Abstract
Background:
Some patients with pelvic organ prolapse may suffer from lower urinary tract symptoms (LUTS), especially stress urinary incontinence (SUI) named de novo SUI after pelvic floor reconstruction. This study aimed to investigate the incidence and risk factors of de novo SUI.
Methods:
This is a nested case-control study of 533 patients who underwent pelvic floor reconstruction due to pelvic organ prolapse (POP) at the Department of Gynecology in Peking University People's Hospital from January 2011 to March 2013. According to the inclusion and exclusion criteria, 401 patients were enrolled in the study with the follow-up rate of 74.8% (101 patients lost to follow-up). There were 75 patients with de novo SUI postoperatively. According to the ratio of 1:3, we ensured the number of control group (n = 225). The preoperative urinary dynamics, POP-quantification scores, and LUTS were compared between the two groups by univariate and multivariate logistic regression analyses to investigate the risk factors of de novo SUI.
Results:
The incidence of de novo SUI was 25% (75/300). Univariate analysis showed that the ratio of lower urinary tract obstruction (LUTO) before surgery in de novo SUI group was significantly higher than the control group (odds ratio [OR] = 2.1, 95% confidence interval [CI] [1.1–4.0], P = 0.022). The interaction test of LUTO and other factors displayed that Aa value was an interaction factor. With the increasing score of Aa, the incidence of de novo SUI become higher (OR = 2.1, 95% CI [1.0–3.7], P = 0.045). After multivariable adjustment, multiple regression analysis showed that LUTO was independently associated with a greater risk of de novo SUI after pelvic floor surgery (OR = 2.3, 95% CI [1.2–4.6], P = 0.013).
Conclusions:
Preoperative LUTO in patients with POP is a high-risk factor of de novo SUI, and high score of Aa-point is related to the occurrence of de novo SUI, which might be due to the outlet obstruction caused by bladder prolapse.
Keywords: De novo Stress Urinary Incontinence, Lower Urinary Tract Obstruction, Pelvic Organ Prolapse, Risk Factor
Introduction
Pelvic organ prolapse (POP) is common in elderly women, with an incidence rate of about 50%.[1] The complex relationship between POP and urinary incontinence (UI) is likely attributed to their pathophysiology. POP, especially the prolapse of anterior vaginal wall, is often accompanied by lower urinary tract symptoms, such as stress urinary incontinence (SUI) and dysuria. In some patients, UI can be reduced due to obstruction of the urethra; however, leakage symptom may reappear or even be more severe than preoperative after prolapse correction. Indeed, some patients without complaint of SUI before surgery develop SUI after POP surgery which is called postoperative stress urinary incontinence (POSUI) or de novo stress urinary incontinence (de novo SUI).[2] Anterior vaginal prolapse may actually function to kink the urethra, maintaining stress continence by causing urethral obstruction. As a consequence, it is common for continent women who have undergone a successful POP surgery to develop SUI postoperatively. This might result from relieving the urethral obstruction caused by prolapse, thereby unmasking a preexisting compromised urethral function. SUI may be revealed only after prolapse reduction and is defined as occult stress urinary incontinence (OSUI).[2] OSUI is generally diagnosed before operation, by urodynamic study (UDS) or pressure-induced experiment after bladder prolapse restore. However, OSUI and POSUI cannot be exactly considered the same definition. Some patients reported that OSUI is the high-risk factor of de novo SUI, and some patients have no OSUI preoperative but develop POSUI, so OSUI and POSUI cannot be equated. In women without SUI, POP surgery may cause postoperative de novo SUI in 16–51%.[3,4,5,6] Therefore, some surgeons prefer to perform anti-SUI surgery in patients undergoing pelvic floor reconstruction surgery. Some surgeons recommend a two-step procedure to avoid unnecessary operation and reduce the medical burden.
There is no uniform standard to gauge the possibility of de novo SUI or SUI aggravation and predict the risk factors. Hence, we performed a nested case-control study of patients with severe POP who underwent pelvic reconstructive surgery to identify the high-risk factors of de novo SUI and try to provide a reference for clinicians while performing pelvic reconstructive surgery for POP patients.
Methods
Data resource
Data were collected from patients who underwent pelvic floor reconstructive surgery between January 2011 and March 2013 at the Department of Gynecology of Peking University People's Hospital. All patients were present of stage ≥3 POP confirmed by pelvic organ prolapse quantification (POP-Q). The total number was 533 cases. Patients who had no leakage but developed postoperative SUI were allocated into de novo SUI group, and in the control group, patients had no SUI before and after surgery. Data collected preoperatively and postoperatively included POP-Q staging, preoperative urodynamic parameters and 1-h pad test, and postoperative 1-h pad test in patients who developed SUI. Postoperative urodynamic test was conducted if necessary. Ethical approval for this study was granted by the Ethics Committee of Peking University People's Hospital (No. 2013-ethic-03). Written informed consent was obtained before sample collection.
Inclusion and exclusion criteria
Inclusion criteria: (1) patients with POP-Q staging ≥III; (2) patients who underwent pelvic reconstructive surgery; (3) preoperative pressure-induced test was negative; (4) preoperative urodynamic and 1-h pad test confirmed no objective SUI.
Exclusion criteria: (1) patients had SUI before operation; (2) patients who underwent anti-SUI surgery preoperatively or simultaneously; (3) patients who took drug treatment for UI.
Data collection and follow-up
The pelvic floor follow-up database in our hospital was used; statistical data and general information of the patients were collected. Among the 533 patients, 401 patients met the inclusion criteria, after excluding 101 cases who lost to follow-up, totally 300 patients recruited in this study with the follow-up time of 3–24 months. Among the 300 patients, 75 underwent postoperative de novo SUI. According to the date of hospitalization of ±7 days and age of ±5 years, we set up the control group using the ratio of 1:3 to match the de novo SUI group and the number of control group was 225 cases [Figure 1]. All patients were asked to visit the gynecological clinic at 3, 12, and 24 months postoperatively. All these 300 patients completed OABSS and ICIQ questionnaires. For those who complained of leakage postoperatively, 1-h pad test and/or urodynamic examination (for serious cases) was conducted during outpatient review.
Figure 1.
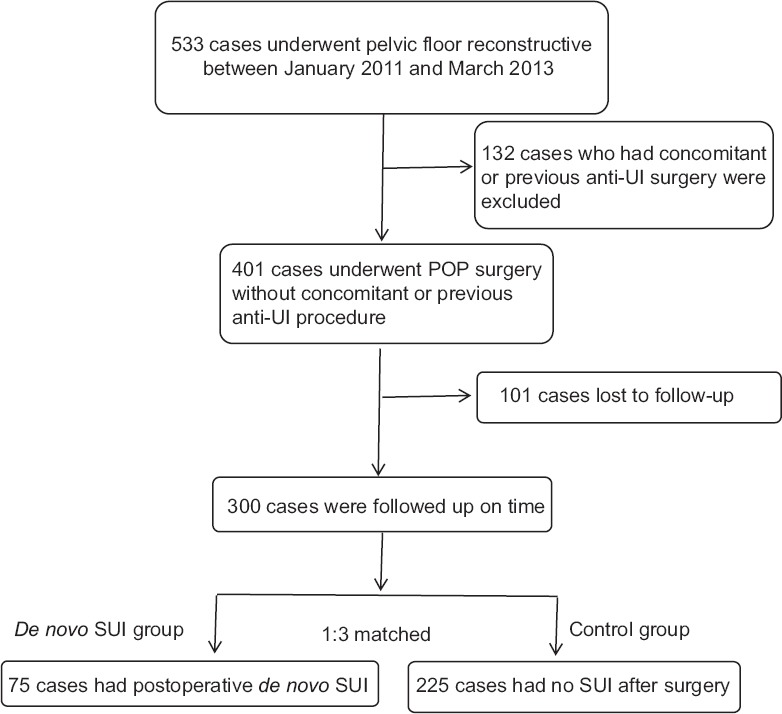
Flowchart of this study. anti-UI: anti-urinary incontinence; SUI: Stress urinary incontinence; POP: Pelvic organ prolapse.
Outcome measures
Main measures: Risk factors of de novo SUI after pelvic floor surgery. Secondary measures: Ratio of de novo SUI after pelvic floor surgery.
Diagnostic criteria
Diagnosis was in accordance with the international standards for urinary control (International Continence Society).[2] SUI diagnostic methods included abdominal pressure at leakage of urine and 1-h pad test and pressure-induced test. Subjective SUI indicated that patients had chief complaint of SUI, with no objective evidence by the detective methods. Postoperative de novo SUI: Patients who had no objective SUI preoperative complained cough leakage postoperatively and had positive 1-h pad test or urodynamics SUI or positive pressure-induced test. Lower urinary tract obstruction (LUTO) was detected by UDS (Qmax ≤12 ml/s and PdetQmax ≥25 cmH2O[7] or residual urine ≥100 ml).
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 software (SPSS Inc., Chicago, USA). The continuous data were described as mean ± standard deviation (SD) or median (quartile) and categorical variables as percentages of number. After testing the normality of the distributions of the variables, Student's t-test was used for the comparisons. Univariate and multivariate logistic regression models were used to examine factors associated with de novo SUI. In multivariate analysis, we included the variable frequency, C point, menopause, and hysterectomy. A value of P < 0.05 was considered statistically significant.
This study is to analyze the relationship between risk factors and de novo SUI. We found that LUTO was the high-risk factor of postoperative de novo SUI. The incontinence rate of patients without LUTO was 22.4% in our preliminary study, to detect an odds ratio (OR) of 2.1 with two-sided 5% significance level and power of 80%, a sample size of 278 in total. In this observation cohort, de novo SUI occurred in 75 patients, so we conducted 1:3 matched nested case-control study; 300 patients in total achieved 83% power at 0.05 significance level to detect an OR of 2.1.
Results
Baseline characteristics of patients
Between January 2011 and March 2013, totally 533 patients underwent pelvic reconstructive surgery due to POP staging ≥III at Peking University People's Hospital, of which 401 cases met the inclusion standard, 300 cases were followed up on time with the follow-up rate of 74.8%. The median age of the patients was 66 years (39–90 years). The median delivery times were 2 (1–7 times). The surgery methods included 43 cases of native tissue repair, 170 of tension-free vaginal mesh (TVM) procedure, 63 of colpocleisis, 10 of sacrospinous ligament fixation, and 14 of sacral colpopexy. Baseline characteristics of the two groups were compared and there was no significant difference between the two groups [Table 1].
Table 1.
Baseline characteristics of de novo SUI group and control group in which who had no SUI before and after surgery
| Characteristics | De novo SUI group (n = 75) | Control group (n = 225) | Statistics | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 64.3 ± 11.0 | 65.4 ± 10.0 | 0.774* | 0.439 |
| Delivery times, mean ± SD | 2.4 ± 1.2 | 2.4 ± 1.3 | 0.079* | 0.937 |
| Prolapse operation mode, n (%) | 6.692† | 0.143 | ||
| Native tissue | 6 (8.0) | 37 (16.4) | ||
| Colpocleisis | 13 (17.3) | 50 (22.2) | ||
| TVM | 51 (68.0) | 119 (52.9) | ||
| SSLF | 1 (1.3) | 9 (4.0) | ||
| Sacral colpopexy | 4 (5.3) | 10 (4.4) | ||
| BMI (kg/m2), mean ± SD | 24.7 ± 3.6 | 24.3 ± 3.4 | −0.820* | 0.413 |
| Hysterectomy, n (%) | 46 (61.3) | 165 (73.3) | 3.882† | 0.058 |
| COPD, n (%) | 5 (6.7) | 9 (4.0) | 0.899† | 0.530 |
| Menopause, n (%) | 59 (78.7) | 201 (85.5) | 2.116† | 0.146 |
| Hypertension, n (%) | 37 (49.3) | 110 (48.9) | 0.004† | 1.000 |
| Diabetes mellitus, n (%) | 17 (22.7) | 55 (24.4) | 0.399† | 0.907 |
| Heart disease, n (%) | 12 (16.0) | 32 (14.2) | 0.142† | 0.851 |
*t values; †Chi-square values. SD: Standard deviation; SUI: Stress urinary incontinence; TVM: Tension-free vaginal mesh; SSLF: Sacrospinous ligament fixation; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease.
Grouping
De novo stress urinary incontinence group
Seventy-five patients among the 300 patients complained of different levels of SUI symptoms postoperatively and confirmed by pressure-induced test and 1-h pad test, those who had severe leakage or were suspected Mixture UI underwent UDS, the incidence rate of de novo SUI was 25%. According to the symptoms and the 1-h pad test, de novo SUI patients were divided into 3 degrees. Mild category only occurs in coughing and sneezing and 1-h pad test was ≥2 g and ≤10 g; moderate occurs in daily activities and 1-h pad was >10 g and ≤30 g; severe occurs in the change of posture and 1-h pad was >30 g. Conservative treatment such as pelvic floor muscle exercise (improved levator ani movement) was recommended for mild and moderate de novo SUI. A total of 46 patients were with mild de novo SUI and the rate was 61.3%, 20 moderate patients with the rate of 26.7%, and 9 severe patients with the rate of 12%. However, only 3 patients with severe de novo SUI underwent anti-incontinence surgery at 3, 6, and 8 months postoperatively. Four patients in de novo group who were repaired by TVM procedure recurred in these 75 cases.
Control group
Two hundred and twenty-five patients underwent POP repair at the same period with no complaint of SUI and were objectively confirmed with no SUI postoperatively, and the ratio was 75%. Ten of the 225 patients had been developed prolapse recurrent.
Analysis of high-risk factors of de novo stress urinary incontinence
There was no significant difference in body mass index (BMI), hysterectomy, history of chronic obstructive pulmonary disease (COPD), and menopause status of the two groups [Table 1]. Comparison of preoperative urine kinetic parameters and urination condition were performed between the two groups by univariate regression analysis. The results indicated that preoperative LUTO was the high-risk factor (P = 0.022) for de novo SUI after surgery [Table 2]. Furthermore, through the analysis of the interactions, Aa value was found to be the interaction factor for the occurrence of new SUI after preoperative urinary tract obstruction affecting POP operation (P < 0.05) [Table 3]. For covariate screening using the multiple regression equation adjusting for variable frequency, C point, menopause, and hysterectomy, P value of LUTO was <0.05, indicating that LUTO was the independent high-risk factor of de novo SUI after operation [Tables 2 and 4]. The results of single-factor analysis showed no significant difference among patients with different pelvic floor surgeries, indicating that the type of prolapse operation mode did not impact the incidence of de novo SUI [Table 1].
Table 2.
Patients' urine, POP-Q staging, and logistic single factor regression analysis of urine kinetic parameters
| Parameters | De novo SUI group (n=75) | Control group (n=225) | OR (95% CI) | P |
|---|---|---|---|---|
| LUTO, n (%) | 19 (25.3) | 31 (13.8) | 2.1 (1.1–4.0) | 0.022 |
| Preoperative detrusor instability, n (%) | 9 (12) | 23 (10.2) | 1.2 (0.5–2.7) | 0.666 |
| Preoperative subjective SUI, n (%) | 17 (22.7) | 61 (27.1) | 0.8 (0.4–1.5) | 0.448 |
| Preoperative UUI, n (%) | 4 (5.3) | 16 (7.1) | 0.7 (0.2–2.3) | 0.594 |
| Aa-point, median (P25, P75) | 2.0 (0.0, 2.5) | 1.5 (0.0, 2.0) | 1.1 (0.9–1.2) | 0.468 |
| Ba point, median (P25, P75) | 3.0 (2.0, 4.8) | 3.0 (2.0, 5.0) | 1.0 (0.9–1.1) | 0.880 |
| C point, median (P25, P75) | 1.0 (0.3, 4.0) | 2.5 (0.0, 4.0) | 0.9 (0.9–1.0) | 0.087 |
| Qmax (ml/s), median (P25, P75) | 17.0 (13.0, 22.6) | 17.6 (12.0, 23.2) | 1.0 (0.9–1.0) | 0.791 |
| MUPP (cmH2O), median (P25, P75) | 59.5 (46.5, 82.5) | 60.0 (46.0, 83.5) | 1.0 (1.0–1.0) | 0.927 |
MUPP: Micturition urethral pressure measurement; UUI: Urgency urinary incontinence; SUI: Stress urinary incontinence; POP-Q: Pelvic organ prolapse quantification; OR: Odds ratio; CI: Confidence interval; LUTO: Lower urinary tract obstruction.
Table 3.
Role of Aa in the development of urinary incontinence in patients with LUTO after pelvic floor surgery
| LUTO | Aa-point | Total | OR (95% CI) | P | P value for interaction | ||
|---|---|---|---|---|---|---|---|
| Low (−3, −1) | Middle (−1, 1) | High (1, 3) | |||||
| No | 30 | 98 | 122 | 250 | 1.0 | 0.045 | 0.044 |
| Yes | 11 | 7 | 32 | 50 | 2.1 (1.0–3.7) | ||
OR: Odds ratio; CI: Confidence interval; LUTO: Lower urinary tract obstruction.
Table 4.
Multivariate regression analysis after LUTO, delivery times, C point, menopause, operation mode, and hysterectomy
| Screening variables | OR (95% CI) | P |
|---|---|---|
| LUTO | 2.3 (1.2–4.6) | 0.013 |
| Delivery times | 1.1 (0.9–1.4) | 0.540 |
| C point | 0.9 (0.9–1.0) | 0.192 |
| Menopause | 0.7 (0.3–1.4) | 0.290 |
| Operation mode | 1.3 (0.9–1.8) | 0.134 |
| Hysterectomy | 0.6 (0.3–1.2) | 0.137 |
LUTO: Lower urinary tract obstruction; OR: Odds ratio; CI: Confidence interval.
Discussion
Symptoms of female pelvic floor dysfunction are diverse, including POP and UI. With the extensive development of pelvic floor surgery, postoperative stress urinary incontinence (POSUI) or de novo SUI is receiving greater attention. Full risk assessment of de novo SUI before operation helps make proper clinical decision. OSUI is closely related to the occurrence of de novo SUI.
Anti-SUI surgery while performing pelvic floor reconstruction in the patients without preoperative SUI remains controversial. Some clinicians believe that all patients with no SUI should undergo middle segment suspension of urethra during vaginal repair.[8] Others believe that the high-risk factors for de novo SUI after surgery need to be identified before performing anti-UI surgery. Some studies have reported that the incidence of de novo SUI was 16–51%[3,4,5,6] in women undergoing prolapse repair surgery without prophylactic anti-UI surgery. In a relatively new review, Al-Mande et al.[9] reported that the incidence of POSUI was 42% (subjective symptoms); they further analyzed the incidence of de novo SUI for 1–3 years after pelvic floor surgery of 100 POP women without previous SUI. The follow-up results showed that 25% (75/300) patients developed postoperative SUI after pelvic floor repair surgery, which was similar to previous reports.
Several attempts were made to find high-risk factors and preventive methods of de novo SUI before surgery. Forsgren et al.[10] analyzed 907 patients and reported that the high-risk factors included preoperative SUI, BMI, and COPD. The recurrence in anterior POP may mask the SUI. The incidence of de novo SUI was high in patients undergoing hysterectomy.[10,11,12,13] Sun et al. summarized 140 cases and found that the high-risk factors of de novo SUI were preoperative SUI, obvious urethral prolapse, severe bladder prolapse, and low micturition urethral pressure measurement.[14] Kuribayashi et al.[15] reported that the ratio of de novo SUI was 37% in 65 patients after TVM operation, and preoperative urinary tract was the high-risk factor for de novo SUI. Single-factor analysis in the present study showed that preoperative urinary tract obstruction in the de novo SUI group was significantly higher than that in the contrast group. This was further confirmed by multiple regression, which showed that preoperative urinary tract obstruction was an independent high-risk factor for de novo SUI after operation, except C point, menopause, operation method, and hysterectomy [Table 4].
The reason for LUTO in POP was the increase of urethral resistance. After the operation, the obstruction is relieved and the urethra distortion is corrected, reducing the urethral pressure, which could change the OSUI to dominant SUI. This study suggested that the interaction of Aa with LUTO had an effect on the occurrence of postoperative SUI [Table 3]. The high score of Aa suggested that the urethra moved down and was highly active. Therefore, these patients were prone to SUI. Bladder prolapse could increase urethral resistance, masking SUI. Hence, SUI could appear after bladder prolapsed corrected.
The results of this study were slightly different from previous studies, which suggested that high BMI and COPD before surgery were high-risk factors for POSUI. In contrast, the results of this study showed that BMI and COPD were not associated with the occurrence of de novo SUI, which might be related to the inclusion criteria and the number of cases.
Several studies suggested methods to prevent the occurrence of de novo SUI. Pelvic floor reconstruction surgery accompanied with anti-SUI surgery could reduce the occurrence of SUI by 10–15%.[16] For patients confirmed with objective SUI after prolapse repair before surgery, it was normal to conduct anti-UI surgery to prevent SUI after surgery.[17,18] However, some doctors prefer to put middle urethral suspension simultaneously for patients without SUI who underwent vaginal repair.[8] After pelvic floor prolapse, SUI could be caused by urethral pressure or curvature change or changes in urinary dynamics. Therefore, POP patients with no SUI may have OSUI, which may appear after the prolapse repair.[19,20] Due to the fact that not all prolapse repair would develop SUI, surgeons need to determine whether the patients have risk of SUI after POP repair and perform anti-UI surgery along with POP repair. The study shows that preoperative LUTO in POP patients is a risk factor for the development of de novo SUI postoperatively. In addition, a greater Aa-point is related to the occurrence of de novo SUI, which might be due to outlet obstruction caused by bladder prolapse. Except other influence factors, patients with urinary obstruction caused by prolapse of bladder, especially whom with greater Aa-point, can be considered for prophylactic anti-incontinence surgery during POP repair surgery.
In this study, the high-risk factor for the occurrence of de novo SUI after surgery was preoperative LUTO which can be mainly identified by urodynamic examination. The urodynamic examination played an important role in identifying OSUI and LUTO, so it was recommended as a routine examination for pelvic floor patients before operation.
This study demonstrated that preoperative urinary tract obstruction was an independent predictor and Aa value was an interaction factor of de novo SUI. Therefore, urodynamic examination was recommended for POP patients with high Aa value. If the UDS shows urinary tract obstruction, simultaneous anti-SUI surgery is recommended to avoid POSUI. As an important method to find the urinary tract obstruction, UDS may help predict de novo SUI before surgery.
Financial support and sponsorship
This work is supported by a grant from the Chinese Preventive Medicine Association (No. 2119000216).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Digesu GA, Chaliha C, Salvatore S, Hutchings A, Khullar V. The relationship of vaginal prolapse severity to symptoms and quality of life. BJOG. 2005;112:971–6. doi: 10.1111/j.1471-0528.2005.00568.x. doi: 10.1111/j.1471-0528.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21:5–26. doi: 10.1007/s00192-009-0976-9. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 3.Visco AG, Brubaker L, Nygaard I, Richter HE, Cundiff G, Fine P, et al. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: The colpopexy and urinary reduction efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:607–14. doi: 10.1007/s00192-007-0498-2. doi: 10.1007/s00192-007-0498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei JT, Nygaard I, Richter HE, Nager CW, Barber MD, Kenton K, et al. Amidurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358–67. doi: 10.1056/NEJMoa1111967. doi: 10.1056/NEJMoa1111967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ennemoser S, Schönfeld M, von Bodungen V, Dian D, Friese K, Jundt K. Clinical relevance of occult stress urinary incontinence (OSUI) following vaginal prolapse surgery: Long-term follow-up. Int Urogynecol J. 2012;23:851–5. doi: 10.1007/s00192-012-1765-4. doi: 10.1007/s00192-012-1765-4. [DOI] [PubMed] [Google Scholar]
- 6.Groutz A, Levin I, Gold R, Pauzner D, Lessing JB, Gordon D. “Inside-out” transobturator tension-free vaginal tape for management of occult stress urinary incontinence in women undergoing pelvic organ prolapse repair. Urology. 2010;76:1358–61. doi: 10.1016/j.urology.2010.04.070. doi: 10.1016/j.urology.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 7.Defreitas GA, Zimmern PE, Lemack GE, Shariat SF. Refining diagnosis of anatomic female bladder outlet obstruction: Comparison of pressure-flow study parameters in clinically obstructed women with those of normal controls. Urology. 2004;64:675–9. doi: 10.1016/j.urology.2004.04.089. doi: 10.1016/j.urology.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 8.Fatton B. Is there any evidence to advocate SUI prevention in continent women undergoing prolapse repair? An overview. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:235–45. doi: 10.1007/s00192-008-0734-4. doi: 10.1016/j.urology.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mandeel H, Ross S, Robert M, Milne J. Incidence of stress urinary incontinence following vaginal repair of pelvic organ prolapse in objectively continent women. Neurourol Urodyn. 2011;30:390–4. doi: 10.1002/nau.20947. doi: 10.1007/s00192-008-0734-4. [DOI] [PubMed] [Google Scholar]
- 10.Forsgren C, Lundholm C, Johansson AL, Cnattingius S, Zetterström J, Altman D. Vaginal hysterectomy and risk of pelvic organ prolapse and stress urinary incontinence surgery. Int Urogynecol J. 2012;23:43–8. doi: 10.1007/s00192-011-1523-z. doi: 10.1007/s00192-011-1523-z. [DOI] [PubMed] [Google Scholar]
- 11.Lakeman MM, Van Der Vaart CH, Van Der Steeg JW, Roovers JP HysVA study group. Predicting the development of stress urinary incontinence 3 years after hysterectomy. Int Urogynecol J. 2011;22:1179–84. doi: 10.1007/s00192-011-1427-y. doi: 10.1007/s00192-011-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman D, Granath F, Cnattingius S, Falconer C. Hysterectomy and risk of stress-urinary-incontinence surgery: Nationwide cohort study. Lancet. 2007;370:1494–9. doi: 10.1016/S0140-6736(07)61635-3. doi: 10.1016/S0140-6736(07)61635-3. [DOI] [PubMed] [Google Scholar]
- 13.Brown JS, Sawaya G, Thom DH, Grady D. Hysterectomy and urinary incontinence: A systematic review. Lancet. 2000;356:535–9. doi: 10.1016/S0140-6736(00)02577-0. doi: 10.1016/S0140-6736(00)02577-0. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Wang S. Analysis of de novo stress urinary incontinence after pelvic reconstructive surgery (in Chinese) Chin J Obstet Gynecol. 2013;14:102–5. doi: 10.3969/j.issn.1672-1861.2013.02.003. [Google Scholar]
- 15.Kuribayashi M, Kitagawa Y, Narimoto K, Urata S, Kawaguchi S, Namiki M. Predictor of de novo stress urinary incontinence following TVM procedure: A further analysis of preoperative voiding function. Int Urogynecol J. 2013;24:407–11. doi: 10.1007/s00192-012-1882-0. doi: 10.1007/s00192-012-1882-0. [DOI] [PubMed] [Google Scholar]
- 16.Haessler AL, Lin LL, Ho MH, Betson LH, Bhatia NN. Reevaluating occult incontinence. Curr Opin Obstet Gynecol. 2005;17:535–40. doi: 10.1097/01.gco.0000183530.03481.64. doi: 10.1097/01.gco.0000183530.03481.64. [DOI] [PubMed] [Google Scholar]
- 17.Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531–4. doi: 10.1016/S0022-5347(05)67918-9. [PubMed] [Google Scholar]
- 18.Gordon D, Groutz A, Wolman I, Lessing JB, David MP. Development of postoperative urinary stress incontinence in clinically continent patients undergoing prophylactic Kelly plication during genitourinary prolapse repair. Neurourol Urodyn. 1999;18:193–7. doi: 10.1002/(sici)1520-6777(1999)18:3<193::aid-nau5>3.0.co;2-e. doi: 10.1002/(SICI)1520-6777(1999)18. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig BA, Pushkin S, Blumenfeld D, Bhatia NN. Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol. 1992;79:539–42. [PubMed] [Google Scholar]
- 20.Bergman A, Koonings PP, Ballard CA. Predicting postoperative urinary incontinence development in women undergoing operation for genitourinary prolapse. Am J Obstet Gynecol. 1988;158:1171–5. doi: 10.1016/0002-9378(88)90248-7. doi: 10.1016/0002-9378(88)90248-7. [DOI] [PubMed] [Google Scholar]


