Introduction
The tetrasomy 18p (OMIM 614290) is a very rare chromosomal abnormality, with a prevalence of 1/140,000–180,000 live births.[1] Although it has been known in some countries, it has been seldom reported in China. Especially, mosaicism for tetrasomy 18p is even rare. Because of a very limited number of cases, the phenotypic spectrum of mosaic tetrasomy 18p, the complications, and prognosis are unknown. In this study, we reported a patient with mosaic tetrasomy 18p by conventional karyotyping analysis, high-resolution single nucleotide polymorphism (SNP) array, and fluorescence in situ hybridization (FISH).
Case Report
A 3-month-old girl was born from unrelated healthy parents with no family history of hereditary disease or mental retardation. She presented with developmental retardation and neonatal jaundice, and referred to the genetic department for genetic counseling. The mother was 30 years old and the father was 32 at the time of her birth. The mother had one induced abortion due to taking medicine accidently during her early pregnancy. Ultrasound scans at 16 weeks, 19 weeks, and 38 weeks of gestation showed that fetal measurements were appropriate to 15, 17, and 35 weeks, respectively, which confirmed to be small for gestational age. The child was born by cesarean section at 42 weeks with the length of 51 cm (80th centile), weight of 3500 g (75th–80th centile), and head circumference of 33 cm (20th centile). Neonatally, she presented with difficult feeding, hemolytic disease of newborn, hemolytic jaundice, and anemia. Poor sucking and uncoordinated swallowing were also observed. At 3 months, she was referred to our hospital for investigation because of developmental delay, with 5500 g in weight (5th–10th centile) and 63 cm in length (75th centile), with a head circumference of 36.5 cm (<3th centile). Meanwhile, she cannot control her head or chase objects with sounds. She had long fingers with Michitsura palms, small hands, lower nasal bridge, bilateral internal strabismus, and microcephaly. The tendon reflex was positive. Laboratory tests, including thyroid profile, blood cell count, blood chemistry and liver function, and urine and blood metabolism screening, were all normal. Auxiliary examinations, including cranial magnetic resonance imaging (MRI), electroencephalogram, and abdominal ultrasonography, were normal. Based on Gesell development schedules, delays in gross motor development, fine motor functions, adaptation, language, and social behavior were also presented. Echocardiography showed that the patient had persistent left superior vena cava. Bilateral brainstem auditory-evoked potentials were normal, but the latency of P100 wave was delayed. We performed telephonic follow-up when the patient was 1½ year old. She was 12 kg in weight (80th centile) and 85 cm in length (75th centile). Moreover, she could sit independently at 15 months old. However, she could not walk yet.
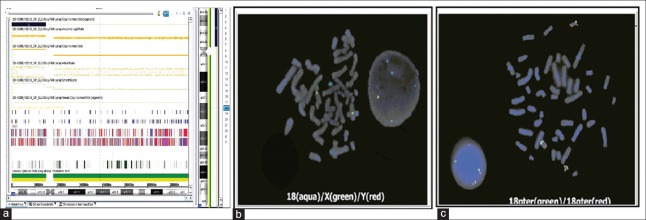
Routine cytogenetic analysis by G-banding techniques at the 400 bands of resolution was done on cultured peripheral blood lymphocytes from the patient and her parents, and fifty metaphase spreads were analyzed. The cytogenetic analysis in the patient revealed the karyotype of 47, XX,+mar [42]/46, XX [8]. Karyotyping results of her parents were 46, XX, and 46, XY, respectively, which indicated that this marker in the patient was a de novo event. We used CytoScan 750K array (Affymetrix, Santa Clara, CA, USA) to examine the copy number variation of the duplication regions. SNP result of the patient showed arr 18p11.32p11.21(136,227-15,106,305)x4 [Figure 1a]. She had four copies of chromosome 18p11.32p11.21, spanning from position 136,227 to 15,106,305 (UCSC Genome Build hg19, Feb. 2009). FISH analysis was performed to confirm the chromosomal origin of extramarker for the patient. A chromosome 18 centromeric probe D18Z1 (CEP 18, Vysis) and two subtelomeric probes (18pter, 18qter, Vysis) were applied. FISH revealed a female tetrasomy 18p pattern in 43 of 50 interphase nuclei scored; the remaining 7 nuclei showed normal female chromosomes (46, XX, [7]/47, ish+i(18)(p10)(D18Z1+,18pter++) [43]) [Figure 1b and 1c].
Figure 1.
Results of G-banded karyotypes, single nucleotide polymorphism array and fluorescence in situ hybridization. Single nucleotide polymorphism array showed 4-fold duplicated region of 18p as a black box in the patient (a). Fluorescence in situ hybridization results with multicolor DNA probes of CEP 18 aqua/X green/Y red (b) and with chromosome 18 subtelomeric probes, 18pter green/18qter red (c).
Discussion
The mosaicism of tetrasomy 18p is rare, mainly reported in prenatal diagnosis followed by termination of the pregnancy. Of interest, it does present in this case. Based on the common mechanism for tetrasomy 18p,[2] we suspect that the mosaicism of 47, XX,+18/46, XX of the patient is first developed from nondisjunction event in an early mitosis after the formation of the zygote. And then, centromeric misdivision in some cells of 47, XX,+18 occurs and results in loss of chromosomes 18q. At last, the two cell lines, including 47, XX,+i(18)(p10)/46, XX, are formed. Moreover, the patient had features not fully consistent with the tetrasomy 18p phenotype. Compared to tetrasomy 18p, her height and weight were not delayed too much. The evaluation of the audiology and endocrine and brain MRI were all normal. Except for small ears, bilateral internal strabismus, and microcephaly, she had less craniofacial characteristic features for tetrasomy 18p. Thus, we suspect that the patients with mosaic tetrasomy 18p might present variable phenotypic features ranging from an apparently normal phenotype to multiple abnormalities. For mosaic tetrasomy 18p, the variability and severity of clinical phenotype might be influenced by euchromatin content, the degree of mosaicism, etc. We suggest that more detailed information regarding the clinical features of individuals with mosaic tetrasomy 18p is worthy to be reported.
For the case, there are 46 OMIM genes located in duplication region. Among them, five genes including TGIF1, LAMA1, PIEZO2, AFG3L2, and MC2R are listed in the Developmental Disorders Genotype-Phenotype Database (DDG2P) (https://decipher.sanger.ac.uk/ddd#ddgenes). Thus, the developmental delay in the patient might be associated with these five genes. Meanwhile, the overexpression of genes in this region, such as MYOM1 (603508) and MYL12B (609211), might be the underlying mechanism of motor developmental delay. In addition, IMPA2 gene (605922), a strong candidate gene for bipolar disorder, might be responsible for aggressive behavior reported in tetrasomy 18p patients although the case is too young to present behavioral regulation problems.[3]
For a small extramarker chromosome, karyotyping is not always possible to determine the chromosomal origin, copy number, and duplication/deletion size of the marker. SNP microarray has been a valuable diagnostic tool for genetic testing of genome-wide copy number changes.[4] In this study, the size and copy number of the duplication region were determined by SNP microarray. Thus, accurate diagnosis of the tetrasomy 18p syndrome, especially for the mosaic tetrasomy 18p, requires the use of combined techniques, such as karyotyping, SNP, and FISH.
In summary, we report a rare mosaic tetrasomy 18p in a female child. When compared to the standard clinical features of tetrasomy 18p, the phenotypic features in the patient are not so classical. The possible explanation for this could be the subtle characterization for mosaic tetrasomy 18p. Pooling the genetic and clinical data would help us learn about genotype/phenotype correlations and aid in the recognition and diagnosis of mosaic tetrasomy 18p. In addition, putting on record of such rare cases of genetic conditions provides an opportunity to explore the mechanisms of anomalies that may be generalizable to other conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Ramegowda S, Gawde HM, Hyderi A, Savitha MR, Patel ZM, Krishnamurthy B, et al. De novo isochromosome 18p in a female dysmorphic child. J Appl Genet. 2006;47:397–401. doi: 10.1007/BF03194651. doi: 10.1007/BF03194651. [DOI] [PubMed] [Google Scholar]
- 2.Bugge M, Collins A, Petersen MB, Fisher J, Brandt C, Tsezou A, et al. Non-disjunction of chromosome 18. Hum Mol Genet. 1998;7:661–9. doi: 10.1093/hmg/7.4.661. [DOI] [PubMed] [Google Scholar]
- 3.Swingle HM, Ringdahl J, Mraz R, Patil S, Keppler-Noreuil K. Behavioral management of a long-term survivor with tetrasomy 18p. Am J Med Genet A. 2006;140:276–80. doi: 10.1002/ajmg.a.31058. doi: 10.1002/ajmg.a.31058. [DOI] [PubMed] [Google Scholar]
- 4.Manning M, Hudgins L. Professional Practice and Guidelines Committee. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12:742–5. doi: 10.1097/GIM.0b013e3181f8baad. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]