Relapse remains a major cause of treatment failure following allogeneic stem cell transplant (HSCT) for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) (1). Attempts at salvage following relapse include withdrawal of immunosuppression, chemotherapy, hypomethylating agents, donor lymphocyte infusions (DLI), and a second HSCT (2). Success rates are variable and no standard of care exists.
Graft-versus-host disease (GVHD) is an important limiting factor for further treatment following relapse. Given the low incidence of GVHD with T-cell depleted TCD-HSCT (3, 4), we postulated that our patient population represents an important model for potential trial eligibility. We conducted a single-center, retrospective analysis of patients who had disease recurrence following their TCD-HSCT for AML or MDS between January 2003 and November 2011 at Memorial Sloan Kettering Cancer Center (MSKCC), New York, USA, to determine their hypothetical eligibility for post-HSCT relapse management trials from clinicaltrails.gov. This review was performed under a waiver of authorization approved by the Institutional Review Board of MSKCC.
Forty of the 255 recipients of TCD-HSCT for AML or MDS, relapsed. All 40 patients had received myeloablative conditioning regimen for their initial transplant. Conditioning regimens included total body irradiation (TBI), thiotepa, fludarabine (n=5); TBI, thiotepa, cyclophosphamide (n=9); busulfan, melphalan, fludarabine (n=25); and 1 with clofarabine, melphalan, thiotepa. In 2 patients, bone marrow grafts were TCD by sequential soybean lectin agglutination and sheep red blood cell (sRBC)-rosette depletion. Thirty patients received peripheral blood stem cell grafts that had undergone CD34+ cell selection using the ISOLEX 300i system (Baxter, Deerfield, IL) followed by sRBC-rosette depletion and 8 underwent CD34+ cell selection using the CliniMACS CD34 Reagent System (Miltenyi etc).
Management of relapse was based on patients’ medical condition and at the discretion of the treating physician and patients’ preference, with 4 groups identified: supportive care (N=6), chemotherapy only (N=15), DLI or Wilms tumor (WT)-1 specific T cells (DLI, N=8, WT-1, N=2), and second HSCT (N=9). All patients in the DLI/WT-1 and the second HSCT groups received additional chemotherapy prior to subsequent cellular infusions. Two patients in the second HSCT group also received DLIs. Ten patients were treated with hypomethylating agents in the chemotherapy only group.
Characteristics of patients in the four groups were compared using Fisher’s exact test when categorical and the Kruskal-Wallis test when continuous. Overall survival (OS) time was calculated from the date of relapse post HSCT to the date of death. Patients still alive were censored at their last follow-up date. Kaplan-Meier methods were used to estimate survival probabilities. Due to the small sample size, a permutation-based log-rank test was used to evaluate differences in OS between groups. Univariable analysis for complete remission (CR)-1 at time of first HSCT, age ≥ 60 years old, Karnofsky-performance status (KPS) ≥80%, ≥ 27% blasts at relapse, and relapse beyond 5 months after initial HSCT was performed based on published results (5, 6). Statistical significance was defined as p<0.05. Statistical analyses were conducted using SAS v.9.4 software (SAS Institute, Cary, NC, USA) and R version 3.1.1 software (R Core Development Team, Vienna, Austria) including the ‘survival’ and ‘clinfun’ packages.
For the hypothetical trial eligibility portion we identified 11, and selected 5 ongoing or recently completed trials from clinicaltrials.gov: azacitidine alone (NCT00422890), cellular adoptive immunotherapy (NCT00107354), decitabine followed by second HSCT (NCT00002832), DLI and dendritic cells (NCT00476177), and azacitidine with DLI (NCT00795548). Trials were selected based on eligibility criteria, and usage of different modalities ranging from hypomethylating agents alone, to intensive chemotherapy, to cellular therapies and a second HSCT for relapse management. The major determinants of trial eligibility were age, KPS, hepatic and renal function, presence of GVHD, and sepsis at the time of relapse. None of these trials were randomized comparisons and none were available at MSKCC. Patient’s records were reviewed for trial eligibility based on inclusion and exclusion criteria associated with each trial listed on clinicaltrials.gov.
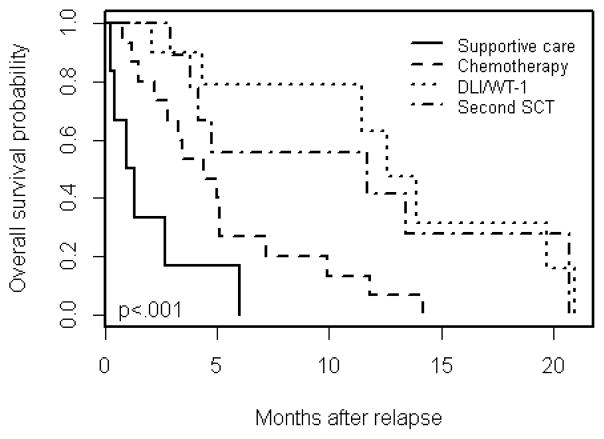
Thirty-two patients were transplanted for AML and 8 for MDS. Twenty-eight (70%) of the 40 patients underwent their first TCD-HSCT on a clinical trial at MSKCC. Upon relapse following the initial transplant, 7 patients were treated on a clinical trial: 4 received a 2nd transplant; 2 received WT-1 specific T-cells and 1 received chemotherapy on clinical trial followed by DLI. Ten patients received DLI (n = 7) or 2nd transplant (n = 3) off protocol. In the 40 total patients, the median follow-up time from relapse amongst survivors was 7.5 months (range, 4.2–16.4 months). During follow-up, 33 patients died of progressive disease, 1 of GVHD and 1 of progressive neurological disorder. Five patients were alive at the time of analysis; 2 in the second HSCT group and 3 in the DLI/WT-1 group. Median OS for all groups was 5.0 months (95% CI, 3.8–11.7). OS differed significantly by treatment group (p<0.001) (Figure 1). Median OS (95% CI) was 1.1, 4.4, 11.7, and 12.6 months among the supportive care, chemotherapy only, second HSCT, and DLI/WT-1 groups, respectively. At 12 months post-relapse, the OS probability (95% CI) was 0%, 6.7% (1.0%, 4.4%), 41.7% (18.5%, 94.0%), and 63.0% (36.3%, 100%) among the supportive care, chemotherapy only, second HSCT, and DLI/WT-1 groups, respectively. Univariable analysis did not show statistically significant improvement in OS for patients who were in CR at time of first TCD-HSCT (p=0.967), ≥ 60 years old (p=0.249), KPS ≥ 80% (p=0.152), or ≥ 27% blasts at relapse (p=0.501). Patients who relapsed beyond 5 months after initial TCD-HSCT had an improved OS post relapse (median 9.9 months vs median 2.7 months; p=0.001). Complete remission was achieved at any time post relapse in 1 patient in the DLI/WT1 group and 3 patients treated with a second HSCT. Of those, 2 remained in remission at the time of analysis, 1 in the DLI/WT-1 group and 1 the second HSCT group. Analysis of whether achievement of CR correlated with outcomes was not possible due to the small number of events.
Figure 1.
Overall survival by treatment group.
Table 1 summarizes the eligibility and exclusion criteria of the 5 selected trials. Three of the 40 patients would not have been eligible for any of these trials due to GVHD (N=1) and sepsis (N=2). Overall, 37/40 (92.5%) of patients were hypothetically eligible for at least one trial. Fifty percent were eligible for 3 or 4 trials. The most common reason for ineligibility was percentage of blasts or myelosuppression in 36 patients (90%) for pre-emptive azacitidine (NCT00422890), lack of available matched related donor (MRD) in 29 (72.5%) for adoptive cellular therapy (NCT00107354), and age greater than 60 in 15 (37.5%) for decitabine plus second HSCT trial (NCT00002832).
Table 1.
Selected clinical trials for eligibility from clinicaltrials.gov
| Trials | Azacitadine alone NCT00422890 |
Cellular adoptive immunotherapy NCT00107354 |
Decitabine plus second SCT NCT00002832 |
DLI plus Dendritic Cells NCT00476177 |
Azacitadine plus DLI NCT00795548 |
|---|---|---|---|---|---|
| Inclusion Criteria | Age>18 Chimerism <80% without hematologic relapse (<5% BM blasts) |
Age>14 KPS 60–100% MRD |
Age<60 ECOG PS 0–2 Relapsed within 12 months |
Age≥18 ECOG PS 0–2 >2months since HSCT |
Age>18 ECOG PS 0–2 Eligible for DLI |
| Exclusion Criteria | Uncontrolled infection Active HBV or HIV AST or ALT >3× normal CrCl<50ml/min WBC <3,000 Platelets <75,000 |
aGVHD grade III or IV cGVHD |
Uncontrolled infection aGVHD grade >2 Any cGVHD Cr >2mg/dl Bilirubin >3mg/dl EF <40% CNS disease |
Uncontrolled infection aGVHD grade II–IV Extensive cGVHD Prior DLI within 8 weeks On IS for <2 weeks prior to enrollment |
Uncontrolled infection Active HBV or HCV Bilirubin > 1.5 xULN GFR <50ml/min Childs Pugh B or C Severe CV disease CNS disease Treatment with other investigational agents after relapse |
KPS – Karnofsky performance status. ECOG PS – Eastern Cooperative Group Performance Status. HSCT – Hematopoietic Stem Cell Transplant. DLI – Donor Lymphocyte Infusion. GVHD – graft versus host disease. Cr – Creatinine. CrCl – Creatinine Clearance. EF – Ejection Fraction. CNS – central nervous system. IS – Immuno suppressions.
Similar to other studies (7–15) we observed an improved OS in patients who underwent a second HSCT and DLI/WT-1 as compared to patients who received supportive care or chemotherapy only. The only statistically significant factor affecting OS post-relapse was time to relapse > 5 months. Based on the standard factors determining trial eligibility such as KPS, renal/hepatic function, GVHD, and presence of sepsis, 92.5% of our patients were eligible for at least one trial, with 54% eligible for 3 to 4 trials. Furthermore, at relapse 88% of our patients had KPS ≥ 80%, 76% had a normal kidney and liver function, and 95% were free of uncontrollable infections. Although our patients would have qualified for a variety of trials, in actuality only 7 were enrolled to trials primarily to undergo a second HSCT or receive cellular therapy. The low number of enrollment in clinical trial, while partly due to patients’ medical condition, was also possibly due to the unavailability of such trials at our center. This emphasizes the importance of opening, and encouraging enrollment on clinical trials for patients with post-transplant relapse. Interestingly, of the 37 patients in this study who would have been ineligible for the pre-emptive azacitidine trial (NCT00422890), 16 received hypomethylating agents. This suggests that perhaps the eligibility criteria in some trials could be broadened.
Small number of patients, lack of a uniform management of relapse, and the retrospective nature of this single center study are its important limitations. Despite these, our study shows majority of patients to be potential candidates for enrollment on a clinical trial given low incidence of GVHD, preserved organ function, and good KPS, and, provides insight in the impact of an intensive treatment in post-transplant relapse setting. As we move forward in our understanding of relapse following HSCT, it is essential that we provide patients with the possibility of trial enrollment and long term disease free survival.
Footnotes
Author Contributions and Disclosure of Conflicts of Interest
P.B.D, and E.M, designed the study, collected, analyzed and interpreted the data and wrote the manuscript; E.C.Z and S.M.D performed the statistics and wrote the manuscript; M.A.P, M.M, H.C.M, R.J.O, E.B.P, and A.A.J wrote the manuscript; S.A.G designed the study and wrote the manuscript.
The authors have no relevant conflicts of interest to declare.
References
- 1.van den Brink MR, Porter DL, Giralt S, Lu SX, Jenq RR, Hanash A, et al. Relapse after allogeneic hematopoietic cell therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(1 Suppl):S138–45. doi: 10.1016/j.bbmt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollyea DA, Artz AS, Stock W, Daugherty C, Godley L, Odenike OM, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2007;40(11):1027–32. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083–90. [PubMed] [Google Scholar]
- 4.Jakubowski AA, Small TN, Kernan NA, Castro-Malaspina H, Collins N, Koehne G, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1343–51. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(4):458–68. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosing C, Saliba RM, Shahjahan M, Estey EH, Couriel D, Giralt S, et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone marrow transplantation. 2005;36(2):157–62. doi: 10.1038/sj.bmt.1705011. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy P, Potter VT, Barber LD, Kulasekararaj AG, Lim ZY, Pearce RM, et al. Outcome of donor lymphocyte infusion after T cell-depleted allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndromes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(4):562–8. doi: 10.1016/j.bbmt.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly RJ, Dao T, Koehne G, Scheinberg D, Doubrovina E. Adoptive transfer of unselected or leukemia-reactive T-cells in the treatment of relapse following allogeneic hematopoietic cell transplantation. Seminars in immunology. 2010;22(3):162–72. doi: 10.1016/j.smim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oran B, Giralt S, Couriel D, Hosing C, Shpall EJ, de Meis E, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia. 2007;21(12):2540–4. doi: 10.1038/sj.leu.2404828. [DOI] [PubMed] [Google Scholar]
- 11.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6):1599–606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 12.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(2):405–12. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 13.Warlick ED, DeFor T, Blazar BR, Burns L, Verneris MR, Ustun C, et al. Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(3):480–6. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Furst S, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leukemia & lymphoma. 2013;54(6):1228–34. doi: 10.3109/10428194.2012.741230. [DOI] [PubMed] [Google Scholar]
- 15.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]