Abstract
BACKGROUND/OBJECTIVES
Osteoporosis affects approximately 2 million men in the U.S., however, few osteoporosis clinical studies include men. The objective of this study was to evaluate the evidence for efficacy of treatment options to reduce osteoporotic fracture risk for men.
DESIGN
Systematic review and meta-analysis.
DATA SOURCES
PubMed, Embase, and Cochrane Library databases.
STUDY SELECTION
Randomized clinical trials that evaluated the efficacy of a treatment for osteoporosis or low bone mineral density for adult male participants and reported fracture outcomes.
DATA EXTRACTION
Information extracted included participant sociodemographic characteristics; number of male participants; treatment/intervention(s) and comparator evaluated; study duration; and fracture outcome(s). Risk of bias of individual studies was assessed using measures recommended by the Cochrane Collaboration.
RESULTS
Twenty-four articles reporting results for 22 different studies (including 4868 male participants) met strict inclusion criteria. Fixed-effects meta-analyses using the Mantel-Haenszel method demonstrated significantly reduced risk of vertebral fractures with alendronate (RR 0.328, 95% CI 0.155–0.692) and risedronate (RR 0.428, 95% CI 0.245–0.746), but not with calcitonin (RR 0.272, 95% CI 0.046–1.608) or denosumab (RR 0.256, 95% CI 0.029–2.238). For bisphosphonates as a treatment category, meta-analyses demonstrated significantly reduced risk of vertebral fractures (RR 0.368, 95% CI 0.252–0.537) and nonvertebral fractures (RR 0.604, 95% CI 0.404–0.904). The meta-analysis finding that bisphosphonates significantly reduce nonvertebral fracture risk was not robust to sensitivity analysis.
CONCLUSION
Bisphosphonates reduce the risk of vertebral and possibly nonvertebral fractures for men with osteoporosis. Further studies are needed to evaluate the efficacy of bisphosphonates for reducing nonvertebral fracture risk and the efficacy of non-bisphosphonates for reducing vertebral and nonvertebral fracture risk for men with osteoporosis.
Keywords: osteoporosis, fractures, systematic review, meta-analysis
INTRODUCTION
Osteoporosis affects 2 million men in the United States,1 and approximately 1 in 5 white men will sustain an osteoporotic fracture in his lifetime.2 Men are estimated to incur 29% of all osteoporotic fractures and account for 25% of total osteoporosis-related costs.3 Furthermore, men have higher mortality rates after hip fracture than women, with nearly 1 in 3 men over the age of 65 years who incur a hip fracture dying within the following year.4 The morbidity, mortality, and costs secondary to osteoporotic fractures in the U.S. are considerable and likely to increase in upcoming years with the aging of the population.2–9
Despite the prevalence of osteoporosis among older men and potential severity of its health consequences, osteoporosis in men is significantly understudied compared with women; most osteoporosis clinical studies to date have not included male participants.10 FDA-approved osteoporosis treatment options for men include alendronate, risedronate, zoledronic acid, teriparatide, and denosumab, and osteoporosis treatment for men is recommended by several organizations.11,12 We performed a systematic review and meta-analysis of the evidence for fracture risk reduction for different osteoporosis treatment options for men.
METHODS
Data sources and search strategies
We developed broad literature search strategies for PubMed, Embase, and Cochrane Library databases to locate randomized clinical trials reporting on the efficacy of osteoporosis treatment options. We performed initial literature searches on 8/15/14 for Embase; 8/28/14 for PubMed; and 11/28/14 for the Cochrane Library. The PubMed search was updated on 3/18/16. The database search strategies are available upon request. We identified additional studies by reviewing the reference lists of topical review papers and studies meeting our inclusion criteria as well as studies identified by experts.
Study selection
We applied inclusion and exclusion criteria to literature identified with the search strategies to select studies of interest. We included studies that evaluated the efficacy of a treatment for adults with osteoporosis or low bone mineral density (BMD); were randomized clinical trials; reported separate data for male participants or had male participants only; and reported fracture outcomes, with provision of either numbers or percentages of men in each study group who sustained incident fractures. We included studies published in any language, and had no restrictions on study participant comorbidities. We excluded studies in which not all participants were identified as having osteoporosis or low BMD (T-score ≤–1). We evaluated studies for inclusion in two stages – we first reviewed titles and abstracts, followed by full-text review of studies that were identified as potentially relevant after title/abstract review.
Data extraction
Information extracted from eligible studies included study participant sociodemographic characteristics; number of male participants; study location; treatment/intervention(s) evaluated; comparator for evaluated treatment/intervention; duration of study/follow-up period for fracture outcomes; fracture outcome(s) evaluated; and results reported for fracture outcomes in intervention and comparator groups. For fracture outcomes, we extracted data on numbers of participants in the intervention and comparator groups who sustained incident fractures.
Data analysis
We qualitatively described included study characteristics and study quality. For study quality assessment, we used measures recommended by the Cochrane Collaboration for assessing risk of bias for individual studies, including criteria to evaluate risk of selection bias, performance bias, detection bias, attrition bias, and reporting bias.13 We also performed fixed-effects meta-analyses using the Mantel-Haenszel method14 to calculate summary relative risk of fracture estimates for each individual treatment option for which there were at least two studies with similar comparators and fracture outcomes assessed; and for bisphosphonates when considered as a treatment category. Between-study heterogeneity in each performed meta-analysis was assessed with I2 values. For studies that reported fracture outcomes for multiple follow-up time periods, we used the fracture outcomes reported for the longest follow-up time period when performing meta-analyses. For meta-analyses that included 3 or more studies, we also performed influence (sensitivity) analysis in which we excluded individual studies one at a time to assess whether meta-analysis findings were robust to exclusion of individual studies. We used Stata version 11.0 (StataCorp, College Station, TX) to perform these analyses.
RESULTS
Literature search and study selection
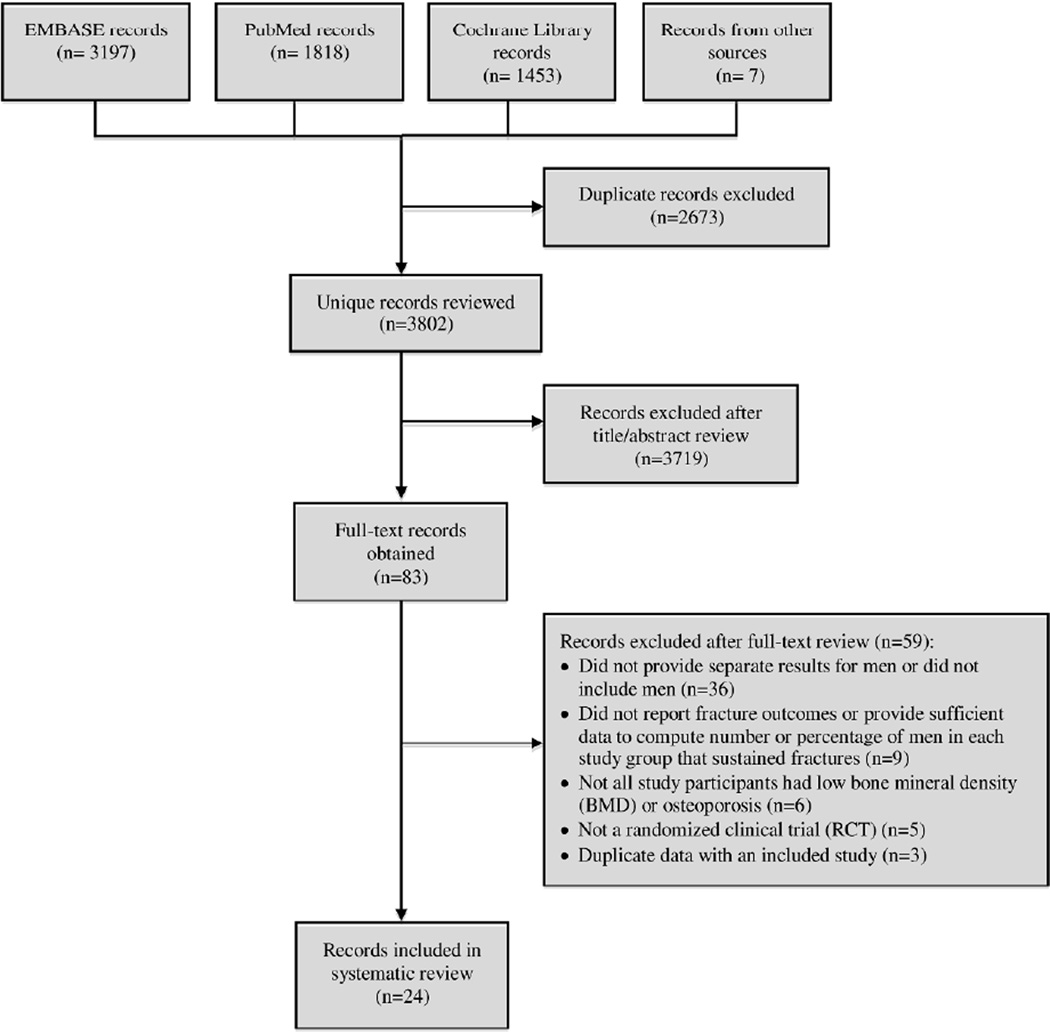
The literature searches identified a total of 6475 records (citations) for review; 2673 of these records were excluded because they were duplicates (same citation found in different databases), leaving 3802 unique records for review. Twenty-four of these records reporting results for 22 different studies met inclusion criteria.15–38 Figure 1 shows a flow diagram of the literature search and study selection.
Figure 1.
Flow diagram of literature search and study selection
Study characteristics
Supplementary Table S1 shows included study characteristics. Included studies were published between 1998 and 2013, number of male study participants ranged from 23 to 1199, and study duration ranged from 1 to 3 years. Approximately half of the included studies evaluated the efficacy of bisphosphonate medications, with more studies assessing alendronate or zoledronic acid than other bisphosphonates. Most included studies compared a treatment option to placebo and/or calcium and vitamin D only; very few had active comparators. A majority of studies included only men with primary osteoporosis and/or hypogonadal osteoporosis, and did not include men with other causes of secondary osteoporosis. Many of the included studies had largely white study participant populations. Commonly assessed osteoporosis fracture outcomes included all vertebral fractures (morphometric – detected by x-rays, including asymptomatic as well as symptomatic vertebral fractures); clinical vertebral fractures (meaning symptomatic vertebral fractures only); nonvertebral fractures; and clinical fractures (clinically symptomatic fractures at any site). Vertebral fractures were the most commonly assessed fracture outcomes, followed by nonvertebral fractures and clinical fractures.
Only one included study, a study by Boonen et al. evaluating zoledronic acid therapy, reported having sufficient statistical power for fracture outcomes for men, for the outcome of morphometric vertebral fractures.36 Only 4 studies reported relative risk, odds ratio, or hazard ratio of fracture for men in the intervention groups compared to the comparator groups;17,18,29,30,35,36 of these, 3 studies evaluating alendronate, risedronate, and zoledronic acid reported a statistically significant reduction in risk of fracture for men receiving the evaluated intervention/treatment compared to the comparator; all for the outcome of vertebral fractures,18,29,30,36 and only one study evaluating risedronate for the outcome of nonvertebral fractures.30 Only two included studies performed head-to-head comparisons of drugs that are FDA-approved for men – one study that compared teriparatide to alendronate,33 and another study that compared zoledronic acid to alendronate;38 neither of these studies reported relative risk for fracture outcomes. A majority of studies reported pharmaceutical company funding.15,16,19,21–25,27,28,31,33–36,38
Study quality and potential sources of bias
Supplementary Table S2 shows findings of our assessment of included study quality using criteria recommended by the Cochrane Collaboration for assessing risk of bias for individual studies. In general, studies did not sufficiently describe their methods of random sequence generation, allocation concealment, and whether all pre-specified outcomes in the study protocol were reported on in the pre-specified way to permit judgment of “low risk” or “high risk” of bias for these domains. Thus, most studies were assessed as “unclear risk” of bias for the categories of selection bias and reporting bias. A majority of studies reported blinding of fracture outcome assessment, and thus were assessed as low risk for detection bias. Studies were mixed with respect to domains of reporting of blinding of participants and personnel, as well as incomplete outcome data, which fall within categories of performance bias and attrition bias, respectively. No included study was assessed as low risk of bias for all evaluated domains, and thus no study received a summary assessment of low risk of bias. A slight majority of studies received a summary assessment of high risk of bias (due to at least one bias domain being assessed as high risk of bias), with the remainder of the studies receiving a summary assessment of unclear risk of bias.
Meta-analyses
Sufficiently similar studies were available for separate meta-analyses for the outcome of vertebral fractures for alendronate, calcitonin, denosumab, and risedronate; for the outcome of nonvertebral fractures for alendronate; and for the outcome of clinical fractures with zoledronic acid. When considering bisphosphonates as a treatment category, sufficiently similar studies were available for outcomes of vertebral fractures, clinical vertebral fractures, nonvertebral fractures, and clinical fractures. Meta-analysis results are shown in Table 1. Forest plots for the meta-analyses are shown in Supplementary Figures S1–S10.
Table 1.
Meta-Analysis Results
| Treatment | Included studies | Summary estimates of fracture relative risk (RR) from meta-analysisa (95%CI; I2 valueb) |
|---|---|---|
| Alendronate | Orwoll 200016,c & Ringe 200418 | Vertebral fractures: 0.328 (0.155–0.692; I2=29.4%); Nonvertebral fractures: 0.751 (0.352–1.602); I2=0.0%) |
| Calcitonin | Toth 200520 & Trovas 200221 | Vertebral fractures: 0.272 (0.046–1.608; I2=0.0%) |
| Denosumab | Nakamura 201323 & Orwoll 201224 | Vertebral fractures: 0.256 (0.029–2.238; I2=0.0%) |
| Risedronate | Boonen 200928 & Ringe 200930 | Vertebral fractures: 0.428 (0.245–0.746; I2=27.4%) |
| Zoledronic acid | Boonen 201135 & Boonen 201236 | Clinical fractures: 0.742 (0.436–1.263; I2=0.0%) |
| Any bisphosphonate (alendronate, ibandronate, risedronate, or zoledronic acid) |
Boonen 200928, Boonen 201236, Orwoll 200016, Orwoll 201025, Ringe 200418, Ringe 200930 (vertebral fractures meta-analysis); Boonen 201236, Orwoll 200016, Orwoll 201025 (clinical vertebral fractures meta-analysis)d; Boonen 201236, Orwoll 200016, Ringe 200418, Ringe 200930 (nonvertebral fractures meta-analysis); Boonen 200928, Boonen 201135, Boonen 201236, Orwoll 201025 (clinical fractures meta-analysis) |
Vertebral fractures: 0.368 (0.252–0.537; I2=0.0%); Vertebral fractures (clinical only): 0.398 (0.105–1.506; I2=0.0%); Nonvertebral fractures: 0.604 (0.404–0.904; I2=0.0%); Clinical fractures: 0.791 (0.500–1.253; I2=0.0%) |
Fixed-effects meta-analysis using Mantel-Haenszel method
Percentage of variation across studies attributable to heterogeneity
For Orwoll 2000 study, vertebral fracture results reported when using quantitative assessment method were used for meta-analysis
Shimon 2005 study excluded from analysis due to no fracture events in intervention or comparator groups
For individual treatment options, the meta-analyses findings demonstrated significantly reduced risk of vertebral fractures with alendronate (RR 0.328, 95% CI 0.155–0.692) and risedronate (RR 0.428, 95% CI 0.245–0.746), but not with calcitonin (RR 0.272, 95% CI 0.046–1.608) or denosumab (RR 0.256, 9 5% CI 0.029–2.238). The meta-analyses findings for individual treatment options did not demonstrate significantly reduced risk of nonvertebral fractures with alendronate (RR 0.751, 95% CI 0.352–1.602) or clinical fractures with zoledronic acid (RR 0.742, 95% CI 0.436–1.263). When considering bisphosphonates as a treatment category, meta-analyses findings demonstrated significantly reduced risk of vertebral fractures (RR 0.368, 95% CI 0.252–0.537) and nonvertebral fractures (RR 0.604, 95% CI 0.404–0.904), but not clinical vertebral fractures (RR 0.398, 95% CI 0.105–1.506) or clinical fractures (RR 0.791, 95% CI 0.500–1.253). Between-study heterogeneity in each performed meta-analysis was low as demonstrated by low I2 values.
The meta-analysis finding that bisphosphonates significantly reduce risk of vertebral fractures was robust to influence analysis, with summary estimates of relative risk of vertebral fractures with bisphosphonate therapy ranging from 0.353–0.391 with removal of individual studies, and the lower limit of the 95% CI ranging from 0.215–0.265 and the upper limit of the 95% CI ranging from 0.518–0.594 with removal of individual studies. However, the finding that bisphosphonates significantly reduce risk of nonvertebral fractures was sensitive to removal of the study by Ringe et al. in 2009 that evaluated risedronate therapy for men30 – when this study was removed from the analysis, the summary estimate for relative risk of nonvertebral fractures with bisphosphonate therapy was 0.715, with a 95% CI of 0.382–1.337. The meta-analysis findings of nonsignificant reduction in the relative risk of clinical vertebral fractures or clinical fractures with bisphosphonates were robust to influence analysis, with results remaining nonsignificant with removal of any individual study in either analysis.
DISCUSSION
Relatively few randomized clinical trials have been performed to date to assess efficacy of osteoporosis treatment options for reducing fracture risk for men. Our meta-analysis findings for individual treatment options demonstrated that alendronate and risedronate significantly reduce risk of vertebral fracture for men; however, our meta-analyses for individual treatment options did not demonstrate evidence of statistically significant reduction in vertebral fracture risk for men with calcitonin or denosumab, nonvertebral fracture risk for men with alendronate, or clinical fracture risk for men with zoledronic acid. Our meta-analyses findings for bisphosphonates when considered as a treatment category demonstrated significantly reduced risk of vertebral fractures and nonvertebral fractures, but not clinical vertebral fractures or clinical fractures. Our results for significant reduction in the relative risk of nonvertebral fractures with bisphosphonate therapy were sensitive to the removal of the study by Ringe et al. in 2009 that demonstrated significantly reduced risk of nonvertebral fracture for men with risedronate therapy.30 There were insufficient data to perform meta-analyses for the efficacy of calcitriol, monoflourophosphate, parathyroid hormone, strontium ranealate, or teriparatide for reducing fracture risk for men.
Our meta-analyses were limited by the number of similar studies assessing each medication, with only 2 studies included in each separate meta-analysis of individual medications that we performed, and 3–6 studies included in each separate meta-analysis we performed for different fracture outcomes when evaluating bisphosphonates as a treatment category. Additionally, many of the included studies in our meta-analyses had small sample sizes. Furthermore, although one included study demonstrated significant reduction in morphometric vertebral fracture risk with zoledronic acid treatment,36 there were not two studies similar enough to perform a meta-analysis for the efficacy of zoledronic acid treatment on fracture outcomes for men. Moreover, our systematic review and meta-analysis findings are limited by the caveat that all studies included in our systematic review and meta-analysis were assessed as having unclear or high risk of bias. However, despite these limitations our findings suggest that in the absence of additional evidence bisphosphonates should preferentially be used as first-line osteoporosis treatment for men given evidence for their efficacy in reducing vertebral fracture risk, and possibly nonvertebral fracture risk as well. Our findings for the evidence of bisphosphonate efficacy to reduce fracture risk for men apply to individuals who have osteoporosis or low BMD by DXA criteria, or who have had a prior osteoporotic fracture, as the studies included in this systematic review and meta-analysis included participants who met these criteria. Further studies are needed to evaluate the efficacy of osteoporosis treatment for men with risk factors for fracture who are not known to have osteoporosis or low BMD by DXA criteria or prior osteoporotic fracture.
Our study highlights the need for additional high-quality, sufficiently powered for fracture outcomes randomized clinical studies of osteoporosis treatment efficacy for men, particularly for nonvertebral fracture outcomes, and for non-bisphosphonate treatment options such as denosumab or teriparatide. Our findings also highlight the lack of active comparator randomized clinical trials of osteoporosis treatment for men; additional studies of osteoporosis treatment for men with active comparators would help clarify the relative efficacy of different treatment options for reducing fracture risk. Additionally, our findings reveal the need for greater diversity of participants in clinical trials of osteoporosis treatment for men; most included studies in our systematic review had largely white study participant populations. Finally, no study included in our systematic review had a duration greater than 3 years, and thus the efficacy of longer osteoporosis treatment durations to reduce fracture risk for men is unknown – additional studies with longer durations would be helpful to evaluate the impact of osteoporosis treatment for longer durations than 3 years on fracture risk for men, similar to longer duration osteoporosis treatment studies that have demonstrated fracture risk reduction benefit for women.39
Our study has several notable strengths. Our study is the most comprehensive systematic review and meta-analysis of randomized clinical trials of osteoporosis treatment efficacy for reducing fracture risk for men to date. A prior systematic review and meta-analysis on the topic of osteoporosis treatment efficacy for men by Schwarz et al. published in 2011 included only five studies that reported fracture outcomes, and concluded that the evidence for osteoporosis treatment for men to reduce fracture risk was inconclusive.40 Our systematic review and meta-analysis, which included 22 randomized clinical studies of osteoporosis treatment for men that reported fracture outcomes, finds evidence for the efficacy of bisphosphonate medications for reducing risk of vertebral fractures and possibly nonvertebral fractures for men. Another strength of our study was the assessment of risk of bias of individual randomized clinical trials included in our systematic review using criteria recommended by the Cochrane Collaboration.
In conclusion, our findings support the use of bisphosphonates to reduce vertebral and possibly nonvertebral fracture risk for men with osteoporosis. Further studies are needed to evaluate the efficacy of bisphosphonates for reducing nonvertebral fracture risk for men, and to evaluate the efficacy of non-bisphosphonate treatment options such as denosumab or teriparatide to reduce vertebral and nonvertebral fracture risk for men.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Nayak was supported by grant R01AR060809 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and a Swedish Foundation Philanthropy at Work award; Dr. Greenspan was supported by NIH grant P30AG024827 from the National Institute on Aging.
Dr. Greenspan is on the scientific advisory board for Merck & Co.
Sponsor’s Role: The sponsors had no role in the design, methods, data collection, analysis, or preparation of the paper.
Additional Contributions: The authors thank Eric S. Orwoll, MD and Arthur C. Santora II, MD, PhD, for providing requested data.
Appendix
Conflict of Interest Checklist:
| Elements of Financial/Personal Conflicts |
SN | SLG | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Employment or Affiliation | X | X | ||
| Grants/Funds | X | X | ||
| Honoraria | X | X | ||
| Speaker Forum | X | X | ||
| Consultant | X | X | ||
| Stocks | X | X | ||
| Royalties | X | X | ||
| Expert Testimony | X | X | ||
| Board Member | X | X | ||
| Patents | X | X | ||
| Personal Relationship | X | X | ||
For “yes”, provide a brief explanation: Dr. Greenspan is on the scientific advisory board for Merck & Co.
Footnotes
Conflict of Interest Statement: Dr. Greenspan is on the scientific advisory board for Merck & Co. Dr. Nayak has no conflicts of interest.
Author Contributions: Study concept and design: SN and SG. Acquisition, analysis, or interpretation of data: SN and SG. Data interpretation: SN and SG. Drafting manuscript: SN. Critical revision of the manuscript for important intellectual content: SN and SG. Approving final version of manuscript: SN and SG.
REFERENCES
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014 Nov;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. Bone Health and Osteoporosis: A Report of the Surgeon General. [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007 Mar;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Bass E, French DD, Bradham DD, et al. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007 Jul;17(7):514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Brenneman SK, Yurgin N, Fan Y. Cost and management of males with closed fractures. Osteoporos Int. 2013 Mar;24(3):825–833. doi: 10.1007/s00198-012-2067-x. [DOI] [PubMed] [Google Scholar]
- 6.MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord. 2003 Oct 31;4:24. doi: 10.1186/1471-2474-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orsini LS, Rousculp MD, Long SR, et al. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005 Apr;16(4):359–371. doi: 10.1007/s00198-004-1694-2. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz-Alonso FJ, Vidan-Astiz M, Alonso-Armesto M, et al. The pattern of recovery of ambulation after hip fracture differs with age in elderly patients. J Gerontol A Biol Sci Med Sci. 2012 Jun;67(6):690–697. doi: 10.1093/gerona/glr231. [DOI] [PubMed] [Google Scholar]
- 9.Venmans A, Klazen CA, Lohle PN, et al. Natural history of pain in patients with conservatively treated osteoporotic vertebral compression fractures: results from VERTOS II. AJNR Am J Neuroradiol. 2012 Mar;33(3):519–521. doi: 10.3174/ajnr.A2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haney EM, Bliziotes MM. Male osteoporosis: new insights in an understudied disease. Curr Opin Rheumatol. 2008 Jul;20(4):423–428. doi: 10.1097/BOR.0b013e3283025eb0. [DOI] [PubMed] [Google Scholar]
- 11.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014 Oct;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012 Jun;97(6):1802–1822. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Smith GD, Altman DG. In: Systematic reviews in health care : meta-analysis in context. 2nd. Egger Matthias, Smith George Davey, Altman Douglas G., editors. London: BMJ Books; 2001. (2003 [printing]) [Google Scholar]
- 15.Miller PD, Schnitzer T, Emkey R, et al. Weekly oral alendronic Acid in male osteoporosis. Clin Drug Investig. 2004;24(6):333–341. doi: 10.2165/00044011-200424060-00003. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000 Aug 31;343(9):604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 17.Ringe JD, Faber H, Dorst A. Alendronate treatment of established primary osteoporosis in men: results of a 2-year prospective study. J Clin Endocrinol Metab. 2001 Nov;86(11):5252–5255. doi: 10.1210/jcem.86.11.7988. [DOI] [PubMed] [Google Scholar]
- 18.Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004 Mar;24(2):110–113. doi: 10.1007/s00296-003-0388-y. [DOI] [PubMed] [Google Scholar]
- 19.Shimon I, Eshed V, Doolman R, et al. Alendronate for osteoporosis in men with androgen-repleted hypogonadism. Osteoporos Int. 2005 Dec;16(12):1591–1596. doi: 10.1007/s00198-005-1879-3. [DOI] [PubMed] [Google Scholar]
- 20.Toth E, Csupor E, Meszaros S, et al. The effect of intranasal salmon calcitonin therapy on bone mineral density in idiopathic male osteoporosis without vertebral fractures--an open label study. Bone. 2005 Jan;36(1):47–51. doi: 10.1016/j.bone.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Trovas GP, Lyritis GP, Galanos A, et al. A randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markers. J Bone Miner Res. 2002 Mar;17(3):521–527. doi: 10.1359/jbmr.2002.17.3.521. [DOI] [PubMed] [Google Scholar]
- 22.Ebeling PR, Wark JD, Yeung S, et al. Effects of calcitriol or calcium on bone mineral density, bone turnover, and fractures in men with primary osteoporosis: a two-year randomized, double blind, double placebo study. J Clin Endocrinol Metab. 2001 Sep;86(9):4098–4103. doi: 10.1210/jcem.86.9.7847. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Matsumoto T, Sugimoto T, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014 Jul;99(7):2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orwoll E, Teglbjaerg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012 Sep;97(9):3161–3169. doi: 10.1210/jc.2012-1569. [DOI] [PubMed] [Google Scholar]
- 25.Orwoll ES, Binkley NC, Lewiecki EM, et al. Efficacy and safety of monthly ibandronate in men with low bone density. Bone. 2010 Apr;46(4):970–976. doi: 10.1016/j.bone.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Ringe JD, Dorst A, Kipshoven C, et al. Avoidance of vertebral fractures in men with idiopathic osteoporosis by a three year therapy with calcium and low-dose intermittent monofluorophosphate. Osteoporos Int. 1998;8(1):47–52. doi: 10.1007/s001980050047. [DOI] [PubMed] [Google Scholar]
- 27.Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000 Sep;85(9):3069–3076. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 28.Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res. 2009 Apr;24(4):719–725. doi: 10.1359/jbmr.081214. [DOI] [PubMed] [Google Scholar]
- 29.Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006 Mar;26(5):427–431. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 30.Ringe JD, Farahmand P, Faber H, et al. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. 2009 Jan;29(3):311–315. doi: 10.1007/s00296-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman JM, Audran M, Bianchi G, et al. Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab. 2013 Feb;98(2):592–601. doi: 10.1210/jc.2012-3048. [DOI] [PubMed] [Google Scholar]
- 32.Ringe JD, Dorst A, Farahmand P. Efficacy of strontium ranelate on bone mineral density in men with osteoporosis. Arzneimittelforschung. 2010;60(5):267–272. doi: 10.1055/s-0031-1296284. [DOI] [PubMed] [Google Scholar]
- 33.Langdahl BL, Marin F, Shane E, et al. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int. 2009 Dec;20(12):2095–2104. doi: 10.1007/s00198-009-0917-y. [DOI] [PubMed] [Google Scholar]
- 34.Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003 Jan;18(1):9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 35.Boonen S, Orwoll E, Magaziner J, et al. Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc. 2011 Nov;59(11):2084–2090. doi: 10.1111/j.1532-5415.2011.03666.x. [DOI] [PubMed] [Google Scholar]
- 36.Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012 Nov;367(18):1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- 37.Kachnic LA, Pugh SL, Tai P, et al. RTOG 0518: randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis. 2013 Dec;16(4):382–386. doi: 10.1038/pcan.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010 Oct;25(10):2239–2250. doi: 10.1002/jbmr.119. [DOI] [PubMed] [Google Scholar]
- 39.Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N Engl J Med. 2012 May 31;366(22):2051–2053. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz P, Jorgensen NR, Mosekilde L, et al. The evidence for efficacy of osteoporosis treatment in men with primary osteoporosis: a systematic review and meta-analysis of antiresorptive and anabolic treatment in men. J Osteoporos. 2011;2011:259818. doi: 10.4061/2011/259818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.