer E., Kurachi K., Söderström G. The molecular-weight dependence of the rate-enhancing effect of heparin on the inhibition of thrombin, factor Xa, factor IXa, factor XIa, factor XIIa and kallikrein by antithrombin. Biochem J. 1981 Feb 1;193(2):395–400. doi: 10.1042/bj1930395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J., Hök M., Linker A., Lindahl U. Anticoagulant activity of heparin: isolation of antithrombin-binding sites. FEBS Lett. 1976 Oct 15;69(1):51–54. doi: 10.1016/0014-5793(76)80651-5. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli J. D. Plasminogen activator in mouse and rat oocytes: induction during meiotic maturation. Cell. 1985 Dec;43(2 Pt 1):551–558. doi: 10.1016/0092-8674(85)90184-9. [DOI] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jaques L. B., Wollin A. A modified method for the colorimetric determination of heparin. Can J Physiol Pharmacol. 1967 Sep;45(5):787–794. doi: 10.1139/y67-093. [DOI] [PubMed] [Google Scholar]
- Kristensen P., Nielsen L. S., Grøndahl-Hansen J., Andresen P. B., Larsson L. I., Danø K. Immunocytochemical demonstration of tissue-type plasminogen activator in endocrine cells of the rat pituitary gland. J Cell Biol. 1985 Jul;101(1):305–311. doi: 10.1083/jcb.101.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L., Leder I. G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Nesheim M., Blackburn M. N., Lawler C. M., Mann K. G. Dependence of antithrombin III and thrombin binding stoichiometries and catalytic activity on the molecular weight of affinity-purified heparin. J Biol Chem. 1986 Mar 5;261(7):3214–3221. [PubMed] [Google Scholar]
- Patterson P. H. On the role of proteases, their inhibitors and the extracellular matrix in promoting neurite outgrowth. J Physiol (Paris) 1985;80(4):207–211. [PubMed] [Google Scholar]
- Pâques E. P., Stöhr H. A., Heimburger N. Study on the mechanism of action of heparin and related substances on the fibrinolytic system: relationship between plasminogen activators and heparin. Thromb Res. 1986 Jun 15;42(6):797–807. doi: 10.1016/0049-3848(86)90116-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Rosenberg R. D., Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1218–1222. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonaeva E. E., Shimonaev G. S., Aleshina T. S. Kompleks geparin--tkanevyi aktivator plazminogena i nekotorye ego svoistva. Biokhimiia. 1983 Oct;48(10):1687–1690. [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Soeda S., Kakiki M., Shimeno H., Nagamatsu A. Localization of the binding sites of porcine tissue-type plasminogen activator and plasminogen to heparin. Biochim Biophys Acta. 1987 Dec 18;916(3):279–287. doi: 10.1016/0167-4838(87)90171-3. [DOI] [PubMed] [Google Scholar]
- Soeda S., Kakiki M., Shimeno H., Nagamatsu A. Rapid and high-yield purification of porcine heart tissue-type plasminogen activator by heparin-sepharose choromatography. Life Sci. 1986 Oct 13;39(15):1317–1324. doi: 10.1016/0024-3205(86)90329-2. [DOI] [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. L., van Zonneveld A. J., Pannekoek H., Strickland S. Structural domains of human tissue-type plasminogen activator that confer stimulation by heparin. J Biol Chem. 1989 Sep 15;264(26):15441–15444. [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen J. H., Mullaart E., Chang G. T., Kluft C., Wijngaards G. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost. 1982 Dec 27;48(3):266–269. [PubMed] [Google Scholar]
- Vinazzer H., Stemberger A., Haas S., Blümel G. Influence of heparin; of different heparin fractions and of a low molecular weight heparin-like substance on the mechanism of fibrinolysis. Thromb Res. 1982 Aug 1;27(3):341–352. doi: 10.1016/0049-3848(82)90081-0. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Biosynthesis of proteoglycans by rat granulosa cells cultured in vitro. J Biol Chem. 1979 Dec 25;254(24):12355–12364. [PubMed] [Google Scholar]




