Abstract
Objective:
Asthma and chronic obstructive pulmonary disease (COPD) are representative chronic inflammatory airway diseases responsible for a considerable burden of disease. In this article, we reviewed the relationship between neutrophil extracellular traps (NETs) and chronic inflammatory airway diseases.
Data Sources:
Articles published up to January 1, 2017, were selected from the PubMed, Ovid Medline, Embase databases, with the keywords of “asthma” or “pulmonary disease, chronic obstructive”, “neutrophils” and “extracellular traps.”
Study Selection:
Articles were obtained and reviewed to analyze the role of NETs in asthma and COPD.
Results:
NETs are composed of extracellular DNA, histones, and granular proteins, which are released from activated neutrophils. Multiple studies have indicated that there are a large amount of NETs in the airways of asthmatics and COPD patients. NETs can engulf and kill invading pathogens in the host. However, disordered regulation of NET formation has shown to be involved in the development of asthma and COPD. An overabundance of NETs in the airways or lung tissue could cause varying degrees of damage to lung tissues by inducing the death of human epithelial and endothelial cells, and thus resulting in impairing pulmonary function and accelerating the progress of the disease.
Conclusions:
Excessive NETs accumulate in the airways of asthmatics and COPD patients. Although NETs play an essential role in the innate immune system against infection, excessive components of NETs can cause lung tissue damage and accelerate disease progression in asthmatics and COPD patients. These findings suggest that administration of NETs could be a novel approach to treat asthma and COPD. Mechanism studies, clinical practice, and strategies to regulate neutrophil activation or directly interrupt NET function in asthmatics and COPD patients are desperately needed.
Keywords: Asthma, Pulmonary Disease, Chronic Obstructive, Extracellular Traps, Neutrophils
Introduction
Neutrophils are an essential component of the host response against invading pathogens, thought to be the first line of defense in the innate immune system against infection and constitute approximately 70% of the leukocytes in the peripheral blood.[1] In response to inflammatory stimuli, neutrophils migrate from the circulating blood to infected tissues, where they can efficiently bind, engulf, and inactivate pathogens through phagocytosis, degranulation, and the release of neutrophil extracellular traps (NETs).[2] The formation of NETs is a recently discovered mechanism of host defense against invading pathogens. When an organism becomes infected or stimulated to induce inflammation, neutrophils are activated and release NETs. These NETs are composed of granules and nuclear constituents that disarm and kill pathogens extracellularly through a series of activated signaling pathways. However, NETs can both also cause potential detriment, depending on the location, timing, and extent of inflammatory response. Therefore, the creation of too many NETs at a particular time or location can cause tissue damage of the host organism.
Asthma and chronic obstructive pulmonary disease (COPD) are representative chronic inflammatory airway diseases responsible for a considerable burden of disease.[3] Both asthma and COPD involve an obstruction in airflow, which is reversible in asthma but progressive and irreversible in patients with COPD.[4] In addition, both diseases are recognized for their heterogeneous nature, particularly regarding the type of inflammation within the lungs.[3] However, the underlying pathogenesis of asthma and COPD remains poorly understood, treatment options contain deficiencies, and further exploration of targeted therapy is urgently required.
Existing research indicated that intra-airway neutrophils are associated with the resistance of corticosteroids in asthmatics,[5] disease progression in COPD,[6] and the clinical severity and exacerbations of COPD.[7] Despite the positive role of NETs in the resistance to infection, one recent study demonstrated that NETs exist in the airways of patients with chronic inflammatory airway diseases.[8] Moreover, the accumulation of NETs is related to the activation of the innate immune response, which contributes to the disease pathogenesis in chronic inflammatory airway diseases.[8] Although it remains unclear how NETs participate in the pathogenesis of chronic inflammatory airway diseases, the significance of NETs in that diseases should not be ignored. This article provides an overview of the recent advances in NETs research, including the composition and functionality of NETs, as well as their relationship with asthma and COPD [Figure 1].
Figure 1.
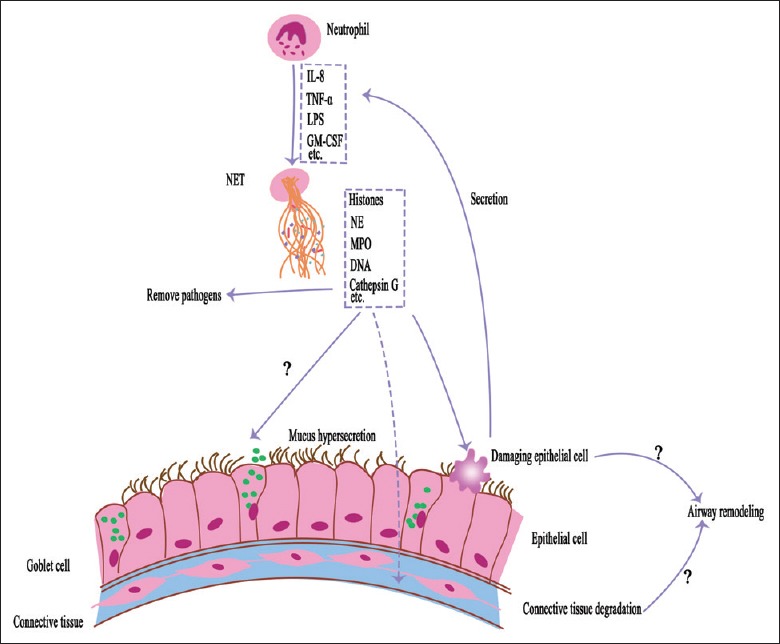
Role of neutrophil extracellular traps in the chronic obstructive airway diseases. In the airways of asthmatic and chronic obstructive pulmonary disease patients, neutrophils can be stimulated by a variety of biological molecules (eg., interleukin-8 [IL-8], tumor necrosis factor-α [TNF-α], lipopolysaccharide [LPS], granulocyte/macrophage colony-stimulating factor [GM-CSF]) produced by organisms or pathogens. Activated neutrophils can release neutrophil extracellular traps (NETs) which are composed of histones, neutrophil elastase (NE), myeloperoxidase (MPO), cathepsin G, and DNA. The components of neutrophil extracellular traps could damage airway epithelium and trigger inflammatory responses (the structure of neutrophil extracellular trap is adapted from Camicia 2014 and Cheng 2013). Furthermore, the components of neutrophil extracellular traps might induce mucus hypersecretion and airway remodeling to exacerbate asthma and chronic obstructive pulmonary disease.
Neutrophil Extracellular Traps
Neutrophil extracellular trap structure
In 2004, Brinkmann et al.[2] first reported that NETs consist of extracellular three-dimensional web-like scaffolds of DNA strands adorned with histones and antimicrobial proteins, which are released from activated neutrophils.[9] DNA is a major structural component of NETs consisting of granule and cytoplasmic proteins (e.g., neutrophil elastase [NE], myeloperoxidase [MPO], cathepsin G, proteinase 3, gelatinase, cathelicidins, lysozyme, defensins, lactoferrin, and calprotectin) as well as histones H1, H2A, H2B, H3, and H4, embedded in the DNA backbone.[1,10] The integrity of the NET structure provides the basis for its functionality and the structure of NET can be seen in recent reports[1,11] [Figure 1].
Neutrophil extracellular trap formation
Multiple studies demonstrated that a variety of biological molecules (e.g., interleukin-8 [IL-8],[11] tumor necrosis factor-α [TNF-α],[12] platelet-activating factor (PAF),[13] lipopolysaccharide [LPS],[14] antineutrophil cytoplasmic antibodies,[15] and granulocyte/macrophage colony-stimulating factor [GM-CSF] with complement factor 5a [C5a].[16]) produced by organisms or pathogens can induce the activation of neutrophils. Subsequently, activated nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2) generates a large amount of reactive oxygen species (ROS) which can lead to fission of the neutrophil nuclear membrane through binding to toll-like receptor 4 (TLR4). Moreover, intracellular NE and MPO migrate to the nucleus through the division of the neutrophil nuclear membrane, where they partially degrade specific histones and promote chromatin decondensation.[17] In addition, the citrullination of histones induced by peptidylarginine deiminases 4 (PAD4) further promotes chromatin decondensation.[18] However, the precise mechanisms that lead to PAD4 activation in this process remain largely unknown. Only one study reported that calcium binding may be involved in the activation of PAD4 and ROS may be involved in regulating PAD4 activation.[19] Following chromatin decondensation, the loose chromatin mixes with the granular cytoplasm and various proteins, and then all of which are ejected into the extracellular space, forming NETs.
Mechanism of neutrophil extracellular trap occurrence
Due to the release of NETs, a novel cell death program termed NETosis, which is distinct from apoptosis and necrosis, is triggered.[10] NETosis is an irreversible process that studies have found to be highly dependent on ROS production, known as NOX-dependent NETosis; however, a recent groundbreaking report revealed that in response to an acute infection with Staphylococcus aureus, neutrophils retain the ability to multitask when releasing NETs, which was found to be NOX-independent.[20] Currently, studies reported that platelet TLR4 and activated PAD4 are crucial to the process of NETs formation,[13,21] while others indicated that the direct contact between neutrophils and LPS without the involvement of platelet TLR4 can also cause NET formation.[22,23,24] To date, the mechanism of NETosis remains poorly understood and requires further study. However, it is known that the activation of NOX plays an important role in NETosis and the NOX-dependent NETosis signaling cascade includes the Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinases (MAPK) pathways.[25,26,27] Furthermore, the TLR4-myeloid differentiation factor 88 (MyD88) signaling pathway is also essential for the release of NETs from neutrophils.[28,29]
Function of Neutrophil Extracellular Traps
Positive effect of neutrophil extracellular traps
NETs have been shown to aid in the entrapment and killing of Gram-positive bacteria, Gram-negative bacteria, fungi, parasites, and protista.[10,21,22,29] They are also formed during viral infections,[30] likely providing a protective role in the infected host. The constructive function of NETs was indirectly demonstrated in chronic granulomatous disease (CGD) patients with severe aspergillosis. CGD is an inherited immunodeficiency disease caused by nonfunctional NOX2;[25] this defect interferes with phagocytic killing and prevents NET formation. The ability of NETs to trap and kill pathogens depends on the integrity of the DNA network structure and its internal bactericidal substances,[2,25] including histones, MPO, serine proteinase, lactoferrin, and lipocalin 2. It has been well established that histones are able to kill bacteria more effectively than common antimicrobials.[31] In particular, NE can cleave a number of enterobacterial virulence factors and prevent bacterial escape from the phagolysosome.[2,32] In addition, serine proteinase is positioned to penetrate and disrupt bacterial membranes through their cationic charge,[33] and lactoferrin and lipocalin 2 can restrict the supply of important nutrients to microbes through chelating iron and interfere with its absorption.[33,34] Furthermore, MPO can catalyze the formation of hypochlorite which is bactericidal. NETs might also help prevent the spread of microbe by forming a physical barrier and scaffold to enhance antimicrobial synergy while minimizing damage to host tissues. However, increasing evidence revealed that many pathogenic microorganisms can avoid entrapment by NETs using diverse methods. For example, Streptococcus pneumonia can escape NETs by changing their surface charge, creating a polysaccharide capsule or secreting deoxyribonuclease (DNase) which can degrade NETs.[35,36] Importantly, one study of gout found that aggregated NETs are formed during the gout inflammatory process when there is a high neutrophil density and are capable of degrading cytokines and chemokines via serine proteases.[37] Furthermore, aggregated NETs constitute an anti-inflammatory mechanism and reduce the recruitment and activation of neutrophils during acute gout.[37]
Negative effects of neutrophil extracellular traps
NETs are important components of the host defense response and provide a novel immune mechanism against infectious agents. While under normal condition, human DNase and monocyte-derived macrophages can clear NETs efficiently,[38] growing evidence suggested that the excessive production of NETs and the inefficient dismantling of these structures might potentially damage the host. Research suggested that the decreased ability of lupus nephritis patients to degrade NETs is due to the production of DNase I inhibitors or anti-NETs antibodies, thereby contributing to disease progression.[39] Moreover, Saffarzadeh et al.[31] found that NETs can directly induce the death of human epithelial and endothelial cells, suggesting that NETs have potentially cytotoxic effects. The general cytotoxic capability of NETs is associated with its components, with histones playing a predominant role in the cytotoxic effect. Other substances, such as NE, can efficiently degrade extracellular matrix components that mediate neutrophil-induced tissue damage,[40] and cathepsin G digests the connective tissue and cell surface proteins resulting in lung injury. Furthermore, MPO can degrade endothelial cell matrix heparan sulfate proteoglycan in concert with NE to induce capillary leakage and proteinuria.[41]
Therefore, there appears to be a balance between pathogen defense and host tissue damage regarding the functionality of NETs. On the one hand, NETs are able to entrap and kill pathogens, degrade cytokines and chemokines, as well as function as a valuable antimicrobial defense mechanism. On the other hand, NETs can lead to organ failure and even death if the regulatory mechanisms are absent or fail. Therefore, it is of great clinical significance to acknowledge the beneficial effects of NETs and simultaneously reduce their potentially harmful effects.
Neutrophil Extracellular Traps and Asthma
Asthma is a highly prevalent chronic inflammatory lung disease characterized by airway hyperresponsiveness to allergens, reversible airflow obstruction, airway edema, and increased mucus secretion.[42] Currently, asthma can be divided into four distinct phenotypes termed eosinophilic, neutrophilic, mixed granulocytic, and pauci-granulocytic phenotypes by investigating granulocyte infiltration in induced sputum.[43] Although the majority of patients with eosinophilic asthma are sensitive to corticosteroids, there is recognition that some asthmatics, particularly those who have severe disease and are resistant to corticosteroids, have elevated neutrophil counts in their airways. The number of neutrophils in the induced sputum of asthmatics is significantly correlated with the degree of airway obstruction and the levels of IL-8.[11] Moreover, the neutrophilic granulocyte count in the bronchoalveolar lavage fluid of asthmatic patients is used to distinguish moderate to severe asthma from mild asthma.[11] Furthermore, it has been shown that neutrophils play a vital role in the exacerbation of asthma by inducing mucus hypersecretion and airway remodeling, which results in acute reversible and progressive irreversible airway obstruction, respectively.[44] In addition, glucocorticoid administration to neutrophilic asthmatics can aggravate lung inflammation and damage lung tissue, since glucocorticoids can augment the potential effect of neutrophils by delaying their apoptosis and ultimate clearance from lung tissue.[45] Although neutrophils are thought to decrease lung function, the specific mechanism of neutrophils affect lung functionality remains unclear. A new perspective has emerged in light of one recent report, which showed that extracellular DNA traps can be generated by eosinophils and neutrophils in human atopic asthmatic airways in vivo.[46] Moreover, it is suggested that both NETs and eosinophil extracellular traps (EETs) are present in the airways of asthmatics.[46] The levels of extracellular DNA and NETs components in the induced sputum are significantly higher in neutrophilic versus nonneutrophilic asthma.[47] In addition, antimicrobial proteins and extracellular DNA were found to be positively correlated with airway neutrophils and negatively associated with lung function, respiratory symptoms, and disease control.[47] These effects lead to the extensive accumulation of NETs which aggravate the condition of asthmatics and promote the progression of the disease. A new study demonstrated that NETs can damage airway epithelium and trigger inflammatory responses, which could aggravate the severity of asthma.[48] However, to the best of our knowledge, the mechanisms by which NETs influence the progression of asthma have not been clearly elucidated. The initiating factor for inducing NETosis remains undefined. A interestingly report showed that although allergen challenge can increase eosinophils and neutrophils in the bronchoalveolar lavage fluid of asthmatics which cannot increase NET or EET formation in the airways of asthmatics.[46] It has been found that the level of LPS in asthmatics is elevated, indicating that NETosis might be related to the presence of LPS.[8] In addition, IL-8 is a potential trigger of NETosis in these airways as it has previously been shown to induce NETosis in other studies.[2,10,48,49] Côté et al.[50] revealed that NETs formation in an equine model of recurrent airway obstruction was significantly reduced in a time- and concentration-dependent manner by secretoglobin family 1A member 1 (SCGB 1A1), a small protein primarily secreted by mucosal epithelial cells of the lungs. Therefore, the low concentration of SCGB 1A1 in asthmatics might promote NET formation. Moreover, SCGB 1A1 might play a role in regulating NETosis in the lung; however, the factors that affect the formation of NETs in the airways of asthmatics are also unclear and require further study.
In the airways of asthmatics, NETs produced by activated neutrophils might enhance resistance to infection; however, the enhanced accumulation of NETs will aggravate the patients’ condition, particularly in neutrophilic asthmatics in whom the ability of alveolar macrophages to degrade and remove NETs is impaired.[38,51] Similarly, the sputum of patients with cystic fibrosis (CF) contains abundant NETs that make it difficult to expectorate thick sputum. Moreover, DNase therapy can degrade the structure of DNA so that to treat CF.[52] Therefore, we consider that reducing the formation of NETs will be beneficial to asthmatics. However, Dubois et al.[53] demonstrated that CF patients treated with DNase resulted in an increasing in NE activity and subsequent injury to the lung tissue. In a mouse model of asthma,[42] it was observed that extracellular DNA in mucous was involved in lower airway obstruction in an ovalbumin-induced model of asthma. In particular, lung functionally was improved by means of treatment with an intranasal administration of recombinant human DNase.[42] This study provides a novel viewpoint that the use of recombinant human DNase is a potential strategy for preventing tissue damage in asthmatics when the NET formation appears to be detrimental.
Neutrophil Extracellular Traps and Chronic Obstructive Pulmonary Disease
COPD is affiliated with smoking and the exposure to environmental fumes and is typically characterized by recurrent bacterial infections, persistent neutrophil infiltration, emphysematous alveolar wall destruction, and persistent airflow limitation.[9,54] It has a substantial impact on the quality of life and life expectancy, as well as currently being the third leading cause of mortality and the fifth leading cause of disability on a global scale.[55,56]
The infection is the main predisposing factor to cause COPD patients to change from being in periods of a stable condition to severe episodes of worsening (exacerbations), leading to an increasing impairment of lung function. The activation and aggregation of neutrophils in the lung is an important part of COPD inflammatory process, and neutrophils can induce chronic airway mucus hypersecretion and the destruction of the lung parenchyma through the release of NE, which is the main constituent of NETs and other active substances. The NE plays a pro-inflammatory role in COPD, particularly by stimulating the secretion of IL-8.[57] Thus, COPD is a prominent candidate for NETs formation and NETosis-mediated tissue damage, with clearly morphological evidence showing that NETs are present in the induced sputum of patients in both stable and exacerbated COPD.[9,58] Furthermore, Grabcanovic-Musija et al.[58] demonstrated that NETs formation in COPD patients were correlated with the severity of the airflow limitation. Therefore, it is assumed that NETs are responsible for the chronic inflammatory and lung function decline in COPD patients. However, the pathophysiology of NETs involved in the airways inflammation and lung injury in patients with COPD remains unclear. NETs, containing a mixture of extracellular DNA, histones, and granular proteins, might be directly cytotoxic to airway epithelial and endothelial cells,[59] or indirectly induce injury to the lung tissue through the promotion of autoimmune reactions against an aberrant amount of NETs components.[60] The level of NETs and NET components in the airways of COPD patients is associated with other markers of activate innate immune responses, including the expression of pro-inflammatory cytokines IL-1β and C-X-C motif chemokine ligand 8 and the inflammasome component NOD-like receptor family, pyrin domain containing 3.[8] Consequently, the positive feedback of pro-inflammatory cytokines and neutrophilic chemokines contributes to the persistent airway neutrophilia observed in COPD and promotes the production of additional NETs, thereby creating a vicious circle. This partially explains a possible mechanism of the substantial number of NETs in the airways of patients with COPD; however, the precise mechanism remains unknown.
Smoking cessation can decrease the rate of lung function decline in patients with COPD;[61] nevertheless, the relationship between smoking and NET formation remains controversial. Some studies revealed that NETosis-induced lung injury might have occurred in smokers who do not exhibit an airflow limitation, and NETs can also be induced by nicotine which is the addictive component of tobacco.[58,62] Moreover, others showed that NET formation was not affected by the current smoking status of COPD patients,[54] which indicates that smoking might not trigger NET formation in COPD patients. Currently, glucocorticoids are the main method of treatment for acutely exacerbated chronic obstructive pulmonary disease (AECOPD). Although some AECOPD patients exhibit a superior response to systemic glucocorticoid therapy, there is an existing portion of patients who do not respond to glucocorticoid.[63] Moreover, the long-term use of glucocorticoids is associated with multiple side effects, including fluid retention, hypertension, diabetes, and osteoporosis. Recent experimental evidence showed that systemic corticosteroid treat for AECOPD patients is insufficient to reduce NET formation.[64] This might be one of the reasons that patients with AECOPD appear to respond poorly to glucocorticoid drugs. Based on these findings, further studies are needed to explore whether modulating NETosis can ease the symptoms of COPD patients who are insensitive to glucocorticoid treatment. Fortunately, previous studies indicated that C-X-C motif chemokine receptor 2 antagonists can reduce neutrophils in the lungs of patients with COPD and limit the harmful effects of neutrophils on the lung tissue.[65]
Conclusion
To date, since their discovery, there has been substantial research progress regarding the antibacterial properties of NETs, and it has been widely accepted that NETs play an essential role in trapping and killing microbes to prevent microbial dissemination. However, growing evidence demonstrates that the formation of these extracellular structures contributes to the pathogenesis of several diseases (e.g., acute lung injury,[66] systemic lupus erythematosus,[67] CF,[68] and thrombosis.[69]). In particular, studying the role of NETs in asthma and COPD has become an area of growing interest. Existing researches indicated that there is a substantial level of NETs present in the airways of asthmatics and COPD patients. In addition, it appears that NETs are beneficial to fighting infection in the presence of chronic inflammatory airway diseases, and fine-tuning of NET formation throughout the course of the above diseases is the goal for the development of novel NET-targeted therapies of infected patients. The excessive NETs in the airways or lung tissue can cause varying degrees of damage to the lung, resulting in impaired pulmonary function and the acceleration of disease progression. The severity of these patients’ condition is positively correlated with the level of NETs in the airways. Thus, inhibiting NET formation is an attractive strategy for preventing the deleterious effects of NETs or their components in patients with asthma or COPD. It is uncertain whether regulating neutrophil activation or directly targeting NETs can inhibit the occurrence and development of disease in such patients; however, we hope to provide new potential therapeutic targets and customize treatment for chronic inflammatory airway diseases in the future. This can be achieved by increasing our understanding of the molecular mechanisms behind NET formation, elucidating the regulation of NETosis and investigating the function of these processes in chronic inflammatory airway diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Camicia G, Pozner R, de Larrañaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286–94. doi: 10.1097/SHK.0000000000000221. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9. doi: 10.1136/thx.2006.073429. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parr DG, White AJ, Bayley DL, Guest PJ, Stockley RA. Inflammation in sputum relates to progression of disease in subjects with COPD: A prospective descriptive study. Respir Res. 2006;7:136. doi: 10.1186/1465-9921-7-136. doi: 10.1186/1465-9921-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaron SD, Angel JB, Lunau M, Wrigth K, Fex C, Saux NL, et al. Granulocyte inflammatory markers and air way infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:349–55. doi: 10.1164/ajrccm.163.2.2003122. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- 8.Wright TK, Gibson PG, Simpson JL, McDonald VM, Wood LG, Baines KJ. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology. 2016;21:467–75. doi: 10.1111/resp.12730. doi: 10.1111/resp.12730. [DOI] [PubMed] [Google Scholar]
- 9.Obermayer A, Stoiber W, Krautgartner WD, Klappacher M, Kofler B, Steinbacher P, et al. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One. 2014;9:e97784. doi: 10.1371/journal.pone.0097784. doi: 10.1371/journal.pone.0097784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol. 2012;198:773–83. doi: 10.1083/jcb.201203170. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng OZ, Palaniyar N. NET balancing: A problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, et al. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS One. 2012;7:e48111. doi: 10.1371/journal.pone.0048111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 14.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187:1856–65. doi: 10.4049/jimmunol.1004201. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 15.Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–18. doi: 10.1182/blood-2012-03-416156. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 16.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–44. doi: 10.1038/cdd.2009.96. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. doi: 10.1083/jcb.201006052. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185:7413–25. doi: 10.4049/jimmunol.1000675. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 21.Marin-Esteban V, Turbica I, Dufour G, Semiramoth N, Gleizes A, Gorges R, et al. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect Immun. 2012;80:1891–9. doi: 10.1128/IAI.00050-12. doi: 10.1128/IAI.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, Rondina MT, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 a. Blood. 2012;120:3118–25. doi: 10.1182/blood-2012-01-405993. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–902. doi: 10.4049/jimmunol.180.3.1895. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–7. doi: 10.1038/nchembio.496. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 27.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J Cell Biochem. 2013;114:532–40. doi: 10.1002/jcb.24391. doi: 10.1002/jcb.24391. [DOI] [PubMed] [Google Scholar]
- 28.Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, et al. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–9. doi: 10.4049/jimmunol.175.9.6042. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 29.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011;79:431–8. doi: 10.1128/IAI.00660-10. doi: 10.1128/IAI.00660-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–16. doi: 10.1016/j.chom.2012.05.015. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91–4. doi: 10.1038/417091a. doi: 10.1038/417091a. [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulos V, Zychlinsky A. NETs: A new strategy for using old weapons. Trends Immunol. 2009;30:513–21. doi: 10.1016/j.it.2009.07.011. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. doi: 10.1038/nature.03104. [DOI] [PubMed] [Google Scholar]
- 35.Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, et al. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–71. doi: 10.1111/j.1462-5822.2006.00857.x. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 36.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–7. doi: 10.1016/j.cub.2006.01.056. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 37.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–7. doi: 10.1038/nm.3547. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 38.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–56. doi: 10.4049/jimmunol.1300436. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 39.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–8. doi: 10.1073/pnas.0909927107. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury-a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med. 2008;14:465–75. doi: 10.2119/2008-00011.Perl. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lögters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, et al. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol. 2009;198:211–9. doi: 10.1007/s00430-009-0121-x. doi: 10.1007/s00430-009-0121-x. [DOI] [PubMed] [Google Scholar]
- 42.da Cunha AA, Nuñez NK, de Souza RG, Moraes Vargas MH, Silveira JS, Antunes GL, et al. Recombinant human deoxyribonuclease therapy improves airway resistance and reduces DNA extracellular traps in a murine acute asthma model. Exp Lung Res. 2016;42:66–74. doi: 10.3109/01902148.2016.1143537. doi: 10.3109/01902148.2016.1143537. [DOI] [PubMed] [Google Scholar]
- 43.Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67:835–46. doi: 10.1111/j.1398-9995.2012.02832.x. doi: 10.1111/j.1398-9995.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 44.Louis R, Djukanovic R. Is the neutrophil a worthy target in severe asthma and chronic obstructive pulmonary disease? Clin Exp Allergy. 2006;36:563–7. doi: 10.1111/j.1365-2222.2006.02493.x. doi: 10.1111/j.1365-2222.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 45.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–6. doi: 10.1016/j.jaci.2010.12.1103. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–8. doi: 10.1136/thx.2006.061358. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pham DL, Ban GY, Kim SH, Shin YS, Ye YM, Chwae YJ, et al. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin Exp Allergy. 2017;47:57–70. doi: 10.1111/cea.12859. doi: 10.1111/cea.12859. [DOI] [PubMed] [Google Scholar]
- 49.Alfaro C, Teijeira A, Oñate C, Pérez G, Sanmamed MF, Andueza MP, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs) Clin Cancer Res. 2016;22:3924–36. doi: 10.1158/1078-0432.CCR-15-2463. doi: 10.1158/1078-0432.CCR-15-2463. [DOI] [PubMed] [Google Scholar]
- 50.Côté O, Clark ME, Viel L, Labbé G, Seah SY, Khan MA, et al. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One. 2014;9:e96217. doi: 10.1371/journal.pone.0096217. doi: 10.1371/journal.pone.0096217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, et al. Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy. 2013;43:29–35. doi: 10.1111/j.1365-2222.2012.04075.x. doi: 10.1111/cea.12159. [DOI] [PubMed] [Google Scholar]
- 52.Huggins JT, Doelken P, Sahn SA. Intrapleural therapy. Respirology. 2011;16:891–9. doi: 10.1111/j.1440-1843.2011.02011.x. doi: 10.1111/j.1440-1843.2011.02011.x. [DOI] [PubMed] [Google Scholar]
- 53.Dubois AV, Gauthier A, Bréa D, Varaigne F, Diot P, Gauthier F, et al. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am J Respir Cell Mol Biol. 2012;47:80–6. doi: 10.1165/rcmb.2011-0380OC. doi: 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen F, Marwitz S, Holz O, Kirsten A, Bahmer T, Waschki B, et al. Neutrophil extracellular trap formation and extracellular DNA in sputum of stable COPD patients. Respir Med. 2015;109:1360–2. doi: 10.1016/j.rmed.2015.08.008. doi: 10.1016/j.rmed.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Calik-Kutukcu E, Savci S, Saglam M, Vardar-Yagli N, Inal-Ince D, Arikan H, et al. A comparison of muscle strength and endurance, exercise capacity, fatigue perception and quality of life in patients with chronic obstructive pulmonary disease and healthy subjects: A cross-sectional study. BMC Pulm Med. 2014;14:6. doi: 10.1186/1471-2466-14-6. doi: 10.1186/1471-2466-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou HX, Ou XM, Tang YJ, Wang L, Feng YL. Advanced chronic obstructive pulmonary disease: Innovative and integrated management approaches. Chin Med J. 2015;128:2952–9. doi: 10.4103/0366-6999.168073. doi: 10.4103/0366-6999.168073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ungurs MJ, Sinden NJ, Stockley RA. Progranulin is a substrate for neutrophil-elastase and proteinase-3 in the airway and its concentration correlates with mediators of airway inflammation in COPD. Am J Physiol Lung Cell Mol Physiol. 2014;306:L80–7. doi: 10.1152/ajplung.00221.2013. doi: 10.1152/ajplung.00221.2013. [DOI] [PubMed] [Google Scholar]
- 58.Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner WD, Steinbacher P, Winterberg N, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radic M, Marion TN. Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. Semin Immunopathol. 2013;35:465–80. doi: 10.1007/s00281-013-0376-6. doi: 10.1007/s00281-013-0376-6. [DOI] [PubMed] [Google Scholar]
- 61.Willemse B, Lesman-Leegte I, Timens W, Postma D, ten Hacken N. High cessation rates of cigarette smoking in subjects with and without COPD. Chest. 2005;128:3685–7. doi: 10.1378/chest.128.5.3685. doi: 10.1378/chest.128.5.3685. [DOI] [PubMed] [Google Scholar]
- 62.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. J Leukoc Biol. 2016;100:1105–12. doi: 10.1189/jlb.3AB0815-379RR. doi: 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes PJ. Inhaled corticosteroids in COPD: A controversy. Respiration. 2010;80:89–95. doi: 10.1159/000315416. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- 64.Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther. 2013;345:430–7. doi: 10.1124/jpet.112.202879. doi: 10.1124/jpet.112.202879. [DOI] [PubMed] [Google Scholar]
- 65.Leaker BR, Barnes PJ, O’Connor B. Inhibition of LPS-induced airway neutrophilic inflammation in healthy volunteers with an oral CXCR2 antagonist. Respir Res. 2013;14:137. doi: 10.1186/1465-9921-14-137. doi: 10.1186/1465-9921-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–21. doi: 10.1096/fj.13-236380. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–23. doi: 10.1038/nm.2209. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]


