Abstract
Background:
The clinical significance of metabolic syndrome (MS) score, MS, and its individual components with respect to risk prediction of coronary artery disease (CAD) remains unclear. The objective of this study was to investigate whether and to what extent MS score, MS, and its individual components were related to the risk of CAD.
Methods:
Among 1191 participants who underwent coronary angiography for the confirmation of suspected myocardial ischemia, 858 were included in this study according to the inclusion criteria from September 2010 to June 2013. MS was diagnosed with the 2005 National Cholesterol Education Program Adult Treatment Panel III criteria. The severity of coronary atherosclerosis was assessed by Gensini score.
Results:
The results showed that the age- and sex-adjusted odds ratios (ORs) for CAD were as follows: MS score, 1.327; MS, 2.013; elevated waist circumference, 1.447; reduced high-density lipoprotein cholesterol, 1.654; and elevated fasting glucose, 1.782; all P < 0.05; whereas for elevated triglycerides, 1.324, and elevated blood pressure, 1.342, both P > 0.05. After multivariate adjustment, results showed that only MS and elevated fasting glucose were significantly associated with CAD (OR, 1.628, 95% confidence interval [CI], 1.151–2.305, P = 0.006 for elevated fasting glucose, and OR, 1.631, 95% CI, 1.208–2.203, P = 0.001 for MS). The study showed that only MS score and elevated fasting glucose were significantly associated with Gensini score (standardized coefficient, 0.101, P = 0.031 for elevated fasting glucose and standardized coefficient, 0.103, P = 0.009 for MS score).
Conclusions:
The present study demonstrated that MS score, MS, and its individual components might have different contributions to CAD prevalence and severity. MS and elevated fasting glucose were independent risk factors for the prevalence of angiographic CAD whereas MS score and elevated fasting glucose were significantly associated with the severity of CAD.
Keywords: Coronary Angiography, Coronary Artery Disease, Metabolic Syndrome, Metabolic Syndrome Score
Introduction
The metabolic syndrome (MS), also known as the insulin resistance, is characterized by a clustering of cardiovascular risk factors including hyperglycemia, obesity, dyslipidemia, and hypertension, and it was first defined by Reaven in 1988 to improve the understanding of links between insulin resistance and vascular disease.[1] MS is now becoming increasingly common[2] and represents a global public health problem.[3] Since its first definition, many studies have focused on whether the MS is associated with an increased risk of cardiovascular disease.[4,5,6,7,8,9] Some studies found that MS had a high prevalence in patients with coronary artery disease (CAD)[5,6,10] and increased the risks of cardiovascular disease and all-cause mortality.[4,5,6,7]
It has been reported that MS is a risk factor of cardiovascular disease, but not above and beyond the risk associated with its individual components.[11,12] Therefore, the number of components of MS might be more useful to predict the severity of CAD than MS per se, and it has been used instead of a binary definition of MS in several studies.[13,14,15,16] Each abnormality promotes atherosclerosis independently, but when clustered together, whether or not these metabolic disorders are increasingly atherogenic and enhance the risk of developing CAD and cardiovascular events, results are controversial.[13,14,15,16] Therefore, the clinical role of MS remains contentious, and its definition is also under debate.
The objective of this study was to investigate whether and to what extent MS score, MS, and its individual components were related to the risk for CAD since few studies have focused on this issue in Chinese population.
Methods
Subjects
The individuals of the present study were recruited from a consecutive sample of 1191 individuals who underwent coronary angiography for the confirmation of suspected myocardial ischemia and the evaluation of CAD at the Department of Cardiology in Shanghai Zhongshan Hospital, Fudan University between September 2010 and June 2013. Patients who had an acute coronary syndrome (n = 264) or had incomplete clinical information were excluded (n = 69) from the study. Thus, 858 individuals participated in this study.
All participants were Chinese and living in Shanghai and its neighboring areas, and all gave their informed written consents. The Institutional Review Board of Shanghai Zhongshan Hospital approved the study protocol.
Clinical and biochemical measurements
All patients were inquired in detail about case histories, which included sex, age, history of smoking, alcohol consumption, hypertension, diabetes, family history of hypertension, diabetes, and CAD, and information on medical treatment. The anthropometric measurements such as body height, body mass, waist circumference, hip circumference, and blood pressure were determined by the same physicians from our department with body mass index (BMI) and waist to hip ratio (WHR) being calculated as well. BMI was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference was calculated as the average of two measurements taken after inspiration and expiration at the highest point of iliac crest. WHR was calculated as the waist circumference divided by the hip circumference. Blood pressure was assessed while the patient was sitting, and the average of three measurements was calculated. Current cigarette smoking was defined as a daily intake of more than five cigarettes. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg (1 mmHg=0.133 kPa), or diastolic blood pressure (DBP) ≥90 mmHg in at least two separate measurements, or a history of hypertension. Diabetes was defined according to the diagnostic criteria of the World Health Organization.[17]
After an overnight fasting, blood samples were collected from all of the patients for the measurements of fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). FPG concentrations were measured by a glucose oxidase method (Beckman Coulter Inc., USA). Serum TG, TC, and HDL-C levels were measured by the enzymatic method (7170A, Hitachi, Japan), and LDL-C levels were calculated according to the Friedewald formula (LDL-C = TC − HDL-C − TG/2.2).[18]
Definition of metabolic syndrome
The presence of MS was determined using the updated 2005 National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria (adapted for Asians).[19] The reason why this study chose NCEP-ATP III diagnostic criteria was that this diagnostic criteria was for the whole population and the criteria for waist was adapted for Asians. Thus, these diagnostic criteria were appropriate for the study and had least influence on the results. In detail, the definition of MS requires the presence of any three or more of the following five criteria: (1) elevated waist circumference: ≥90 cm in men or ≥80 cm in women; (2) elevated TGs: ≥1.7 mmol/L or on drug treatment for elevated TGs; (3) reduced HDL-C: <1.03 mmol/L in men or <1.3 mmol/L in women or on drug treatment for reduced HDL-C; (4) elevated blood pressure: SBP ≥130 mmHg or DBP ≥85 mmHg or on antihypertensive drug treatment in a patient with a history of hypertension; and (5) elevated fasting glucose: ≥5.6 mmol/L or on drug treatment for elevated glucose. According to the existence of these criteria, points were summed and MS scores[14] varying from 0 to 5 were calculated in patients who underwent coronary angiography.
Coronary angiography and diagnostic criteria for coronary artery disease
Selective coronary angiography was performed using standard Judkins techniques in all of the patients. Angiography analysis was conducted by two experienced interventional cardiologists who were blinded to the study protocol. Significant angiographic CAD was defined as ≥50% lumen diameter reduction in at least one major coronary artery.[20] Gensini score[21] was used to assess the severity of CAD as follows: it grades narrowing of the lumen of the coronary artery and scores it as 1 for 1–25% narrowing, 2 for 26–50% narrowing, 4 for 51–75%, 8 for 76–90%, 16 for 91–99%, and 32 for a totally occluded artery. This score was then multiplied by a factor according to the importance of the coronary artery. The multiplication factor for a left main stem lesion is 5. It is 2.5 for proximal left anterior descending artery (LAD) and proximal circumflex (CX) artery lesions, 1.5 for a mid-LAD lesion, and 1 for distal LAD, mid/distal CX, and right coronary artery lesions. The multiplication factor for any other branch is 0.5.[22,23]
Statistical analysis
Statistical analysis was performed with SPSS version 13.0 software (SPSS, Chicago, IL, USA). Continuous variables were presented as means ± standard deviation (SD) and were compared with an analysis of variance (ANOVA). Discrete variables were presented as total number (percentage) and were compared by the Chi-square test. Univariate and multivariate logistic regression models were used to evaluate whether MS score, MS, and its individual components were related to the prevalence of angiographic CAD. Spearman's rank correlation and multivariate linear stepwise regression analyses were used to evaluate to what extent MS score, MS, and its individual components were related to the severity of angiographic CAD assessed by the Gensini score after adjustment for age, sex, and the use of lipid-lowering drugs, antidiabetic drugs, and antihypertensive drugs. Analysis of covariance was performed to adjust for the effect of age, sex, and the use of lipid-lowering drugs, antidiabetic drugs, and antihypertensive drugs. Logarithmic transformation was used for Gensini score because of the high degree of skewing. A P < 0.05 was considered statistically significant.
Results
Baseline characteristics
In this study, all of the patients were aged 33–82 years (mean 59 years), of which 626 (73.0%) were male. A total of 471 (54.9%) patients had MS. Most frequent component of MS determined in the patients was elevated blood pressure. A total of 638 (74.4%) patients had elevated blood pressure, followed by reduced HDL-C (59.8%), elevated TGs (57.2%), waist circumference (41.1%), and fasting glucose (29.0%).
Relative frequency according to the metabolic syndrome score
The distribution of patients with a 0 to 5 MS score is listed in Table 1. In most groups, elevated blood pressure was the most frequent abnormality, followed by reduced HDL-C, elevated TGs, waist circumference, and fasting glucose.
Table 1.
Relative frequency of various components of MS according to the MS score
| Variables | MS score | χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|
| 0 (n = 40) | 1 (n = 140) | 2 (n = 207) | 3 (n = 253) | 4 (n = 159) | 5 (n = 59) | |||
| Elevated waist circumference | 0 | 9 (6.4) | 50 (24.2) | 116 (45.8) | 119 (74.8) | 59 (100) | 283.591 | <0.001 |
| Elevated TGs | 0 | 19 (13.6) | 91 (44.0) | 183 (72.3) | 139 (87.4) | 59 (100) | 304.302 | <0.001 |
| Reduced HDL-C | 0 | 35 (25.0) | 102 (49.3) | 175 (69.2) | 142 (89.3) | 59 (100) | 246.042 | <0.001 |
| Elevated blood pressure | 0 | 70 (50.0) | 140 (67.6) | 218 (86.2) | 151 (95.0) | 59 (100) | 238.745 | <0.001 |
| Elevated fasting glucose | 0 | 7 (5.0) | 31 (15.0) | 67 (26.5) | 85 (53.5) | 59 (100) | 266.586 | <0.001 |
Data are shown as n (%). MS: Metabolic syndrome; HDL-C: High-density lipoprotein cholesterol; TGs: Triglycerides.
Clinical and biochemical characteristics in relation to the metabolic syndrome score
Table 2 outlines the demographic, clinical, and biochemical characteristics of the patients in relation to the MS score. As the MS score rose, so did age, BMI, waist circumference, WHR, SBP, DBP, and FPG (P < 0.05 or <0.001). TG increased and HDL-C decreased gradually with the MS score from 0 to 5 (P < 0.001). There were significant differences with respect to history of hypertension and diabetes, family history of hypertension, diabetes, and CAD, and the medical treatment (P < 0.05 or <0.001). No significant differences were found with respect to sex, TC, LDL-C, smoking status, and alcohol consumption (all P > 0.05).
Table 2.
Clinical and biochemical characteristics of patients in relation to the MS score
| Variables | MS score | Statistics | P | |||||
|---|---|---|---|---|---|---|---|---|
| 0 (n = 40) | 1 (n = 140) | 2 (n = 207) | 3 (n = 253) | 4 (n = 159) | 5 (n = 59) | |||
| Sex (male/female), n | 32/8 | 108/32 | 156/51 | 187/66 | 104/55 | 39/20 | 8.971† | 0.110 |
| Age (years) | 57 ± 9 | 58 ± 9 | 58 ± 9 | 60 ± 9 | 59.4 ± 9 | 61 ± 9 | 2.546* | 0.027 |
| BMI (kg/m2) | 21.7 ± 2.9 | 22.9 ± 3.3 | 24.1 ± 2.7 | 25.1 ± 2.7 | 25.6 ± 2.5 | 27.0 ± 3.1 | 35.227* | <0.001 |
| Waist circumference (cm) | 78.3 ± 8.1 | 81.5 ± 6.7 | 84.9 ± 7.9 | 89.1 ± 7.9 | 90.9 ± 7.2 | 95.7 ± 7.5 | 45.864* | <0.001 |
| WHR | 0.86 ± 0.06 | 0.88 ± 0.05 | 0.90 ± 0.06 | 0.94 ± 0.06 | 0.94 ± 0.05 | 0.96 ± 0.06 | 31.986* | <0.001 |
| SBP (mmHg) | 115 ± 7 | 127 ± 18 | 130 ± 17 | 134 ± 17 | 138 ± 16 | 139 ± 17 | 17.652* | <0.001 |
| DBP (mmHg) | 72 ± 7 | 78 ± 10 | 80 ± 10 | 82 ± 11 | 83 ± 10 | 83 ± 11 | 10.285* | <0.001 |
| Glycemic status | ||||||||
| FPG (mmol/L) | 4.6 ± 0.4 | 4.8 ± 0.8 | 5.0 ± 1.3 | 5.2 ± 1.0 | 5.8 ± 1.7 | 6.5 ± 1.3 | 24.813* | <0.001 |
| Lipid profile | ||||||||
| TG (mmol/L) | 1.0 ± 0.3 | 1.3 ± 0.8 | 1.8 ± 1.5 | 2.1 ± 1.1 | 2.6 ± 2.2 | 2.5 ± 1.3 | 18.936* | <0.001 |
| TC (mmol/L) | 4.4 ± 0.9 | 4.2 ± 0.9 | 4.4 ± 1.0 | 4.4 ± 0.8 | 4.4 ± 0.9 | 4.5 ± 1.1 | 1.420* | 0.214 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.9 ± 0.1 | 33.455* | <0.001 |
| LDL-C (mmol/L) | 2.6 ± 0.9 | 2.4 ± 0.7 | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.4 ± 0.8 | 2.7 ± 1.0 | 0.866* | 0.503 |
| Coronary risk factors | ||||||||
| Former smoker | 2 (5.0) | 19 (13.6) | 26 (12.6) | 30 (11.9) | 21 (13.2) | 8 (13.6) | 2.441† | 0.785 |
| Current smoker | 16 (40.0) | 50 (35.7) | 69 (33.3) | 94 (37.2) | 56 (35.2) | 19 (32.2) | 1.368† | 0.928 |
| Alcohol consumption | 6 (15.0) | 37 (26.4) | 48 (23.2) | 65 (25.7) | 45 (28.3) | 14 (23.7) | 3.681† | 0.596 |
| Hypertension | 0 | 47 (33.6) | 102 (49.3) | 171 (67.6) | 117 (73.6) | 53 (89.8) | 144.866† | <0.001 |
| Diabetes mellitus | 0 | 3 (2.3) | 22 (10.9) | 37 (14.8) | 49 (30.8) | 29 (50.0) | 109.261† | <0.001 |
| Family history of hypertension | 9 (22.5) | 57 (40.7) | 112 (54.1) | 143 (56.5) | 97 (61.0) | 41 (69.5) | 35.276† | <0.001 |
| Family history of diabetes | 6 (15.0) | 22 (15.7) | 32 (15.5) | 47 (18.6) | 40 (25.2) | 20 (33.9) | 15.139† | 0.010 |
| Family history of CAD | 4 (10.0) | 24 (17.1) | 66 (31.9) | 64 (25.3) | 56 (35.2) | 23 (39.0) | 24.869† | <0.001 |
| Medical treatment | ||||||||
| Aspirin | 1 (2.5) | 51 (36.4) | 95 (45.9) | 139 (54.9) | 108 (67.9) | 46 (78.0) | 88.667† | <0.001 |
| β-blocker | 1 (2.5) | 32 (22.9) | 72 (34.8) | 108 (42.7) | 82 (51.6) | 35 (59.3) | 61.746† | <0.001 |
| Calcium blocker | 0 | 24 (17.1) | 56 (27.1) | 78 (30.8) | 65 (40.9) | 28 (47.5) | 47.082† | <0.001 |
| ACEI | 0 | 27 (19.3) | 52 (25.1) | 73 (28.9) | 68 (42.8) | 29 (49.2) | 50.522† | <0.001 |
| Statin | 0 | 29 (20.7) | 56 (27.1) | 89 (35.2) | 75 (47.2) | 36 (61.0) | 68.253† | <0.001 |
Data are shown as means ± SD or n (%) or as indicated. *F value; †χ2 value. MS: Metabolic syndrome; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; TGs: Triglycerides; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; CAD: Coronary artery disease; ACEI: Angiotensin converting enzyme inhibitor; WHR: Waist to hip ratio; SD: Standard deviation; 1 mmHg=0.133 kPa.
Relative frequency according to with and without metabolic syndrome
The distribution of patients with and without MS is listed in [Table 3]. In the MS group, elevated blood pressure was also the most frequent abnormality, followed by elevated TGs, reduced HDL-C, waist circumference, and fasting glucose.
Table 3.
Relative frequency of various components of MS for patients with and without MS
| Variables | Without MS (n = 387) | With MS (n = 471) | χ2 | P |
|---|---|---|---|---|
| Elevated waist circumference | 59 (15.2) | 294 (62.4) | 195.243 | <0.001 |
| Elevated TGs | 110 (28.4) | 381 (80.9) | 238.924 | <0.001 |
| Reduced HDL-C | 137 (35.4) | 376 (79.8) | 174.433 | <0.001 |
| Elevated blood pressure | 210 (54.3) | 428 (90.9) | 147.655 | <0.001 |
| Elevated fasting glucose | 38 (9.8) | 211 (44.8) | 126.189 | <0.001 |
Data are shown as n (%). MS: Metabolic syndrome; HDL-C: High-density lipoprotein cholesterol; TGs: Triglycerides.
Clinical and biochemical characteristics in relation to with and without metabolic syndrome
Table 4 outlines the demographic, clinical, and biochemical characteristics of patients with and without MS. There were significant differences between the two groups in most variables, especially the individual components of MS, whereas the TC, LDL-C, smoking status, and alcohol consumption were not significantly different (P > 0.05).
Table 4.
Clinical and biochemical characteristics of patients with and without MS
| Variables | Without MS (n = 387) | With MS (n = 471) | Statistics | P |
|---|---|---|---|---|
| Sex (male/female), n | 296/91 | 330/141 | 4.441† | 0.035 |
| Age (years) | 58 ± 9 | 60 ± 9 | 9.265* | 0.002 |
| BMI (kg/m2) | 23.4 ± 3.1 | 25.5 ± 2.8 | 107.950* | <0.001 |
| Waist circumference (cm) | 83.0 ± 7.8 | 90.7 ± 7.9 | 158.987* | <0.001 |
| WHR | 0.89 ± 0.06 | 0.94 ± 0.06 | 125.543* | <0.001 |
| SBP (mmHg) | 127 ± 17 | 136 ± 17 | 54.950* | <0.001 |
| DBP (mmHg) | 78 ± 10 | 82 ± 10 | 32.751* | <0.001 |
| FPG (mmol/L) | 4.9 ± 1.1 | 5.5 ± 1.4 | 56.677* | <0.001 |
| Lipid profile | ||||
| TG (mmol/L) | 1.5 ± 1.2 | 2.3 ± 1.6 | 60.436* | <0.001 |
| TC (mmol/L) | 4.3 ± 0.9 | 4.4 ± 0.9 | 3.406* | 0.065 |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.0 ± 0.3 | 103.894* | <0.001 |
| LDL-C (mmol/L) | 2.5 ± 0.8 | 2.5 ± 0.8 | 0.073* | 0.786 |
| Coronary risk factors | ||||
| Former smoker | 47 (12.1) | 59 (12.5) | 0.029† | 0.886 |
| Current smoker | 135 (34.9) | 169 (35.9) | 0.092† | 0.761 |
| Alcohol consumption | 91 (23.5) | 124 (26.3) | 0.895† | 0.344 |
| Hypertension | 149 (38.5) | 341 (72.4) | 99.660† | <0.001 |
| Diabetes mellitus | 25 (6.5) | 115 (24.4) | 50.164† | <0.001 |
| Family history of hypertension | 178 (46.0) | 281 (59.7) | 15.947† | <0.001 |
| Family history of diabetes | 60 (15.5) | 107 (22.7) | 7.053† | 0.008 |
| Family history of CAD | 94 (24.3) | 143 (30.4) | 3.917† | 0.048 |
| Medical treatment | ||||
| Aspirin | 147 (38.0) | 293 (62.2) | 49.896† | <0.001 |
| β-blocker | 105 (27.1) | 225 (47.8) | 38.234† | <0.001 |
| Calcium blocker | 80 (20.7) | 171 (36.3) | 25.089† | <0.001 |
| ACEI | 79 (20.4) | 170 (36.1) | 25.357† | <0.001 |
| Statin | 85 (22.0) | 200 (471) | 40.243† | <0.001 |
Data are shown as means ± SD or n (%) or as indicated. *F value; †χ2 value. MS: Metabolic syndrome; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; TG: Triglyceride; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; CAD: Coronary artery disease; ACEI: Angiotensin converting enzyme inhibitor; SD: Standard deviation; WHR: Waist to hip ratio; 1 mmHg=0.133 kPa.
Frequency of various combinations of metabolic syndrome components
Frequency of various combinations of MS components of patients with and without MS is listed in Table 5. In patients with three components, the most frequent combination is elevated TGs combined with reduced HDL-C and elevated blood pressure. In patients with four components, the most frequent combination is elevated waist circumference and elevated TGs combined with reduced HDL-C and elevated blood pressure.
Table 5.
Frequency of various combinations of MS components of patients with MS
| Combinations | MS components | n (%) | ||||
|---|---|---|---|---|---|---|
| Elevated waist circumference | Elevated TGs | Reduced HDL-C | Elevated blood pressure | Elevated fasting glucose | ||
| Three components of MS | ||||||
| 3.1 | ✓ | ✓ | ✓ | – | – | 20 (4.2) |
| 3.2 | ✓ | ✓ | – | ✓ | – | 36 (7.6) |
| 3.3 | ✓ | ✓ | – | – | ✓ | 6 (1.3) |
| 3.4 | ✓ | – | ✓ | ✓ | – | 40 (8.5) |
| 3.5 | ✓ | – | ✓ | – | ✓ | 1 (0.2) |
| 3.6 | ✓ | – | – | ✓ | ✓ | 13 (2.8) |
| 3.7 | – | ✓ | ✓ | ✓ | – | 90 (19.1) |
| 3.8 | – | ✓ | ✓ | – | ✓ | 8 (1.7) |
| 3.9 | – | ✓ | – | ✓ | ✓ | 23 (4.9) |
| 3.10 | – | – | ✓ | ✓ | ✓ | 16 (3.4) |
| Four components of MS | ||||||
| 4.1 | ✓ | ✓ | ✓ | ✓ | – | 74 (15.7) |
| 4.2 | ✓ | ✓ | ✓ | – | ✓ | 8 (1.7) |
| 4.3 | ✓ | ✓ | – | ✓ | ✓ | 17 (3.6) |
| 4.4 | ✓ | – | ✓ | ✓ | ✓ | 20 (4.2) |
| 4.5 | – | ✓ | ✓ | ✓ | ✓ | 40 (8.5) |
| Five components of MS | ||||||
| 5.1 | ✓ | ✓ | ✓ | ✓ | ✓ | 59 (12.5) |
–: Not applicable; ✓: Present; MS: Metabolic syndrome; HDL-C: High-density lipoprotein cholesterol; TGs: Triglycerides.
Association between metabolic syndrome score, metabolic syndrome, and its individual components and the prevalence of angiographic coronary artery disease
Coronary angiography revealed that 533 (62.1%) patients had significant angiographic CAD. Figure 1 depicts CAD prevalence in relation to the MS score. As the MS score rose, the CAD prevalence before and after adjustment for age and sex increased. Individuals with an MS score of 0 had the lowest CAD prevalence (45.0%) while the other five groups had significantly higher prevalence (51.4%, 57.0%, 68.4%, 70.4%, and 72.9%, respectively, P < 0.001). Figure 2 depicts CAD prevalence in relation to patients with and without MS. Compared to patients without MS, patients with MS had significantly higher prevalence of CAD (69.6% vs. 53.7%, P < 0.001). By univariate logistic regression analyses adjusted for age and sex, MS score, MS, and three of its individual components including elevated waist circumference, reduced HDL-C, and elevated fasting glucose were significantly associated with CAD [Table 6]. After adjustment for age, sex, MS score, MS and its components each other, and drug using, multivariate logistic stepwise regression analysis showed that only MS and its components of elevated fasting glucose were significantly associated with CAD (odds ratio [OR], 1.628, 95% confidence interval [CI], 1.151–2.305, P = 0.006 for elevated fasting glucose and OR, 1.631, 95% CI, 1.208–2.203, P = 0.001 for MS) [Table 6].
Figure 1.
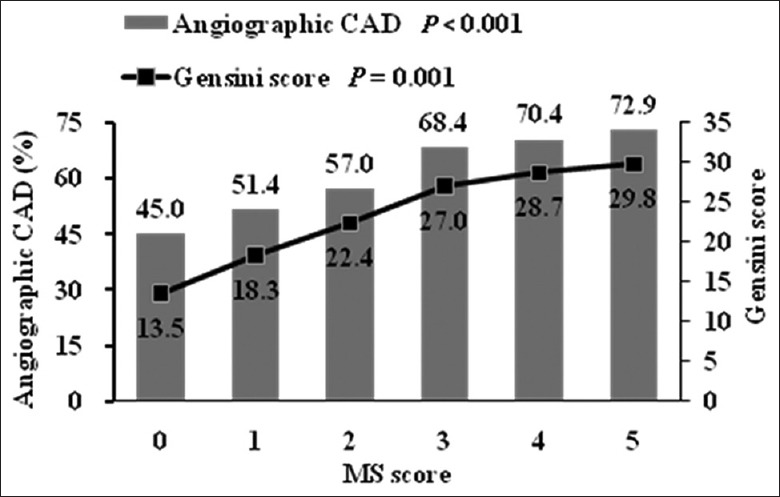
CAD prevalence and Gensini score according to the MS score. Data are adjusted for age and sex. CAD: Coronary artery disease; MS: Metabolic syndrome.
Figure 2.
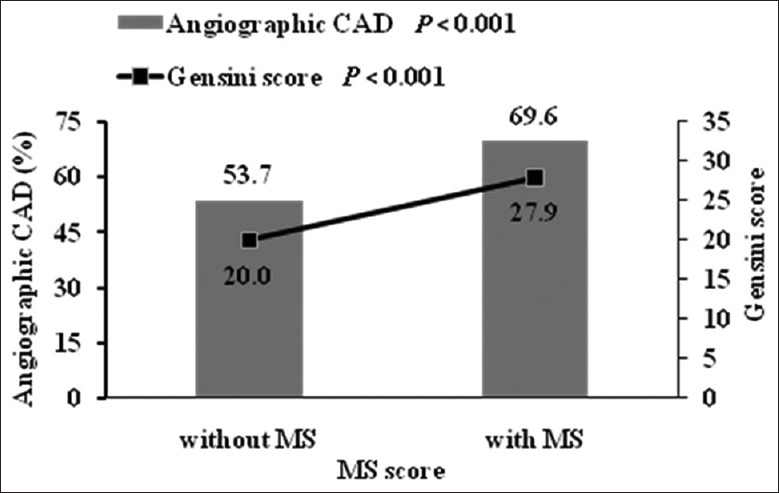
CAD prevalence and Gensini score of patients with and without MS. Data are adjusted for age and sex. CAD: Coronary artery disease; MS: Metabolic syndrome.
Table 6.
Estimates of relative risk for angiographic CAD of MS score, MS, and its individual components
| Items | Univariate (enter) | Multivariate (stepwise) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Elevated waist circumference | 1.447 | 1.067–1.963 | 0.017 | – | – | – |
| Elevated TGs | 1.324 | 0.985–1.778 | 0.063 | – | – | – |
| Reduced HDL-C | 1.654 | 1.226–2.230 | 0.001 | – | – | – |
| Elevated blood pressure | 1.342 | 0.961–1.873 | 0.084 | – | – | – |
| Elevated fasting glucose | 1.782 | 1.269–2.503 | 0.001 | 1.628 | 1.151–2.305 | 0.006 |
| MS score | 1.327 | 1.178–1.494 | <0.001 | – | – | – |
| MS | 2.013 | 1.494–2.713 | <0.001 | 1.631 | 1.208–2.203 | 0.001 |
Data are adjusted for age and sex in univariate analysis and are adjusted for age, sex, MS score, MS and its components each other, and drug using in multivariate analysis. –: Not applicable; CAD: Coronary artery disease; HDL-C: High-density lipoprotein cholesterol; OR: Odds ratio; CI: Confidence interval; MS: Metabolic syndrome; TGs: Triglycerides.
We then explored that to what extent MS score, MS, and its individual components were related to the severity of angiographic CAD. Figure 1 depicts CAD severity assessed by the Gensini score in relation to the MS score. The Gensini score increased gradually with the MS score before and after adjustment for age and sex. Individuals with an MS score of 0 had the lowest Gensini score while the other five groups had significantly higher Gensini score (P = 0.001). Figure 2 depicts Gensini score in relation to patients with and without MS. Compared to patients without MS, patients with MS had significantly higher Gensini score (P < 0.001). By Spearman's rank correlation analyses adjusted for age and sex, MS score, MS, and three of its individual components including elevated waist circumference, reduced HDL-C, and elevated fasting glucose were significantly associated with the Gensini score [Table 7]. After adjustment for age, sex, MS score, MS and its components each other, and drug using, multivariate linear stepwise regression analysis showed that only MS score and its components of elevated fasting glucose were significantly associated with the Gensini score (standardized coefficients, 0.103, P = 0.009 for MS score, and standardized coefficients, 0.101, P = 0.031 for elevated fasting glucose) [Table 7].
Table 7.
Spearman's rank correlation and multivariate linear stepwise regression analyses for the Gensini score
| Items | Spearman’s rank correlation | Linear stepwise regression | ||
|---|---|---|---|---|
| r | P | Standardized coefficients | P | |
| Elevated waist circumference | 0.070 | 0.041 | – | |
| Elevated TGs | 0.066 | 0.054 | – | – |
| Reduced HDL-C | 0.102 | 0.003 | – | – |
| Elevated blood pressure | 0.039 | 0.256 | – | – |
| Elevated fasting glucose | 0.103 | 0.003 | 0.101 | 0.031 |
| MS score | 0.142 | <0.001 | 0.103 | 0.009 |
| MS | 0.131 | <0.001 | – | – |
Data are adjusted for age and sex in Spearman’s rank correlation analysis and are adjusted for age, sex, MS score, MS and its components each other, and drug using in linear stepwise regression analysis. –: Not applicable; HDL-C: High-density lipoprotein cholesterol; MS: Metabolic syndrome; TGs: Triglycerides.
Discussion
The aim of the present study was to investigate whether and to what extent MS score, MS, and its individual components were related to risk for CAD. This study found the following results. (1) In Chinese high-risk patients of the present study, among all the MS components, elevated waist circumference, reduced HDL-C, and elevated fasting glucose might have the relatively higher association with CAD, whereas elevated TGs and elevated blood pressure might have weaker association. Besides, MS score and MS were also related with CAD. After adjustment for age, sex, MS score, MS and its components each other, and drug using, results showed that only MS and elevated fasting glucose were significantly associated with CAD. (2) By Spearman's rank correlation analyses, MS score, MS, and three of its individual components including elevated waist circumference, reduced HDL-C, and elevated fasting glucose were significantly associated with the severity of CAD assessed by the Gensini score, whereas no significant associations were found in elevated TGs and elevated blood pressure. After adjustment as above, results showed that only MS score and elevated fasting glucose were significantly associated with the Gensini score. This study indicated that MS score, MS, and its individual components might have different values in respect of the prevalence and severity of CAD.
The MS is determined by the presence of three or more of quantitatively identified components. From the clinical prospective, it has been questioned whether or not MS improves cardiovascular risk prediction, beyond previously used tools for CAD.[4,5,6,7,8,9] Many studies have confirmed the predictive effects of MS to CAD in different population. For example, in a Dutch population-based cohort study which was named the Hoorn study, the NCEP definition of MS was associated with about a 2-fold increase in age-adjusted risk of fatal CVD in men and nonfatal CVD in women.[4] Moreover, in a longitudinal cohort study in Dubbo, Australia of 2805 men and women ≥60 years followed for 16 years from 1988, prediction of CAD and all-cause mortality is genuinely driven by the MS and independently of its component variables.[24] Both studies indicated a clinical value in diagnosing the MS. A similar conclusion was also obtained in two Chinese population groups.[5,25] However, despite such findings, the predictive value of MS for CAD was poor in some other studies.[8,9,26,27,28] The results from an American population showed that although accelerated coronary atherosclerosis progression is observed in the setting of MS, this is due to the presence of individual component risk factors rather than to the presence of the syndrome itself.[27] Furthermore, a recent prospective study in Finland also showed that during the 9-year follow-up, 422 deaths occurred, and after multivariable adjustment, no significant differences were found between patients with and without MS for all-cause, CVD, or CAD mortality in all study participants or by gender.[28] These findings indicated that the MS is a marker of CAD risk but no above and beyond the risk associated with its individual components. Therefore, the number of components of MS might be more useful than MS per se to predict the severity of CAD. The results of the present study showed that MS score, MS, and three of its individual components including elevated waist circumference, reduced HDL-C, and elevated fasting glucose were associated with the severity of CAD assessed by the Gensini score. After adjustment for age, sex, MS score, MS and its components each other, and drug using, only MS score and elevated fasting glucose were still significantly associated with the Gensini score. These results are partly in line with some previous studies.[15,25] In Korea, Kim et al.[15] found that the MS score correlates with the angiographic severity of CAD, but different from this study, they obtained their results without a multiple regression analysis. In China, Zhang et al.[25] also found that MS patients had significant increases in CAD Gensini score, but unfortunately, MS score was not used and multivariate analysis was not performed in their studies.
Whether or not MS score, MS, and its individual components were related to the prevalence of angiographic CAD was also evaluated in the present study. Results showed that elevated waist circumference, reduced HDL-C, and elevated fasting glucose might have the relatively higher association with CAD, as well as MS score and MS, whereas elevated TGs and elevated blood pressure might have weaker association. After adjustment as above, results showed that only MS and elevated fasting glucose were significantly associated with CAD. Therefore, all these results indicated that different components of MS might have different contributions with respect to CAD. MS and elevated fasting glucose were independent risk factors for the prevalence of angiographic CAD, whereas MS score and elevated fasting glucose were significantly associated with the severity of CAD in the present study population.
In this study, however, we did not find any association of elevated TGs and elevated blood pressure with the prevalence of CAD as well as the severity of it assessed by the Gensini score. These results can be supported by some previous studies, in which elevated TGs and/or elevated blood pressure was not associated with CAD.[15,25,29] For example, in a Japanese study,[29] the ORs of the NCEP definition of elevated TGs for CAD were 1.12 (0.76–1.68) in male and 2.16 (0.91–5.13) in female, and the ORs of elevated blood pressure for CAD were 1.39 (0.96–2.01) in male and 1.73 (0.87–3.46) in female. However, Tanomsup et al.[30] drew different conclusions. This cohort study in Thailand showed that when all individual components were cross-adjusted for each other in the same model, only high blood pressure and elevated fasting glucose were independently associated with CVD and mortality. An additional analysis, excluding those with diabetes at baseline, found that only high blood pressure was independently associated with each outcome. Tanomsup et al.[30] observed CVD including fatal or nonfatal myocardial infarction or stroke, and no coronary angiography was observed to assess the severity of CAD, and no antihypertensive drugs were recorded, which were all different from the present study. This might partially explain the reason why different conclusion was drawn.
The primary novelty of this study included that it was investigated whether and to what extent MS score, MS, and its individual components were related to risk for CAD in a much more detail way than before.[15,25] Besides, patients with acute myocardial infarction were excluded from the study avoiding some selection bias such as stress hyperglycemia.
However, there are still several limitations to be found in the present study. First, it is a retrospective study, therefore not directly allowing the prediction of future cardiovascular risk in patients at a given baseline status. Second, all the patients included were middle aged or elderly and most of them were referred to the Department of Cardiology because of the occurrence of cardiac symptoms. These might have resulted in a higher prevalence of CAD in this study population than those in the common population. Third, although having been adjusted for drug use, results might still be affected by it more or less in the study population inevitably.
In conclusion, in this study, MS score, MS, and its individual components might have different values in respect of the prevalence and severity of CAD in the high-risk Chinese patients. MS and elevated fasting glucose were independent risk factors for the prevalence of angiographic CAD, whereas MS score and elevated fasting glucose were significantly associated with the severity of CAD in the present study population. These findings might be helpful for other investigators to reevaluate the predictive effects of MS on CAD.
Financial support and sponsorship
This work was supported by grants from the Shanghai Municipal Health Project (No. 2013ZYJB0802), and the Shanghai Health and Family Planning Commission Foundation (No. 2013SY005).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. doi: 10.2337/diab. 37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Yang F, Bots ML, Guo WY, Zhao B, Hoes AW, et al. Prevalence of the metabolic syndrome among employees in Northeast China. Chin Med J. 2015;128:1989–93. doi: 10.4103/0366-6999.161337. doi: 10.4103/0366-6999.161337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James PT, Rigby N, Leach R International Obesity Task Force. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11:3–8. doi: 10.1097/01.hjr.0000114707.27531.48. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 4.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn study. Circulation. 2005;112:666–73. doi: 10.1161/CIRCULATIONAHA.104.516948. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Jiang B, Wang J, Feng K, Chang Q, Fan L, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47:1588–94. doi: 10.1016/j.jacc.2005.11.074. doi: 10.1016/j.jacc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 6.Varounis C, Rallidis LS, Franco OH, Lekakis J. Prevalence of metabolic syndrome and association with burden of atherosclerotic disease in patients with stable coronary artery disease. Curr Med Res Opin. 2016;32:1175–81. doi: 10.1185/03007995.2016.1163257. doi: 10.1185/03007995.2016.1163257. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Guo Y, Wang H, Gu B, Liu G, Zhou C, et al. The angiographic and clinical outcomes after coronary stenting in patients with metabolic syndrome. Atherosclerosis. 2012;221:416–21. doi: 10.1016/j.atherosclerosis.2011.12.016. doi: 10.1016/j.atherosclerosis.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. doi: 10.1016/j.jacc.2006.09.032. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Hadaegh F, Mohebi R, Cheraghi L, Tohidi M, Moghaddam NB, Bozorogmanesh M, et al. Do different metabolic syndrome definitions predict cerebrovascular events and coronary heart disease independent of their components? 9 years follow-up of the Tehran lipid and glucose study. Stroke. 2012;43:1669–71. doi: 10.1161/STROKEAHA.112.650812. doi: 10.1161/STROKEAHA.112.650812. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Li D, Chu X, An Y, Song T, Feng H, et al. Coronary heart disease: Incidence, risk factors and interventions in Jiaozhou of Shandong province. Chin Med J. 2014;127:2275–8. doi: 10.3760/cma.j.issn.0366-6999.20133323. [PubMed] [Google Scholar]
- 11.Iribarren C. The metabolic syndrome is no better than its components. Minerva Cardioangiol. 2007;55:487–9. [PubMed] [Google Scholar]
- 12.Wang J, Ruotsalainen S, Moilanen L, Lepistö P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: A 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–64. doi: 10.1093/eurheartj/ehl524. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- 13.Solymoss BC, Bourassa MG, Campeau L, Sniderman A, Marcil M, Lespérance J, et al. Effect of increasing metabolic syndrome score on atherosclerotic risk profile and coronary artery disease angiographic severity. Am J Cardiol. 2004;93:159–64. doi: 10.1016/j.amjcard.2003.09.032. doi: 10.1016/j.amjcard.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Yavuz B, Kabakci G, Aksoy H, Tulumen E, Deveci OS, Aytemir K, et al. Determining the relationship between metabolic syndrome score and angiographic severity of coronary artery disease. Int J Clin Pract. 2008;62:717–22. doi: 10.1111/j.1742-1241.2008.01702.x. doi: 10.1111/j.1742-1241.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Mun HS, Lee BK, Yoon SB, Choi EY, Min PK, et al. Impact of metabolic syndrome and its individual components on the presence and severity of angiographic coronary artery disease. Yonsei Med J. 2010;51:676–82. doi: 10.3349/ymj.2010.51.5.676. doi: 10.3349/ymj.2010.51.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SE, Ahn SG, Kim JY, Park JS, Shin JH, Tahk SJ, et al. Differential relationship between metabolic syndrome score and severity of coronary atherosclerosis as assessed by angiography in a non-diabetic and diabetic Korean population. J Korean Med Sci. 2011;26:900–5. doi: 10.3346/jkms.2011.26.7.900. doi: 10.3346/jkms.2011.26.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. doi: 10.1002/(SICI)1096-9136(199807)15: 7%3C539: AID-DIA668%3E3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Fiévet C, Nuttens MC, Ducimetière P, Fruchart JC, Bertrand M, Salomez JL. Relation of arteriographically defined coronary artery disease to serum lipoprotein particles mapped with monoclonal antibodies. Circulation. 1991;84:153–9. doi: 10.1161/01.cir.84.1.153. doi: 10.1161/01.CIR.84.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. doi: 10.1016/S0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 22.Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gui MH, Li X, Lu ZQ, Gao X. Fasting plasma glucose correlates with angiographic coronary artery disease prevalence and severity in Chinese patients without known diabetes. Acta Diabetol. 2013;50:333–40. doi: 10.1007/s00592-012-0405-2. doi: 10.1007/s00592-012-0405-2. [DOI] [PubMed] [Google Scholar]
- 24.Simons LA, Simons J, Friedlander Y, McCallum J. Is prediction of cardiovascular disease and all-cause mortality genuinely driven by the metabolic syndrome, and independently from its component variables? The Dubbo study. Heart Lung Circ. 2011;20:214–9. doi: 10.1016/j.hlc.2010.12.005. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Hong J, Gu W, Gui M, Chen Y, Zhang Y, et al. Impact of the metabolic syndrome and its individual components on risk and severity of coronary heart disease. Endocrine. 2009;36:233–8. doi: 10.1007/s12020-009-9214-y. doi: 10.1007/s12020-009-9214-y. [DOI] [PubMed] [Google Scholar]
- 26.Kamalesh M, Campbell S, Ligler L, Meda M, Eckert GJ, Sawada S. Metabolic syndrome does not predict an increased risk of coronary disease in patients with traditional risk factors referred for stress imaging study. Metab Syndr Relat Disord. 2010;8:223–8. doi: 10.1089/met.2009.0079. doi: 10.1089/met.2009.0079. [DOI] [PubMed] [Google Scholar]
- 27.Bayturan O, Tuzcu EM, Lavoie A, Hu T, Wolski K, Schoenhagen P, et al. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch Intern Med. 2010;170:478–84. doi: 10.1001/archinternmed.2009.551. doi: 10.1001/archinternmed.2009.551. [DOI] [PubMed] [Google Scholar]
- 28.Salminen M, Kuoppamäki M, Vahlberg T, Räihä I, Irjala K, Kivelä SL. The metabolic syndrome defined by modified International Diabetes Federation criteria and mortality: A 9-year follow-up of the aged in Finland. Diabetes Metab. 2010;36(6 Pt 1):437–42. doi: 10.1016/j.diabet.2010.05.002. doi: 10.1016/j.diabet.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Kasai T, Miyauchi K, Kubota N, Tamura H, Kojima T, Yokoyama K, et al. The relationship between the metabolic syndrome defined by various criteria and the extent of coronary artery disease. Atherosclerosis. 2008;197:944–50. doi: 10.1016/j.atherosclerosis.2007.08.023. doi: 10.1016/j.atherosclerosis.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Tanomsup S, Aekplakorn W, Sritara P, Woodward M, Yamwong S, Tunlayadechanont S, et al. A comparison of components of two definitions of the metabolic syndrome related to cardiovascular disease and all-cause mortality in a cohort study in Thailand. Diabetes Care. 2007;30:2138–40. doi: 10.2337/dc07-0388. doi: 10.2337/dc07-0388. [DOI] [PubMed] [Google Scholar]


