To The Editor
Cutaneous T cell lymphoma (CTCL) is commonly manifested as skin-restricted mycosis fungoides (MF) or Sézary syndrome (SS), a leukemic variant characterized by erythroderma and circulating malignant CD4+ T cells with features of Th2 cells (Kim et al., 2005).
TIGIT (T cell immunoreceptor with Ig and ITIM domains) is a recently identified co-inhibitory receptor expressed on the cell surface of activated or regulatory T cells, and NK cells. Emerging data suggests that TIGIT plays an inhibitory role by downregulating Th1 and Th17 while enhancing Th2 immune responses (Johnston et al., 2015; Joller et al., 2014; Kourepini et al., 2016; Kurtulus et al., 2015; Zhang et al., 2016). Helios is a transcription factor in the Ikaros family present in regulatory T cells. Like TIGIT, Helios has inhibitory activity and plays a role in anti-tumor immunity (Khaitan et al., 2016; Muto et al., 2015; Sebastian et al., 2016). The expression and role of Helios and TIGIT in SS has not been explored, but given the immunosuppressive nature of the disease, both molecules may potentially contribute to this process.
We reported high expression of FCRL3 on CD26− CD4+T cells in SS patients with high blood tumor burden. A microarray analysis of global gene expression that identified FCRL3, also showed significantly increased expression of Helios in CD4+ cells from SS patients with high tumor burden (data not shown) (Wysocka et al., 2014). A recent report linked the expression of FCRL3 with the expression of TIGIT and Helios (Bin Dhuban et al., 2015).
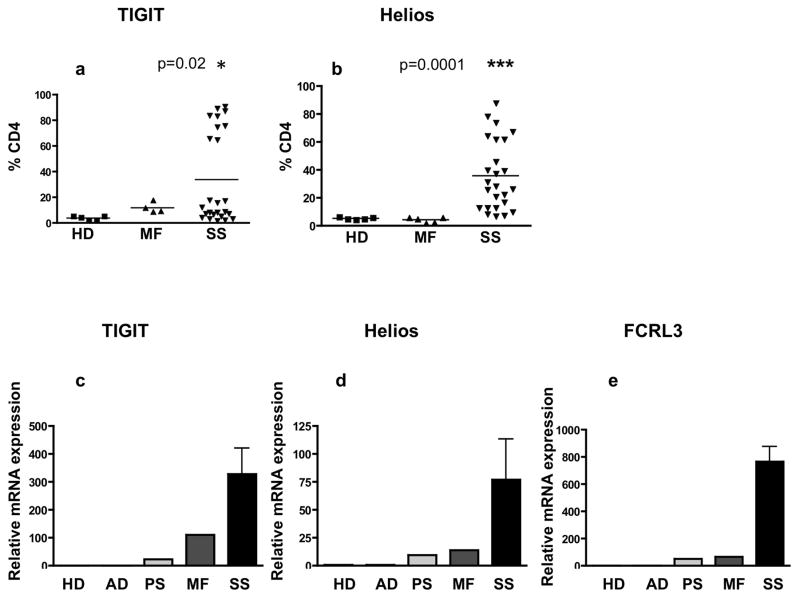
Our data show significantly increased percentages of TIGIT+ and Helios+ CD4+ T cells in patients with SS (mean: 33.6%, p=0.02, mean: 35.7%, p=0.0001, respectively) when compared to patients with MF and healthy donors (HDs) (Figure 1a,b,). TIGIT and Helios were also highly expressed in the skin of SS and MF patients compared with atopic dermatitis (AD), psoriasis (PS) and control skin (HD) (Figure 1c–e). TIGIT mRNA in skin from SS and MF patients was 327 and 110 fold increased, respectively, compared with levels in AD patients or control skin (Figure 1c). Similarly, Helios mRNA was increased in the skin of SS and MF patients, 77 and 13.6-fold, respectively, (Figure 1d), along with FCRL3, 765 and 68.3-fold for SS and MF, respectively, when compared to HD, (Figure 1e).
Figure 1. Helios, TIGIT and FCRL3 are highly expressed in skin and PBMC of Sézary syndrome patients.
(a,b) The cell surface expression of TIGIT (a) and intracellular expression of Helios (b) was assessed by flow cytometry in CD4+ T cells from SS patients (n=25) MF patients (n=5) and healthy donors HDs (n=5). Results are expressed with the mean. (c,d,e) Skin samples from 11 SS patients, 7 MF patients, 4 atopic dermatitis (AD), 3 psoriasis (PS) and 8 normal controls (HDs, pooled samples) were examined for mRNA expression of TIGIT (c), Helios (d) and FCRL3 (e) by qRT-PCR.
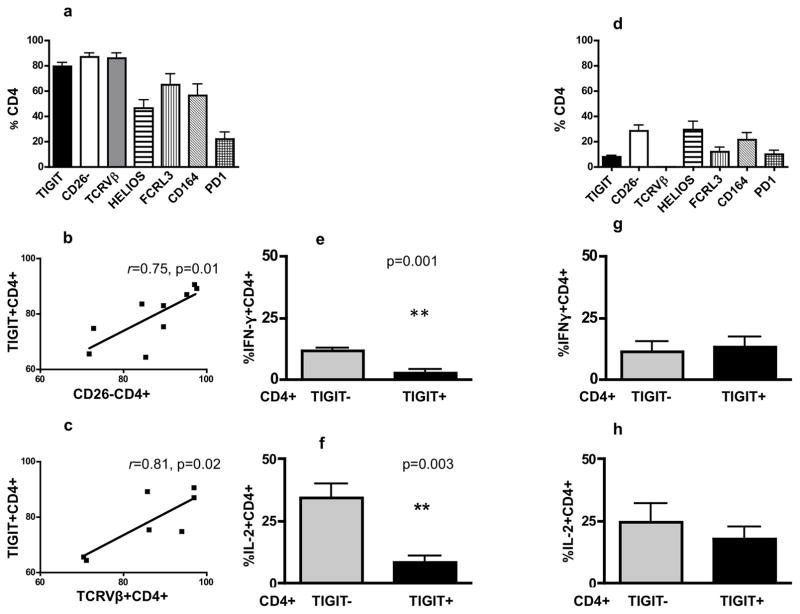
The data presented in Figure 1a, distinguishes patients with a high and low percentage of TIGIT+ CD4+ T cells. A thorough analysis of patients with high TIGIT expression (mean: 79.3%, SD: 9.7, Figure 2a) revealed that patients’ CD4+ T cells also demonstrate a high percentage of CD26 negative cells (mean: 87.1%, SD: 9.6), expresses a single TCRVβ (mean: 85.9%, SD: 11.3), highly express Helios (mean: 46.7%, SD: 19.4), FCRL3 (mean: 64.9% SD: 25.7), and CD164 (mean: 56.7%, SD: 26.6), but low PD1 (mean: 22.1%, SD: 15.8). Importantly, TIGIT expression correlated positively and significantly with CD26 negativity and with a single TCRVβ, (Figure 2b, c) but not with the expression of Helios, FCRL3 and CD164 due to the variability between patients.
Figure 2. High TIGIT expression correlates with the high percentage single TCRVβ+CD4+ T cells, loss of CD26 expression and is associated with impaired cytokines production.
(a) Peripheral blood mononuclear cells (PBMC) from patients with high TIGIT expression (n=9 including 7 patients with defined TCRVβ) were analyzed by flow cytometry to assess the expression of molecules on CD4+ T cells. (b,c) High TIGIT expression in CD4+ T cells correlates significantly with high percentage of CD26 negative (b, r=0.75, p=0.01, n=9) and single TCRVβ positive CD4+ T cells (c, r=0.81, p=0.02, n=7). (d) Flow cytometry analysis of PBMC from patients with low TIGIT expression to assess the expression of molecules on patients’ CD4+ T cells. In this group of patients, there is a significant decrease in the percentages of CD4+ T cells expressing tested molecules compared to percentages for corresponding molecules in patients with high TIGIT expression (p<0.05). (e,f) TIGIT+CD4+ T cells from patients with high TIGIT expression produce significantly less IFN-γ (e, p=0.001, n=6) and IL-2 (f, p=0.003, n=6) compared with TIGIT−CD4+ T cells from these patients. (g,h) In patients with low TIGIT expression there were no differences in levels of IFN-γ (g) and IL-2 (h) production between TIGIT+ and TIGIT− CD4+ T cells (n=6).
Patients with low expression of TIGIT on CD4+ T cells (mean: 7.9 %, SD: 5.12, Figure 2d) also demonstrate a low percentage of CD26 negativity (mean: 28.5%, SD: 18.7), no detectable single TCRVβ, low expression of Helios (mean: 29.6%, SD: 26.1), FCRL3 (mean: 11.9%, SD: 13.5), CD164 (mean: 21.5%, SD: 22.7) and PD1 (mean: 10.0%, SD: 12.6). This group of patients lacks a well defined cell surface malignant phenotype with the percentages of CD4+ T cells expressing the aforementioned molecules being significantly lower compared to high TIGIT expressing patients (p<0.05 for all tested molecules), implying that low TIGIT expressing patients have a low circulating tumor burden. We analyzed the clinical differences between the high and low TIGIT/Helios groups in terms of tumor burden in the blood, stage, and LDH and found that all patients in the high TIGIT group (n=9) were diagnosed with advanced B2 disease, whereas among 16 patients with low TIGIT expression, 10 patients were diagnosed as B0 (62.5%) and 6 patients were diagnosed as B1 (37.5%).
TIGIT is a known activation marker and is expressed on chronically activated, exhausted CD4+T cells (Chew et al., 2016; Le Mercier et al., 2015; Pauken and Wherry, 2014). Furthermore, as we demonstrate, it is highly expressed on CD26− TCRVβ+ CD4+T cells, defined as the malignant population in SS. We observed significantly decreased production of IFN-γ and IL-2 by TIGIT+ compared with TIGIT− CD4+T cells from patients with high TIGIT expression and advanced B2 disease (Figure 2e,f). This is consistent with the reported function of TIGIT positive, exhausted T cells and is strongly suggestive that TIGIT is associated with an immunosuppressive phenotype typically observed in advanced SS by being expressed on both exhausted and malignant CD4+ T cells (Joller et al., 2014; Kourepini et al., 2016; Kurtulus et al., 2015). In contrast to patients with high TIGIT expression, TIGIT+ CD4+ T cells from patients with low TIGIT expression produce IFN-γ and IL-2 at levels comparable to their TIGIT negative cells (Figure 2g, h). These results suggest that low TIGIT expression in these patients may be linked to activation status of CD4 T cells rather than to an exhausted phenotype.
The precise pathogenic nature of cells expressing TIGIT and Helios molecules in CTCL currently remains unknown. TIGIT+ and/or Helios+ CD4+ T-cells may represent: malignant cells, exhausted cells or non-conventional CD4+T regulatory cells, lacking Foxp3 and CD25 (data not shown). Malignant Foxp3+CD25− CD4+ T cells with suppressive activity have been identified in SS patients, further suggesting that the typical phenotype of T regulatory cells may be challenged in the context of CTCL (Heid et al., 2009).
High expression of TIGIT and Helios identifies CD4+ T cells with impaired immunological functions, primarily among patients with an advanced stage of Sézary syndrome, suggesting that high expression of these molecules may correlate with a poor prognosis. However, further studies are needed to define in detail the functional and biological significance of TIGIT and Helios expressing CD4+ T cells in SS and the potential use of these markers as therapeutic targets.
Acknowledgments
Donation of blood and skin samples by patients and HDs, with written informed consent conformed to the protocols approved by Institutional Review Board of the University of Pennsylvania and Washington University. This work was supported by a translational research grant from the Leukemia and Lymphoma Society (AHR), by a developmental project on grant 5P50CA174523-02 from the National Cancer Institute (AHR), National Institute of Arthritis and Musculoskeletal and Skin Diseases, K23-AR68433 to J.T., and K08-AR065577-03 to B.S.K., American Skin Association Research Grant to B.S.K., an R01 CA 132098 (LCS), and by the Penn Cutaneous Lymphoma Charitable Research Fund. Inquiries about presented work, particularly concerning clinical aspects of the work, should be addressed to Dr. Alain H. Rook: arook@mail.med.upenn.edu.
Abbreviations
- CTCL
Cutaneous T-cell Lymphoma
- SS
Sezary Syndrome
- MF
mycosis fungoides
- AD
Atopic Dermatitis
- PS
Psoriasis
- HDs
healthy donors
Footnotes
Conflict of Interest
The authors state no conflict of interest
References
- Bin Dhuban K, d’Hennezel E, Nashi E, Bar-Or A, Rieder S, Shevach EM, et al. Coexpression of TIGIT and FCRL3 identifies Helios+ human memory regulatory T cells. J Immunol. 2015;194:3687–96. doi: 10.4049/jimmunol.1401803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid JB, Schmidt A, Oberle N, Goerdt S, Krammer PH, Suri-Payer E, et al. FOXP3+CD25− tumor cells with regulatory function in Sezary syndrome. J Invest Dermatol. 2009;129:2875–85. doi: 10.1038/jid.2009.175. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Yu X, Grogan JL. The checkpoint inhibitor TIGIT limits antitumor and antiviral CD8 T cell responses. Oncoimmunology. 2015;4:e1036214. doi: 10.1080/2162402X.2015.1036214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–81. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaitan A, Kravietz A, Mwamzuka M, Marshed F, Ilmet T, Said S, et al. FOXP3+Helios+ regulatory T cells, immune activation and advancing disease in HIV infected children. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. I mmunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourepini E, Paschalidis N, Simoes DC, Aggelakopoulou M, Grogan JL, Panoutsakopoulou V. TIGIT Enhances Antigen-Specific Th2 Recall Responses and Allergic Disease. J Immunol. 2016;196:3570–80. doi: 10.4049/jimmunol.1501591. [DOI] [PubMed] [Google Scholar]
- Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053–62. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, et al. Clinical significance of expanded Foxp3(+) Helios(−) regulatory T cells in patients with non-small cell lung cancer. Int J Oncol. 2015;47:2082–90. doi: 10.3892/ijo.2015.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. TIGIT and CD226: tipping the balance between costimulatory and coinhibitory molecules to augment the cancer immunotherapy toolkit. Cancer Cell. 2014;26:785–7. doi: 10.1016/j.ccell.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios Controls a Limited Subset of Regulatory T Cell Functions. J Immunol. 2016;196:144–55. doi: 10.4049/jimmunol.1501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka M, Kossenkov AV, Benoit BM, Troxel AB, Singer E, Schaffer A, et al. CD164 and FCRL3 are highly expressed on CD4+CD26− T cells in Sezary syndrome patients. J Invest Dermatol. 2014;134:229–36. doi: 10.1038/jid.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhao W, Li H, Chen Y, Tian H, Li L, et al. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol Immunother. 2016;65:305–14. doi: 10.1007/s00262-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]