Abstract
Meiosis is often described as a special case of cell division since it differs from mitosis in having two nuclear divisions without an intervening S-phase. It will be of great interest to uncover what molecular mechanisms underlie these special features of meiosis. We previously reported that the tardy asynchronous meiosis (tam) mutant of Arabidopsis (Arabidopsis thaliana) is slower in cell cycle progression in male meiosis. Here we report that TAM encodes the A-type cyclin, CYCA1;2. The point mutation in tam replaced a conserved threonine with an isoleucine in the linker region between the α4 and α5 helices of the first cyclin fold. By studying the dynamics of a CYCA1;2-green fluorescent protein fusion protein under the control of the CYCA1;2 promoter, we found that the fusion protein was most abundant at pachytene, but was undetectable from late prophase I until telophase II. Nonetheless, cell cycle progression in tam was delayed in both pachytene and meiosis II. We conclude either that the CYCA1;2 produced in prophase I indirectly regulates meiosis II progression, or that a very low level of CYCA1;2 directly regulates meiosis II progression. Either of these scenarios is a deviation from the typical mode of action of mitotic cyclins in mitosis and meiosis I, in which each nuclear division is coupled with a peak of expression of mitotic cyclins.
A hallmark of cell cycle progression in mitosis is that mitotic cyclins oscillate once with each cell cycle; a high level at the G2/M transition or some time into the M-phase is followed by an abrupt decline before exit from M-phase (Nurse, 2002). Meiosis, in contrast to mitosis, consists of two consecutive nuclear divisions without an intervening S-phase for chromosome duplication, defining an M-M phase transition between meiosis I and meiosis II (Kishimoto, 2003). However, at the molecular level, if or how the meiosis I-meiosis II transition differs from the transition between two consecutive mitotic cell cycles remains largely unknown.
There are two types of mitotic cyclins: A and B. There is some information about meiotic modulation of the oscillation of B-type cyclins. It has been found that degradation of cyclin B is not required for the meiosis I-meiosis II transition (Taieb et al., 2001). This phenomenon suggests that the meiosis I-meiosis II transition does not likely involve an exit from an earlier M-phase and reentry into a subsequent M-phase, because degradation of mitotic cyclins is required for exit from M-phase in mitosis (Nurse, 2002). In fact, incomplete degradation of cyclin B at the end of meiosis I is essential for preventing the entry into S-phase in meiosis II in animals (Picard et al., 1996; Sakamoto et al., 1998; Iwabuchi et al., 2000; Taieb et al., 2001; Perez et al., 2002). In female meiosis of some animal species that normally undergo an arrest in metaphase II, depletion of cyclin B leads to the absence of the meiosis II spindle, underscoring the importance of new cyclin B synthesis for stabilizing the metaphase II spindle in these species (Hunt et al., 1992; Hochegger et al., 2001; Perez et al., 2002). However, it is worth noting in these cases that even when cyclin B is depleted just prior to the onset of meiosis II, chromosome condensation and nuclear envelope breakdown are still maintained (Hunt et al., 1992; Hochegger et al., 2001; Perez et al., 2002). These observations indicate that at least some features of M-phase persist into meiosis II, which is independent of de novo synthesis of cyclin B during the meiosis I-meiosis II transition. These lines of evidence thus suggest that the meiosis I-meiosis II transition fundamentally differs from the transition between two consecutive mitotic cell cycles.
Analysis of A-type cyclins could provide another test of the special nature of the meiosis I-meiosis II transition. Like B-type cyclins, A-type cyclins are in general involved in the G2/M transition in eukaryotes (Lehner and O'Farrell, 1989; den Elzen and Pines, 2001; Geley et al., 2001) and have been found to function in meiotic prophase I in animals (Wolgemuth et al., 2002). However, no A-type cyclin has been found to exist in a significant amount during the meiosis I-meiosis II transition (Gönczy et al., 1994; Okano-Uchida et al., 1998; Wolgemuth et al., 2002). An idea that a meiosis I-expressed A-type cyclin indirectly regulates progression of meiosis II was proposed by Gönczy et al. (1994); they presented evidence that the Roughex protein regulates meiosis II in Drosophila spermatogenesis via a dosage-dependent suppression of cyclin A, but not of cyclin B. Roughex seemed to modulate the function of cyclin A only in G2 of meiosis I, and yet it somehow influenced progression of meiosis II. However, no direct genetic test of the role of cyclin A in meiosis II has been reported, perhaps partly because mutations in cyclin A in animals result in apoptosis in prophase I, precluding tests of the functions of cyclin A in later meiotic stages (Wolgemuth et al., 2002). In contrast, reduced cyclin B activities have not been found to result in apoptosis of meiocytes. Such a difference might be attributed to a difference in expression timing or location, rather than to differences in protein structure between A- and B-type cyclins (Murray, 2004). Furthermore, it is notable that apoptosis or cell cycle arrest usually does not occur in plant meiotic mutants but often do occur in meiotic mutants in yeast (Saccharomyces cerevisiae) and animals. For example, a failure in synapsis in yeast and animals is likely to activate the pachytene checkpoint, resulting in cell cycle arrest or apoptosis, but this is not the case in Arabidopsis (Arabidopsis thaliana) asynaptic mutants (Peirson et al., 1997; Couteau et al., 1999; Caryl et al., 2000; Grelon et al., 2001; Li et al., 2004; Puizina et al., 2004; Y. Wang et al., 2004). Therefore, both meiosis I and meiosis II can be characterized in most plant meiotic mutants, an advantage over their yeast and animal counterparts.
Higher plants contain both A- and B-type cyclins, but nothing is known about their roles in meiosis. Only the SOLO DANCERS protein, perhaps representing a new type of cyclin in Arabidopsis, has been found to regulate synapsis in prophase I (Azumi et al., 2002; G. Wang et al., 2004). We previously reported that the Arabidopsis mutant tardy asynchronous meiosis (tam) is slowed in the progression of male meiosis. This slowing frequently resulted in the formation of dyads and triads before formation of tetrads (Magnard et al., 2001), which is in contrast with the direct formation of tetrads in wild type. The meiotic phenotype of tam suggested that the TAM protein normally regulates both meiosis I and meiosis II. In this report, we demonstrate that tam is defective in the A-type cyclin CYCA1;2. By expressing a CYCA1;2-green fluorescent protein (GFP) fusion protein under the control of the CYCA1;2 promoter, we show that CYCA1;2-GFP is only detectable in prophase I. We also demonstrate that the durations of pachytene and meiosis II are longer in tam than in wild type. Therefore, we conclude that CYCA1;2 regulates both meiosis I and meiosis II even though there is little or no CYCA1;2 present during the meiosis I-meiosis II transition. These results further support the idea that the meiosis I-meiosis II transition fundamentally differs from the transition between two consecutive mitotic cell cycles.
RESULTS
TAM Encodes the A-type Cyclin CYCA1;2
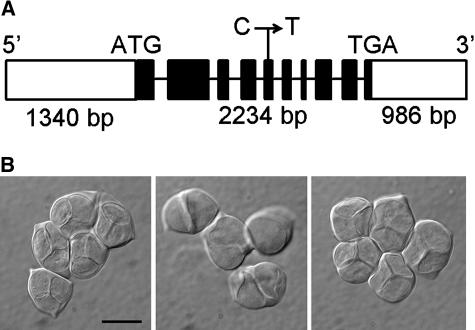
To learn the molecular identity of TAM, we mapped tam to a bacterial artificial chromosome clone corresponding to a region of approximately 130 kb on the bottom arm of chromosome 1. Two promising candidates in this region, a gene predicted to encode a protein kinase (AT1G77280) and a gene predicted to encode an A-type cyclin (AT1G77390; Vandepoele et al., 2002), were chosen for complementation of tam. A wild-type genomic fragment including the coding region and the upstream and downstream regions of each of the genes was introduced into tam via Agrobacterium-mediated transformation. A total of eight independent transformations were performed for each of the transgenes. Because the kinase gene failed to complement tam in transformants (T1 generation) and its cDNA sequence from wild type and tam were identical, we concluded that TAM is not the kinase. However, every transformant with the fragment containing the cyclin gene (Fig. 1A) produced only tetrads in male meiosis at either 22°C or 27°C (Fig. 1B). This phenotype was in sharp contrast to that in tam plants, where there is typically a good portion of dyads at 22°C and nearly 100% dyads at 27°C (Magnard et al., 2001). We obtained the cDNA for CYCA1;2 from both the wild-type and tam RNA samples by reverse transcription (RT)-PCR, using primers outside the predicted coding region. Comparison of the wild-type cDNA with that from tam revealed a 1-nucleotide change from C to T in exon 5, resulting in a Thr to Ile replacement at amino acid 283 (Fig. 1A). These results indicate that the meiotic defect in tam results from the mutation in CYCA1;2.
Figure 1.
Complementation of tam with CYCA1;2. A, Genomic fragment that complemented the tam meiotic phenotype. White bars indicate noncoding regions, black bars indicate exons, and lines indicate introns. ATG and TGA, start and stop codons, respectively. C→T denotes position of the point mutation in tam. bp, Base pairs. B, Meiotic products. Left, Wild-type tetrads. Middle, Dyads in tam. Note the prominent median wall in the dyads. Right, Normal tetrads in a tam plant containing the DNA fragment illustrated in A. All plants were grown at 27°C. Scale bar = 10 μm.
Mutation in CYCA1;2 Corresponds to Temperature Sensitivity of tam Phenotype
Analysis of the wild-type genomic and cDNA sequences indicated that CYCA1;2 has 10 exons and encodes a protein of 442 amino acids as correctly predicted by gene modeling in the Arabidopsis database (www.arabidopsis.org). CYCA1;2 has the two typical cyclin folds at the C terminus (Noble et al., 1997) that account for approximately 60% of the protein. Thr-283, where the substitution in tam occurs, is presumably the first amino acid in the linker region between the α4 and α5 helices of the first fold (Jeffrey et al., 1995). This Thr is conserved in A-, B-, D-, and E-type cyclins (Jeffrey et al., 1995). The linker region is predicted to be essential for forming the correct angle between the α4 and α5 helices. The substitution of a hydrophobic Ile for a hydrophilic Thr presumably results in incorrect folding of the α4 and α5 helices, which likely leads to a loss of function of CYCA1;2. This interpretation is consistent with the recessive nature and temperature-sensitivity of the tam mutation (Magnard et al., 2001). Based on this analysis, we predicted that tam plants might exhibit a less severe meiotic defect if grown at a temperature lower than the 22°C customarily used for Arabidopsis growth. Indeed, when we transferred mature tam plants from 22°C to 17°C and examined the meiotic products 2 d later, tam plants produced only tetrads (not shown), further supporting that the Ile to Thr replacement causes a temperature-sensitive phenotype.
CYCA1;2 Is Transcribed in Various Organs
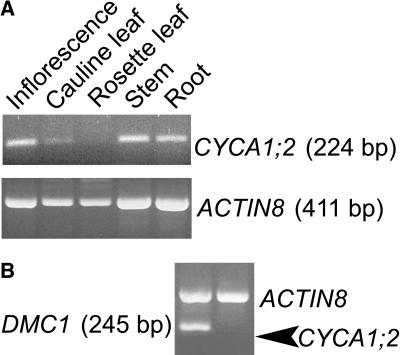
In addition to the meiotic phenotype, we consistently observed that tam plants were delayed in bolting by approximately 1 week, suggesting that CYCA1;2 might be expressed in cells other than microsporocytes. To test this prediction, we performed RT-PCR using CYCA1;2-specific primers and total RNA samples from different tissues. The transcript could be detected after 40 PCR cycles, but hardly at all after 30 PCR cycles, in roots, rosette leaves, stems, and inflorescences (Fig. 2A; data not shown). From the results of this and eight other RT-PCR experiments, the expression level of CYCA1;2 appeared low in all tissues tested. To confirm this, we compared its level in the inflorescence with that of the DMC1 transcript. DMC1 has a conserved role in recombination in eukaryotes (Masson and West, 2001). DMC1 can be regarded as a gene with a low or moderate expression level in total RNA isolated from the inflorescence, because it is expressed predominantly in meiocytes in early meiosis (Klimyuk and Jones, 1997; Y. Wang et al., 2004). Our RT-PCR experiments (repeated three times) indicated that while the DMC1 transcript could be detected simultaneously with an actin control in the same reaction tube, the CYCA1;2 PCR fragment, whose length was similar to that of the DMC1 PCR fragment, was undetectable when the actin control was in the same reaction tube (Fig. 2B). This result demonstrates that the level of CYCA1;2 transcript is lower than that of the DMC1 transcript. Microarray analysis of cyclin gene transcription (G. Wang et al., 2004) indicated that the peak level of the CYCA1;2 transcript was much lower than the peak levels of most of the other cyclins. Interestingly, the microarray data also indicated that the highest level of the CYCA1;2 transcript was in the anther as compared with the levels in the other tested tissues, including leaf, root, stem, inflorescence, stage 12 flowers, and silique. The overall low level of the CYCA1;2 transcript perhaps reflects that the gene is expressed only in certain type(s) of cells and/or in a particular period of the cell cycle.
Figure 2.
RT-PCR analysis of the CYCA1;2 transcript. A, RT-PCR amplification of CYCA1;2 and ACTIN8 (control) in five different organs. The amplifications for CYCA1;2 and ACTIN8 were in separate tubes, with 40 cycles for CYCA1;2 and 20 cycles for ACTIN8. A premix of solution for RT-PCR for each RNA sample was divided equally for CYCA1;2 and ACTIN8. B, In wild-type inflorescences, the CYCA1;2 transcript level was less than the DMC1 transcript level. The same RNA sample was used for both lanes, and the PCR was for 40 cycles. The amplifications for CYCA1;2 and ACTIN8 were in the same tube, and so were the amplifications for DMC1 and ACTIN8.
TAM Exists Predominantly in Pachytene during Male Meiosis
To further investigate the expression pattern of CYCA1;2 in male meiosis, we generated a CYCA1;2-GFP fusion construct, under the control of the same promoter region used for the tam complementation, and introduced the gene construct into both wild-type and tam plants. The CYCA1;2-GFP transgene complemented the tam phenotype, suggesting that the subcellular localization and stability dynamics of the fusion protein likely mimicked that of endogenous CYCA1;2. The GFP signal was observed in microsporocytes dissected from some, but not all, young anthers. This observation suggested that CYCA1;2 might be present in the microsporocytes only at particular stages.
Determining the precise stages of CYCA1;2 expression in microsporocytes is critical for understanding its function. However, it was difficult to observe distinct chromosome morphology after 4′,6-diamidino-2-phenylindole (DAPI) staining of fresh microsporocytes, and conversely, fixed microsporocytes had autofluorescence in the GFP channel that was further intensified after DAPI staining. These limitations hindered observation of the GFP signal and meiotic stage in the same microsporocytes. Therefore, we developed a method to indirectly assess the meiotic stage of each sample examined for the GFP signal. We determined the meiotic stage in a medial anther by examining a portion of the microsporocytes from the same anther or from another medial anther of the same bud. This method is based on the observations that the four medial anthers of Arabidopsis are essentially simultaneous in development (Smyth et al., 1990) and that microsporocytes in an anther of angiosperms are closely synchronous in cell division (Esau, 1976). These observations have long been the basis for routine determination of meiotic stages in Arabidopsis male meiosis (Ross et al., 1996; Armstrong and Jones, 2003). However, as a precautionary measure, and as required for determination of the durations of several cell cycle stages (see next section), we investigated whether the medial anthers within the same buds were highly synchronous in wild-type and tam plants at both 22°C and 27°C. We examined paraformaldehyde-fixed and DAPI-stained medial anthers (3–4 medial anthers per bud) at various cell cycle stages (5–13 buds per stage). Both the morphology of microsporocytes (Ross et al., 1996) and the percentage of binucleate tapetal cells (Y. Wang et al., 2004) were used to determine the cell cycle stages. We found that the medial anthers within a bud were indeed highly synchronous in cell cycle progression in wild-type and tam plants at both 22°C and 27°C. Two or more cell cycle stages in one bud were only observed during the transitions between consecutive stages, or around homolog separation, presumably reflecting the transient nature of these processes.
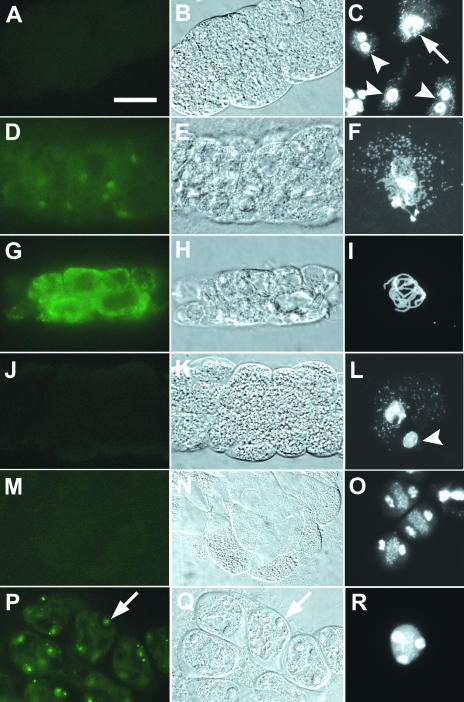
To detect the CYCA1;2-GFP signal, we recorded the status of the GFP signal in fresh microsporocytes within 3 min after dissection, then almost simultaneously fixed a portion of microsporocytes from the same anther or from another medial anther in the same bud to determine the meiotic stage. The meiotic stage was determined after DAPI staining of chromosomes of the dissected microsporocytes, or, for precise identification of zygotene and pachytene, by spreading the chromosomes before DAPI staining (Y. Wang et al., 2004). The incidence of binucleate tapetal cells, which form when microsporocytes are in prophase I, also helped in determining the meiotic stage (Y. Wang et al., 2004). Bleaching of the GFP signal occurs upon prolonged UV illumination; thus, for a valid semiquantitative comparison, all GFP images were taken with a 1-s exposure without prior UV illumination, and all images were subjected to the same extent of digital adjustment of contrast. No GFP signal was detected in microsporocytes before zygotene (Fig. 3, A–C; data not shown), a weak signal appeared at zygotene (Fig. 3, D–F), and the strongest signal was seen in pachytene (Fig. 3, G–I). GFP was not detectable from late diplotene to anaphase II (Fig. 3, J–O; data not shown). The GFP signal reappeared in microsporocytes at telophase II (Fig. 3, P–R), presumably indicating that CYCA1;2 plays a role in processes occurring after the second nuclear division. This timing of CYCA1;2-GFP expression was verified in 13 T2- and T3-generation transgenic plants derived from 2 different T1 plants. These results, that CYCA1;2 is predominantly expressed in pachytene and is essentially not expressed again until telophase II, raised the possibility that a one-time expression of CYCA1;2 in prophase I was sufficient for normal progression of both meiosis I and meiosis II.
Figure 3.
CYCA1;2-GFP during male meiosis in wild type. Left, GFP signal. Middle, Corresponding bright-field images to the images on the left. Right, DAPI-staining of fixed samples showing the corresponding cell cycle stages of the fresh microsporocytes on the left. A to C, No GFP signal, late leptotene, or early zygotene (arrow). Three tapetal cells (arrowheads), two binucleate and one uninucleate, can be seen, consistent with the stage being leptotene or zygotene (Y. Wang et al., 2004). D to F, Weak GFP signal, late zygotene, or early pachytene. G to I, Strong GFP signal, pachytene. J to L, Weak GFP signal, diplotene. A nucleus presumably from an anther wall cell (not a tapetal cell) is also present (arrowhead). M to O, No GFP signal, prophase II. Three microsporocytes are shown in O. The two nuclei near the right margin are from a tapetal cell. P and R, GFP signal in telophase II microsporocytes. Arrows denote one of the nuclei. Cells in F and I were fixed and enzyme-treated for spreading chromosomes; cells in C, L, O, and R were fixed but not enzyme-treated. Scale bar = 20 μm.
CYCA1;2-GFP Is Localized in Both the Cytoplasm and Nucleus
A-type cyclins have been found to exist in both the cytoplasm and nucleus (Castro et al., 1994; Chaubet-Gigot, 2000; Dienemann and Sprenger, 2004), and the function of cyclin A in Drosophila mitosis is independent of its subcellular localization (Dienemann and Sprenger, 2004). Sections P and Q in Figure 3 indicate that the GFP signal appeared to be in both the cytoplasm and the nucleus in the tetrad, with a slightly higher level in the nucleolus. The subcellular localization pattern of the signal in the zygotene-pachytene microsporocytes (Fig. 3, D and G) appeared consistent with that in the tetrad, but it was difficult to clearly visualize the nuclei in the microsporocytes because the microsporocytes were tightly associated. To further detect the subcellular localization of CYCA1;2-GFP in pachytene microsporocytes, which contained the highest level of signal (Fig. 3), we looked for and recorded the GFP signal in separate but intact microsporocytes after dissection of the anther. Our data confirmed that the signal was in both the cytoplasm and nucleus in pachytene microsporocytes (Fig. 4).
Figure 4.
CYCA1;2-GFP is located in both the cytoplasm and nucleus. A, Unfixed microsporocyte showing the GFP signal. B, The differential interference contrast image corresponding to A. C, Spread chromosomes showing that the stage is pachytene. This cell and the cell in A were from separate medial anthers in the same bud. Arrows, Nucleus. Arrowheads, Nucleolus. Bar = 10 μm.
tam Plants Are Slowed in Progression of Both Pachytene and Meiosis II
Although we previously inferred that male meiosis in tam was slowed (Magnard et al., 2001), we had not determined precisely which meiotic stages were affected, or how much slowing occurred at each stage. To accurately determine when cell cycle slowing occurs in tam, we used two different methods to assess the durations of different cell cycle stages in tam, relative to those in wild type. First, we assumed that the duration of a stage would be positively correlated with the frequency of its occurrence, i.e. in randomly sampled buds, stages that last longer will be found more frequently, while those that are shorter will be found infrequently. This assumption requires that no cell cycle arrest occurs at any stage of male meiosis in tam, which was previously established (Magnard et al., 2001). We investigated the relative frequencies of four consecutive stages of meiosis: leptotene, zygotene, pachytene, and early diplotene to early cytokinesis. Early cytokinesis is early tetrad stage in wild type, but corresponds to early dyad stage in tam. The frequency at leptotene was set to 1. The results (Fig. 5) indicated that meiocytes in pachytene were found more frequently in tam than in wild type, suggesting that pachytene in tam is longer than that in wild type. This conclusion is consistent with the finding that CYCA1;2 was predominantly expressed in pachytene in prophase I.
Figure 5.
Distribution of buds at different stages of meiosis. The frequency of buds at leptotene is defined as 1 in both wild type and tam, and the frequencies relative to that of leptotene were calculated for the other stages. The wild-type sample included 42 buds and the tam sample 59 buds. Lep, Leptotene; Zyg, zygotene; Pac, pachytene; Dip, early diplotene; Cyt, early cytokinesis. Dip to cyt corresponds to early diplotene to early tetrad stage in wild type, or to early diplotene to early dyad stage in tam.
We next determined the durations of several phases of the meiotic cell cycle in wild type and tam using a technically challenging method. This method is also based on the synchrony of cell cycle progression in microsporocytes in medial anthers of the same bud. We carefully removed one medial anther at one time point and determined its meiotic stage by fixation and DAPI staining, and similarly determined the meiotic stage in another medial anther in the same bud at a later time point. The difference between the two time points thus defined the time needed to proceed from the early stage to the later stage. The extent of chromosome synapsis in the anthers was determined by spreading the chromosomes to distinguish late zygotene, pachytene, or early diplotene. To practice the technique for minimizing damage of the second anther during the removal of the first anther and to obtain preliminary estimates of timing, experiments on hundreds of buds were performed. The first and second anthers were chosen from the opposite sides of the bud to minimize the chances of damage to the second anther. Because tam plants flower about 1 week later than wild-type plants and have a more severe phenotype at 27°C, our formal analysis of the duration of each stage was carried out on 4- to 5-week-old wild-type plants and on -5 to 6-week-old tam plants, each maintained at 27°C.
Our data (Fig. 6) indicate that the duration of zygotene in both genotypes was essentially the same, whereas pachytene in tam was approximately 6.2 h, significantly longer than in wild type (4.5 h). Notably, the durations from zygotene to early cytokinesis in wild type and in tam were approximately the same (14 h), suggesting that the timing of cytokinesis was not affected in tam. The longer pachytene and the normal timing of cytokinesis apparently accounted for the initial formation of dyads in tam. Furthermore, these dyads did not become tetrads quickly. The duration of the second nuclear division in tam, i.e. the time required for early dyads to become early tetrads, was approximately 4.7 h (Fig. 6). In wild type, approximately 4.6 h were needed for transit from diplotene to early tetrad stage (Fig. 6). We also estimated that the duration from diplotene to early prophase II in wild type was approximately 3 h, thereby limiting the duration of the second nuclear division in wild type to about 1.6 h. Thus, the second nuclear division in tam was approximately 3 h longer than that in wild type. These data indicate that progression through both pachytene and meiosis II in tam is slowed.
Figure 6.
Durations of meiotic stages. Means ± ses are shown. Each mean ± se was derived from four to seven samples. Cyt2, early stage of the second cytokinesis observed only in tam. Cyt to Cyt2 corresponds essentially to the duration of the second nuclear division in tam male meiosis. Other abbreviations are the same as in Figure 5.
DISCUSSION
The Function of CYCA1;2 and the Relationship between Meiosis I and Meiosis II
We characterized the timing of expression and function of CYCA1;2 in Arabidopsis male meiosis. We demonstrated that CYCA1;2 is required for the normal progression of male meiosis but that CYCA1;2 (i.e. the CYCA1;2-GFP fusion protein) is only detectable in prophase I before telophase II. These results suggest that progression of meiosis II in Arabidopsis does not require significant up-regulation of CYCA1;2 during the meiosis I-meiosis II transition. Both the timing of expression of CYCA1;2 and its function in male meiosis support the idea that the meiosis I-meiosis II transition differs from the transition between two consecutive mitotic cell cycles. Perhaps meiosis I and meiosis II are more equivalent to one continuous cell cycle.
Redefining meiosis conceptually as one cell cycle may better explain what is known about the meiosis I-meiosis II transition. The meiosis I-meiosis II transition has been considered as a transition from one mitotic phase to another mitotic phase, i.e. an M/M transition (Iwabuchi et al., 2000), a wording that is itself somewhat inconsistent: if still an M-phase, how can it be a transition? The concept of continuity between meiosis I and meiosis II fits readily with the fact that chromosome duplication does not occur during this period, since this concept requires that the meiosis I-meiosis II transition be part of an extended M-phase. If we consider chromosome segregation, chromatids only finish segregation by telophase II, consistent with an end of a nuclear division, as defined in mitosis. Incomplete degradation of B-type cyclins during the meiosis I-meiosis II transition has been observed (Picard et al., 1996; Sakamoto et al., 1998; Iwabuchi et al., 2000; Taieb et al., 2001; Perez et al., 2002), consistent with the notion that the M-phase extends from meiosis I into meiosis II. Interestingly, the levels of mitogen-activated protein kinase, Polo-like kinase1, and cdc25 remained high from meiosis I to meiosis II in Xenopus oocytes (Kishimoto, 2003), as if there was only one continuous cell cycle. In yeast, it has been suggested that the role of the MEN (mitotic exit network) in meiosis is reduced so that only a moderate decline in cyclin-dependent kinase1 (Cdk1) activity occurs at the end of meiosis I (Stern, 2003). Even in the yeast mutants of SPO12, SLK19, CDC14, and ESP1, which contain a stabilized meiosis I spindle but do not form subsequent meiosis II spindles, chromosome behavior typical of meiosis II occurs on the meiosis I spindle (Buonomo et al., 2003; Marston et al., 2003). These lines of evidence suggest that the “two” M phases of meiosis likely proceed in an all-or-none fashion. Indeed, a naturally occurring cell cycle arrest between late meiosis I and early meiosis II has never been observed in diverse organisms (Russo et al., 1998). Another relevant observation is that a mutant with normal meiosis I but abnormal meiosis II has not been identified. Undoubtedly, meiosis I bears a special relationship with meiosis II.
Possible Mechanisms of Meiosis II Regulation by CYCA1;2
How does CYCA1;2, which is predominantly expressed in prophase I, regulate progression through both meiosis I and meiosis II? One possibility is that the microsporocytes might “inherit” the influence of CYCA1;2 for an extended period, including meiosis II, even when CYCA1;2 is no longer present at a significant level. Indeed, no significant amount of A-type cyclins has been found after meiotic prophase I in mammalian male meiosis (Wolgemuth et al., 2002). The timing of disappearance of CYCA1;2-GFP in Arabidopsis microsporocytes also agrees with the timing of disappearance of A-type cyclins in mammalian spermatocytes (Wolgemuth et al., 2002). Furthermore, the anaphase-promoting complex, which is known to target the degradation of mitotic cyclins in yeast and animals (Zachariae and Nasmyth, 1999), is also required for degradation of Arabidopsis cyclin A3 (Capron et al., 2003), suggesting that degradation of A-type cyclins is regulated by a mechanism that is conserved in eukaryotes.
The scenario of a prolonged effect of CYCA1;2 without its presence would require the persistence of phosphorylated substrates of the Cdk complex(es) in meiosis II. About 200 potential substrates of Cdk1 in budding yeast have been identified (Ubersax et al., 2003). Although little is known about the substrates of cyclin/Cdk complexes in plants, a large number of substrates are expected to exist. A mechanism for inactivating these substrates must exist in mitosis, and such inactivation might be suppressed to maintain the active substrates during meiosis I and meiosis II.
However, we cannot rule out yet an alternative scenario, i.e. that a very low level of CYCA1;2 in meiosis II could still be sufficient for normal progression of meiosis II. This scenario would require that meiosis II be much more sensitive to the levels of cyclin/Cdk activity than are meiosis I and mitosis. To determine which of the two scenarios is correct, a more accurate quantitative assessment of the dynamics of mitotic cyclins and their effects on the meiotic cell cycle progression would be needed.
tam Is Useful for Studying Cell Cycle Progression
Because cell cycle progression in tam plants is slowed but not arrested, tam provides a useful system for identifying other components that modulate the pace of cell cycle progression. For example, screens for mutants that enhance or suppress the delayed bolting and dyad formation seen in tam plants, or direct tests of candidate proteins by artificial up- or down-regulation in tam plants, might reveal how CYCA1;2 regulates cell cycle progression.
MATERIALS AND METHODS
Plants and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type and tam (Columbia ecotype) plants were grown in artificial soil (Metro-Mix 200, Scotts-Sierra Horticultural Products, Marysville, OH) in a Percival growth chamber (model AR-36L; Percival Scientific, Perry, IA) under a 16-h-light/8-h-dark regime at 17°C, 22°C, or 27°C, as specified in the text. Main inflorescences of 4- to 5-week-old wild-type plants and 5- to 6-week-old tam plants were used for determining the durations of meiotic stages. Plants of similar or older ages were used in the other experiments.
Mapping and Complementation of tam
The mapping procedure was as described (Magnard et al., 2001). The location of tam was narrowed down to a region delineated by cleaved-amplified polymorphic sequence marker ADH (Konieczny and Ausubel, 1993) and RFLP marker AGP15E (http://www.arabidopsis.org) on chromosome 1. Agrobacterium tumefaciens strain GV3101 and vector pPZP121 (Hajdukiewcz et al., 1994) were used for genetic transformation using the floral dip method (Clough and Bent, 1998).
RNA Preparation, PCR, RT-PCR, and Gene Cloning
Total RNA from various tissues were prepared using the RNeasy Plant Mini kit (Qiagen, Valencia, CA). The PCR and RT-PCR conditions were essentially as previously described (Y. Wang et al., 2004), except that longer extension times were used for amplification of longer PCR products. Primers for the CYCA1;2 expression analysis were 5′-TCTTCTTCGTCGAGAAATCTATCT3′, and 5′-TTACGAGATAAAGCAGGTTTTGGT. Primers for amplification of full-length CYCA1;2 cDNA were 5′-AACCCTAAATCTCACCGGAAAAC and 5′-TGCCTGACCCGACTGATGAATT. Other internal primers were also used for sequencing the cDNA. Primers for the genomic fragment used for tam complementation were 5′-AGAGTTAGAGCACGCGGTGTTT and 5′-TGCATGCATGCATGGTTGGACT. Primers for the genomic fragment for the GFP fusion were 5′-AGAGTTAGAGCACGCGGTGTTT and 5′-ACAATATGTACAATACACGAGGGT. Primers to amplify the GFP fragment from the CAMBIA vector 1302 (http://www.cambia.org/) were 5′-ATGGTAGATCTGACTAGTAAAGGA and 5′-TCACACGTGGTGGTGGTGGTG. RT-PCR was performed using the SuperScript One-Step RT-PCR kit (Invitrogen, Carlsbad, CA). Appropriate restriction enzyme sites were added to the primers for cloning the products. Long fragments were amplified with PfuTurbo DNA polymerase (Stratagene, La Jolla, CA).
Anther Removal for Determination of Durations of Meiotic Stages
An inflorescence on a plant was held with one hand while observing it under a dissecting microscope. One or two sepals of a meiotic-stage floral bud (estimated by size) were gently peeled back to expose the anthers, using a dissecting tool (a 30G1/2 syringe needle attached to a 1-mL syringe). Care was taken to avoid injuring the other anthers, although this was not an issue for the one being removed for observation of developmental stage. An exposed medial anther (easily recognized by height) was carefully removed from the bud using the needle. This anther was fixed immediately in paraformaldehyde and dissected as described (Yang and Ma, 2001). The plant being operated on was then returned to the growth chamber until it was time to remove the second medial anther. The second medial anther was selected from the side of the gynoecium opposite to that of the first anther.
Light Microscopy
Chromosome spreads, DAPI staining, and the microscope and digital camera settings used were as described (Y. Wang et al., 2004). A FITC-HYG filter cube (Nikon, Tokyo) was used for fluorescence microscopy for detection of the GFP signal. All digital images were processed using software SPOT version 2.2 and Adobe Photoshop 6.0.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Jessica Rivers and Aiko Mori for technical assistance, Enamul Huq for the pPZP121 plasmid for plant transformation, and David Meinke and Sheila Johnson-Brousseau for comments on the manuscript.
This work was supported by Oklahoma State University, by the Energy Center at the Environmental Institute, Oklahoma State University (grant to M.Y.), and by the U.S. Department of Agriculture Current Research Information System (grant no. 5335–21000–020–00D to S.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.051201.
References
- Armstrong SJ, Jones GH (2003) Meiotic cytology and chromosome behavior in wild-type Arabidopsis thaliana. J Exp Bot 54: 1–10 [DOI] [PubMed] [Google Scholar]
- Azumi Y, Liu D, Zhao D, Li W, Wang G, Hu Y, Ma H (2002) Homolog interaction during meiotic prophase I in Arabidopsis requires the SOLO DANCERS gene encoding a novel-cyclin-like protein. EMBO J 21: 3081–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo SBC, Rabitsch KP, Fuchs J, Gruber S, Sullivan M, Uhlmann F, Petronczki M, Toth A, Nasmyth K (2003) Division of the nucleolus and its release of CDC14 during anaphase of meiosis I depends on separase, SPO12, and SLK19. Dev Cell 4: 727–739 [DOI] [PubMed] [Google Scholar]
- Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, Lecureuil A, Guerche P, Kondorosi E, Scheres B, et al (2003) The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell 15: 2370–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caryl AP, Armstrong SJ, Jones GH, Franklin FC (2000) A homologue of the yeast HOP1 gene is inactivated in the Arabidopsis meiotic mutant asy1. Chromosoma 109: 62–71 [DOI] [PubMed] [Google Scholar]
- Castro A, Jaumot M, Verges M, Agell N, Bachs O (1994) Microsomal localization of cyclin A and cdk2 in proliferating rat liver cells. Biochem Biophys Res Commun 201: 1072–1078 [DOI] [PubMed] [Google Scholar]
- Chaubet-Gigot N (2000) Plant A-type cyclins. Plant Mol Biol 43: 659–675 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Couteau F, Belzile F, Horlow C, Grandjean O, Vezon D, Doutriaux MP (1999) Random chromosome segregation without meiotic arrest in both male and female meiocytes of a dmc1 mutant of Arabidopsis. Plant Cell 11: 1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Elzen N, Pines NJ (2001) Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol 153: 121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienemann A, Sprenger F (2004) Requirements of cyclin A for mitosis are independent of its subcellular localization. Curr Biol 14: 1117–1123 [DOI] [PubMed] [Google Scholar]
- Esau K (1976) Anatomy of Seed Plants, Ed 2. John Wiley & Sons, New York, pp 405
- Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T (2001) Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol 153: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P, Thomas BJ, DiNardo S (1994) roughex is a dose-dependent regulator of the second meiotic division during Drosophila spermatogenesis. Cell 77: 1015–1025 [DOI] [PubMed] [Google Scholar]
- Grelon M, Vezon D, Gendrot G, Pelletier G (2001) AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J 20: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewcz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Hochegger H, Klotzbücher A, Kirk J, Howell M, le Guellec K, Fletcher K, Duncan T, Sohail M, Hunt T (2001) New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development 128: 3795–3807 [DOI] [PubMed] [Google Scholar]
- Hunt T, Luca FC, Ruderman JV (1992) The requirement for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol 116: 707–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi M, Ohsumi K, Yamamoto TM, Sawada W, Kishimoto T (2000) Residual Cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J 19: 4513–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massagué J, Pavletich NP (1995) Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376: 313–320 [DOI] [PubMed] [Google Scholar]
- Kishimoto T (2003) Cell-cycle control during meiotic maturation. Curr Opin Cell Biol 15: 54–63 [DOI] [PubMed] [Google Scholar]
- Klimyuk VI, Jones JD (1997) AtDMC1, the Arabidopsis homologue of the yeast DMC1 gene: characterization, transposon-induced allelic variation and meiosis-associated expression. Plant J 11: 1–14 [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403–410 [DOI] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH (1989) Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell 56: 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chen C, Markmann-Mulisch U, Timofejeva L, Schmelzer E, Ma H, Reiss B (2004) The Arabidopsis AtRAD51 gene is dispensable for vegetative development but required for meiosis. Proc Natl Acad Sci USA 101: 10596–10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnard J-L, Yang M, Chen Y-CS, Leary M, McCormick S (2001) The Arabidopsis gene Tardy Asynchronous Meiosis is required for the normal pace and synchrony of cell division during male meiosis. Plant Physiol 127: 1157–1166 [PMC free article] [PubMed] [Google Scholar]
- Marston AL, Lee BH, Amon A (2003) The Cdc14 phosphatase and the FEAR network control meiotic spindle disassembly and chromosome segregation. Dev Cell 4: 711–726 [DOI] [PubMed] [Google Scholar]
- Masson JY, West SC (2001) The Rad51 and Dmc1 recombinases: a non-identical twin relationship. Trends Biochem Sci 26: 131–136 [DOI] [PubMed] [Google Scholar]
- Murray AW (2004) Recycling the cell cycle: cyclins revisited. Cell 116: 221–234 [DOI] [PubMed] [Google Scholar]
- Noble MEM, Endicott JA, Brown NR, Johnson LN (1997) The cyclin box fold: protein recognition in cell-cycle and transcriptional control. Trends Biochem Sci 22: 482–487 [DOI] [PubMed] [Google Scholar]
- Nurse PM (2002) Nobel lecture. Cyclin dependent kinases and cell cycle control. Biosci Rep 22: 487–499 [DOI] [PubMed] [Google Scholar]
- Okano-Uchida T, Sekiai T, Lee K, Okumura E, Tachibana K, Kishimoto T (1998) In vivo regulation of cyclin A/Cdc2 and cyclin B/Cdc2 through meiotic and early cleavage cycles in starfish. Dev Biol 197: 39–53 [DOI] [PubMed] [Google Scholar]
- Peirson BN, Bowling SE, Makaroff CA (1997) A defect in synapsis causes male sterility in a T-DNA-tagged Arabidopsis thaliana mutant. Plant J 11: 659–669 [DOI] [PubMed] [Google Scholar]
- Perez LH, Antonio C, Flament S, Vernos I, Nebreda AR (2002) Xkid chromokinesin is required for the meiosis I to meiosis II transition in Xenopus laevis oocytes. Nat Cell Biol 4: 737–742 [DOI] [PubMed] [Google Scholar]
- Picard A, Galas S, Peaucellier G, Doree M (1996) Newly assembled cyclin B-cdc2 kinase is required for suppress DNA replication between meiosis I and meiosis II in starfish oocytes. EMBO J 15: 3590–3598 [PMC free article] [PubMed] [Google Scholar]
- Puizina J, Siroky J, Mokros P, Schweizer D, Riha K (2004) Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell 16: 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KJ, Fransz P, Jones GH (1996) A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res 4: 507–516 [DOI] [PubMed] [Google Scholar]
- Russo GL, Wilding M, Marino M, Dale B (1998) Ins and outs of meiosis in ascidians. Semin Cell Dev Biol 9: 559–567 [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Takahara K, Yamashita M, Iwao Y (1998) Changes in cyclin B during oocyte maturation and early embryonic cell cycle in the newt, Cynops pyrrhogaster: requirement of germinal vesicle for MPF activation. Dev Biol 195: 60–69 [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern BM (2003) FEARless in meiosis. Mol Cell 11: 1123–1125 [DOI] [PubMed] [Google Scholar]
- Taieb FE, Gross SD, Lewellyn AL, Maller JL (2001) Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from meiosis I to II in Xenopus oocytes. Curr Biol 11: 508–513 [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864 [DOI] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14: 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kong H, Sun Y, Zhang X, Zhang W, Altman N, dePamphilis CW, Ma H (2004) Genome-wide analysis of the cyclin family in Arabidopsis and comparative phylogenetic analysis of plant cyclin-like proteins. Plant Physiol 135: 1084–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu H, Liang G, Yang M (2004) Defects in nucleolar migration and synapsis in male meiosis prophase I in the ask1-1 mutant of Arabidopsis. Sex Plant Reprod 16: 273–282 [Google Scholar]
- Wolgemuth DJ, Laurion E, Lele KM (2002) Regulation of the mitotic and meiotic cell cycles in the male germ line. Recent Prog Horm Res 57: 75–101 [DOI] [PubMed] [Google Scholar]
- Yang M, Ma H (2001) Male meiotic spindle lengths in normal and mutant Arabidopsis cells. Plant Physiol 126: 622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae W, Nasmyth K (1999) Whose end is destruction: cell division and anaphase-promoting complex. Genes Dev 13: 2039–2058 [DOI] [PubMed] [Google Scholar]