Abstract
Accurate and consistent determination of cause of death is challenging in chronic obstructive pulmonary disease (COPD) patients. TIOSPIR (N=17 135) compared the safety and efficacy of tiotropium Respimat 5/2.5 µg with HandiHaler 18 µg in COPD patients. All-cause mortality was a primary end-point. A mortality adjudication committee (MAC) assessed all deaths. We aimed to investigate causes of discordance in investigator-reported and MAC-adjudicated causes of death and their impact on results, especially cardiac and sudden death.
The MAC provided independent, blinded assessment of investigator-reported deaths (n=1302) and assigned underlying cause of death. Discordance between causes of death was assessed descriptively (shift tables).
There was agreement between investigator-reported and MAC-adjudicated deaths in 69.4% of cases at the system organ class level. Differences were mainly observed for cardiac deaths (16.4% investigator, 5.1% MAC) and deaths assigned to general disorders including sudden death (17.4% investigator, 24.6% MAC). Reasons for discrepancies included investigator attribution to the immediate (e.g. myocardial infarction (MI)) over the underlying cause of death (e.g. COPD) and insufficient information for a definitive cause.
Cause-specific mortality varies in COPD, depending on the method of assignment. Sudden death, witnessed and unwitnessed, is common in COPD and often attributed to MI without supporting evidence.
Short abstract
Investigator-attributed causes of death may lead to unreliable estimates of cause-specific mortality in COPD http://ow.ly/uzt9308TePH
Introduction
Accurate determination of cause of death is challenging in patients with chronic obstructive pulmonary disease (COPD) because of the high comorbidities and complex clinical scenarios at the time of death. Consistent attribution of cause of death is important in all clinical trials, especially when mortality is an outcome measure and causes of death are of interest. The TIOtropium Safety and Performance In Respimat (TIOSPIR) trial [1] was conducted to clarify a potential risk for increased death with tiotropium Respimat, because a higher number of deaths was observed in studies with tiotropium Respimat versus placebo, albeit on a limited number of deaths [1–3]. However, tiotropium HandiHaler was associated with a lower probability of death versus placebo in the 4 year Understanding the Potential Long-term Impacts on Function with Tiotropium (UPLIFT) study as well as in the overall HandiHaler database [4, 5]. As the imbalance in the Respimat studies appeared to be prominent in patients with arrhythmias at study entry, cardiovascular deaths, including sudden (cardiac) deaths, were of special interest for two main reasons. First, sudden deaths are not always caused by cardiac disease [6], despite often being mislabelled as such and despite evidence that patients with COPD are at increased risk of sudden cardiac death. Second, sudden death presents unique difficulties because it commonly occurs outside the hospital and is frequently unwitnessed [7, 8].
Similar to the UPLIFT trial [4, 9], a mortality adjudication committee (MAC) was established in TIOSPIR to provide a systematic, independent, blinded assessment of cause-specific mortality reported by the investigators. We therefore undertook a post hoc analysis of the data on mortality and attributed causes of death in the TIOSPIR trial.
Here, we describe the process of mortality adjudication in the TIOSPIR trial and compare investigator-reported versus adjudicated results for system organ class (SOC) and specific causes of death of interest, with a specific focus on cardiovascular death, including sudden death. In particular, we aimed to evaluate the impact of a standardised adjudication process on the assessment of cause-specific mortality.
Materials and methods
TIOSPIR study design
The TIOSPIR study was a randomised, double-blind, parallel-group trial (17 135 patients with COPD) comparing the safety and efficacy of tiotropium Respimat 2.5 or 5 μg versus tiotropium HandiHaler 18 μg. The primary safety end-point was time to all-cause mortality. Other outcome measures included time to first major adverse cardiovascular event (MACE), which comprised stroke, myocardial infarction (MI), sudden death, cardiac death, sudden cardiac death and fatal event in the SOCs for cardiac and vascular disorders [1, 3].
The trial was performed in accordance with the Declaration of Helsinki, and the study protocol and procedures were approved by relevant institutional review boards and ethics committees, as described previously [1]. All patients provided written informed consent.
MAC documentation and adjudication processes in the TIOSPIR study
Adverse events were triaged by medical doctors (medical reviewers/event monitors) and reviewed in priority order for completeness and medical consistency before sending to MAC (supplementary material).
The MAC comprised three pulmonologists (including the chair of the MAC), three cardiologists, an oncologist and a general medicine member (internist). A set of questions was developed for the medical reviewers/event monitors as a resource for the investigational sites to maximise information from patients who initially presented with no or minimal information. The objective of the MAC was to provide continuous, systematic, independent, blinded, expert assessment of investigator-reported deaths. The MAC assigned a single primary cause of death coded to preferred term level using the Medical Dictionary for Regulatory Activities (MedDRA) according to specific rules devised for the TIOSPIR trial within their principles of operation (supplementary material), which also incorporated many of the general principles and methods generally used in the classification of primary cause of death. However, the site investigators were not made aware of the principles of operation utilised by the MAC [10]. A quality control process evaluated the reliability of the adjudication process (supplementary material).
Based on definitions by the World Health Organization, the American College of Cardiology and the American Heart Association [11–13], the MAC defined sudden cardiac deaths as those occurring within 1 h of an abrupt change of a person's clinical state without other obvious non-cardiac cause, while sudden death referred to deaths occurring more than 1 h but less than 24 h after the patient was last observed alive and without evidence of a deteriorating medical condition. The site investigators did not have access to the principles of operation utilised by the MAC.
Statistical analysis
Concordance and discordance between investigator-reported and MAC-adjudicated causes of death (SOC and preferred term) were assessed using shift tables. Given that the analysis was not treatment-related, data from the three treatment arms were pooled.
Additionally, a sensitivity analysis of MACE, including the term “death unknown”, was performed. Investigator-reported and MAC-adjudicated fatal events of MACE and its individual components were compared for the tiotropium Respimat 5 µg and HandiHaler 18 µg treatment groups using rate ratios.
Results
Numbers of deaths and autopsies
Vital status at the conclusion of the trial was ascertained in 99.7% of participants. There were 1302 deaths: 440 in the tiotropium Respimat 2.5 μg group, 423 in the Respimat 5 μg group and 439 in the tiotropium HandiHaler 18 μg group. Although about 382 autopsies were performed, their results were frequently not available for review and, in many cases, were uninformative.
Causes of death
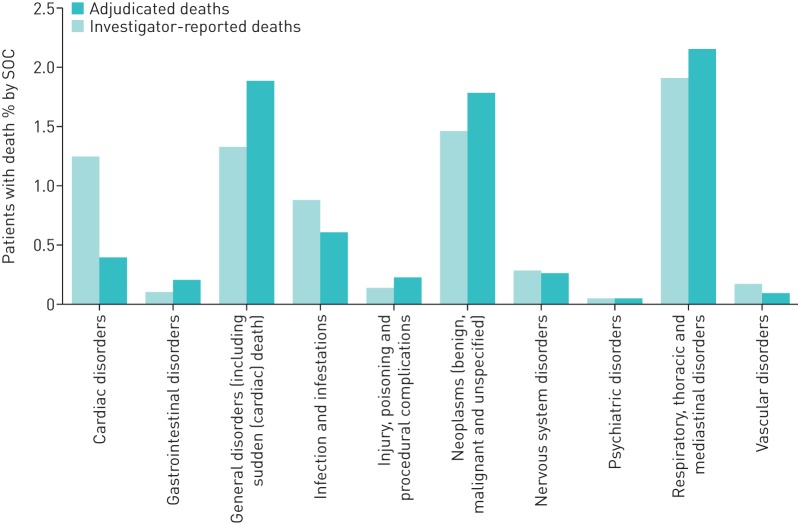
The classification by SOC of investigator-reported versus MAC-adjudicated cause of death as a percentage of deaths is summarised in figure 1. The three leading causes of death, at the SOC level, according to both the investigators and the MAC, were respiratory, neoplasm and general (figure 1, table S1). However, there were notable differences in the number of deaths attributed to each of the SOC categories by the MAC compared with the investigators (figure 1, table S1). Differences were especially marked for the cardiac deaths SOC, where the number of deaths was higher when investigator-reported (n=214; 16.4%) than when MAC-adjudicated (n=66; 5.1%). On the other hand, deaths due to general disorders and neoplasms were higher when MAC-adjudicated (n=320; 24.6% and n=305; 23.4%, respectively) than when investigator-reported (n=226; 17.4% and 249; 19.1%, respectively). The number of deaths due to respiratory causes was also higher when MAC-adjudicated (n=369; 28.3%) than when investigator-reported (n=326; 25.0%) (table S1). There was agreement (concordance), at the SOC level, of the MAC-adjudicated cause of death with the investigator-reported cause of death in 904 of 1302 (69.4%) cases. Concordance was highest for cancer-related deaths (neoplasms) (247 of 249 cases, 99.2%). However, due to the “cancer rule” (supplementary material), the MAC found 58 additional cancer cases, of which 23 were assigned MedDRA terms for lung cancer. Concordance was lowest for cardiac deaths: just 58 of the 214 cases (27.1%). Of the 156 discordant cases, 74 (47.4%) were adjudicated to sudden death or sudden cardiac death, 36 to respiratory (23.1%), 11 to neoplasm (7.1%), 10 to infections (6.4%) and the remaining 6 cases to other causes (3.9%), while 19 were identified by the investigator as cardiorespiratory arrest (n=6), cardiopulmonary failure (n=8) or cor pulmonale (n=5). Moreover, of the 36 cases initially determined as cardiac by the investigator, but adjudicated to respiratory by the MAC, 33 were assigned COPD as the specific cause of death by the MAC.
FIGURE 1.
Classification of investigator-reported and MAC-adjudicated causes of death, as a percentage of patients with death by system organ class (SOC). MAC: mortality adjudication committee.
Discordance was high for the SOC category of infections (70 of 148 (47.3%)) and respiratory deaths (63 of 326 cases (19.3%)) (table S1), although discordance in the SOC respiratory was much less than in SOC cardiac (156 of 214 (72.9%)). Thirty-one (49.2%) of the discordant respiratory cases were assigned to general, which includes sudden cardiac death (n=9) or sudden death (n=12), by the MAC; another 16 cases to cancer (lung neoplasm malignant, small-cell lung cancer, lung cancer metastatic); and 6 cases to infection (pneumonia). On the other hand, the MAC reassigned 36 cardiac deaths, 30 general deaths and 33 infections deaths to respiratory causes, with 96 of these 99 cases assigned to the preferred term COPD.
Detailed assessment of cardiac deaths focused on just the investigator-reported cases of fatal MI (n=46), comprising the specific diagnoses acute MI/MI, post-procedural MI and acute coronary syndrome (table 1). The MAC-adjudicated cause of death, comprising seven specific diagnostic terms, was concordant in 21 of 46 cases (45.7%). The two main reasons for the differences in assignment of cause of death between investigators and adjudicators were that the MAC protocol required that the underlying cause of death be recorded and that specific documentation be cited in order to assign certain causes of death. In four cases where there was discordance between an investigator-reported fatal MI and the adjudicated cause of death, an underlying cause of death (e.g. aortic aneurysm, brain neoplasm or COPD) was provided. In 21 cases, insufficient documentation was available to assign an underlying cause of death, leading to the assignment of a more general term (death, sudden death or sudden cardiac death).
TABLE 1.
Comparison of investigator-reported fatal myocardial infarction (MI)# with adjudicated terms
| Causes of death by MAC | ||||||||
| Concordance | Discordance | |||||||
| Underlying cause of death | Lack of information or evidence¶ | |||||||
| Causes by investigator | Acute MI/MI | Aortic aneurysm | Brain neoplasm | Chronic obstructive pulmonary disease | Death | Sudden cardiac death | Sudden death | Total |
| Acute MI/MI | 21 | 1 | 1 | 1 | 4 | 8 | 6 | 42 |
| Post-procedural MI | 1 | 1 | ||||||
| Acute coronary syndrome | 1 | 2 | 3 | |||||
| Total | 21 | 2 | 1 | 1 | 4 | 9 | 8 | 46 |
MAC: mortality adjudication committee. #: MI is SMQ (standardised MedDRA query) ischaemic heart disease, sub-SMQ MI. ¶: there was insufficient information/evidence for the MAC to assign a specific underlying cause of death so the categorical assignment was made based on MAC protocol-defined criteria. Sudden cardiac deaths were those occurring within 1 h of an abrupt change of a person's clinical state without other obvious non-cardiac cause, and sudden death refers to those occurring more than 1 h but less than 24 h of the patient last being observed alive and without evidence of a deteriorating medical condition. “Death” refers to unexplained death.
Sensitivity analysis
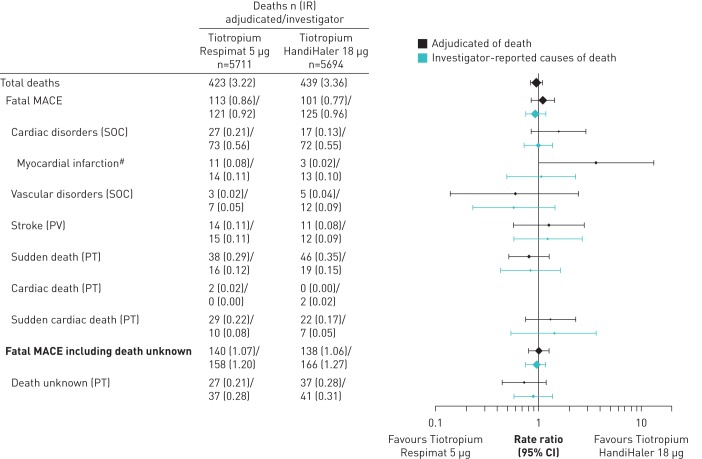
The composite end-points of fatal MACE and fatal MACE plus unknown death showed similar numbers of investigator-reported and MAC-adjudicated deaths for the tiotropium Respimat 5 µg and HandiHaler 18 µg treatment groups (figure 2). Incidence rates for the individual components within these composites, including sudden death and sudden cardiac death, were also similar, with the 95% CI of most of the rate ratios including 1. However, although numbers were very small, there was a between-treatment difference for MAC-adjudicated MI deaths: more deaths were attributed to MI in the tiotropium Respimat group than in the HandiHaler group (n=11 versus 3; rate ratio (95% CI) 3.64 (1.02‒13.06) for tiotropium HandiHaler 18 µg versus tiotropium Respimat).
FIGURE 2.
Comparison of investigator-reported and mortality adjudication committee-adjudicated fatal events (myocardial infarction, sudden death, sudden cardiac death and death) in the tiotropium Respimat 5 µg and HandiHaler 18 µg treatment groups. IR: incidence rate per 100 patient-years; MACE: major cardiovascular event; SOC: system organ class; PV: pharmacovigilance term; PT: preferred term; #: standardised MedDRA query (SMQ) ischaemic heart disease, sub-SMQ myocardial infarction.
Discussion
Reliable reporting of causes of death in clinical trials is essential to ensure the safety of medicines. However, it can be difficult to accurately determine cause of death in patients with COPD due to the presence of comorbidities and the tendency of attributing sudden death, especially when unwitnessed, to a cardiac cause [14]. Studies have concluded that a MAC [9] (or a clinical end-point committee) [14] can provide reliable, standardised adjudication of COPD mortality, which may differ from investigator-assessed cause of death. Moreover, the quality control exercise used to assess the reliability of adjudicated cardiovascular causes of death within the TIOSPIR trial exceeded the target, with a proportion agreement of 94% (“almost perfect”), confirming the reliability and reproducibility of the assignment. This level of reproducibility cannot be expected by investigator assessments, as noted in this study, although investigators had access to the same level of information as the MAC.
Here, we have shown that discordance between investigator-reported and MAC-adjudicated causes of death exists, in particular for cardiovascular deaths, with investigators attributing more deaths to cardiac causes (SOC) than the MAC, and the MAC assigning more to sudden death and sudden cardiac deaths (both categorised as general disorders SOC by MedDRA). The inclusion of sudden death in general versus cardiovascular SOC could be a reason for the shift in attribution from cardiovascular to general disorders SOC in the MAC-adjudicated versus investigator-reported causes of death. The difference between the number of MAC-adjudicated and investigator-reported cases of cardiac death in the UPLIFT trial was similar to that reported here for TIOSPIR; in both trials there was a three-fold greater incidence of investigator-reported cardiac deaths [9].
Moreover, the MAC shifted 58 deaths from other categories to cancer as the underlying cause of death. This is due to the MAC policy that deaths of all patients with incurable cancers should be attributed to the cancer, regardless of the terminal event, which may have been related to secondary complications of the cancer or treatment for the cancer. There was also a shift from general, infectious and cardiovascular causes to respiratory, largely due to the policy that all patients whose terminal illness was initiated by a COPD exacerbation should have the death attributed to respiratory, regardless of the terminal event, which may have been pneumonia, sepsis, MI or sudden death. Thus, although MAC adjudication changed the attribution of cause of death in the TIOSPIR study in just over 30% of cases, this resulted primarily from attribution of death to the underlying cause of the terminal illness (e.g. COPD) rather than the immediate cause of death (e.g. MI).
In the Towards a Revolution in COPD Health (TORCH) study, causes of death were adjudicated by a three-physician clinical end-point committee [14]. Because there were no generally agreed definitions, they developed their own set of working definitions for categories of death in a COPD clinical trial population. Based on this experience, several modifications were recommended for future clinical trials of COPD mortality, including the suggestion that sudden death should be considered as a separate entity, rather than one linked necessarily to a cardiovascular cause [14]. Therefore, adjudication in TIOSPIR used the MedDRA classification. The apparent preferential categorisation of a COPD death as cardiac by investigators in the TORCH, UPLIFT and TIOSPIR studies may also reflect a general tendency of investigators to report the mechanism of death (such as MI), rather than the underlying cause of death. Cause of death is often attributed to MI when the actual cause of death is unknown. This could be reflected in the tendency of medical examiners to label sudden deaths as MI. In fact, sudden cardiac death is usually overestimated. Previous studies have confirmed that pre-existing coronary heart disease or congestive heart failure increase the risk of sudden death [15–17] and account for almost 75% of sudden cardiac deaths in the western world [18–20]. It is noteworthy that the deaths classified as sudden cardiac are often non-cardiac [21] and a significant proportion of out-of-hospital cardiac arrests in COPD patients is attributable to underlying lung disease [22]. Likewise, a study by Huiart et al. and the Rotterdam study emphasised that COPD was associated with an increased risk of sudden cardiac death [8] and a two- to three-fold higher risk of cardiovascular disease, thereby contributing to almost 50% of deaths [8, 23]. Although the MAC distinguished between sudden cardiac death (last seen alive within 1 h), sudden death (last seen alive between 1 and 24 h) and death (last seen alive >24 h ago), these distinctions may depend more on the circumstances of death than the actual cause of death and therefore are not useful distinctions. Moreover, death that occurs more than 1 h and less than 24 h from last being seen clinically stable should not routinely be assigned to a cardiac cause, especially in a study of COPD patients, because of the likelihood that a non-trivial percentage of these cases will be determined to have had a respiratory cause of death [6, 24].
An analysis of data from death certificates from England and Wales (1993–1999) reported that using the immediate cause of death underestimates the contribution of obstructive lung disease to mortality [24]. This was supported by a TORCH death certificate analysis, which found that COPD was often not mentioned [25]. In contrast, in death certificates that mention MI, it was the underlying cause in 94% of cases [26]. Although this difference did not alter the interpretation of the between-treatment analyses in the UPLIFT trial, such discrepancies could potentially alter the interpretation of a clinical trial in which there was a smaller difference between treatment arms. In TIOSPIR, there were more deaths attributed to MI by the MAC in the tiotropium Respimat arm than in the HandiHaler arm (11 versus 3). This difference arose because there were a larger number of cases in the tiotropium Respimat arm for which sufficient data were available to actually make the diagnosis of MI according to the MAC rules (such as electrocardiogram results and cardiac enzyme levels), whereas this was less often the case in the HandiHaler arm. This between-treatment result was not observed when all cardiovascular deaths (fatal MACE) were considered, or when proportions of sudden deaths and sudden cardiac deaths were compared, demonstrating the usefulness of composite MACE end-points. For periods up to 48 weeks, tiotropium maintenance therapy administered using Respimat or HandiHaler once daily was not associated with an increased risk of cardiac arrhythmia in patients with COPD [27]. Furthermore, mortality and subsequent cardiac events were not increased versus placebo in the UPLIFT trial in patients with an acute arrhythmia event [28].
Death may be the end result of a complex series of sequential and contemporaneous factors. Therefore, attributing a single cause of death is often artificial. However, attributing death to the final illness is both logical and leads to consistency of adjudication. Unknown cause of death will always remain an issue in some cases, either because there is a lack of source documentation to assign a definitive cause or because the cause is genuinely unknown despite adequate documentation. In addition, the multiple comorbidities present in most COPD patients make the assignment of a single cause of death particularly difficult. These limitations with respect to assigning a specific cause of death suggest that the results of the MAC adjudication process, while reliable, may not be accurate if documentation is not available. Moreover, adjudication of the underlying cause of death may underestimate the relevance of factors contributing to the immediate cause of death. Therefore, small differences in cause-specific deaths in COPD clinical trials should be interpreted with caution. Although the approach outlined in this study provides for a consistent and reliable ascertainment of cause of death for studies of COPD, it should be noted that this approach may not be applicable for all clinical trials. Furthermore, we suggest that study coordination staff and investigators should be trained to scrutinise the nature and timing of events preceding an unexpected sudden death for future trials.
The findings from this study confirm the value of using all-cause mortality and composite cardiovascular end-points in COPD trials, such as MACE, grouping specific causes of death like sudden (cardiac) death, rather than cause-specific mortality as an outcome in COPD trials, given the difficulty in ascertaining a reliable cause of death in individual cases.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00073-2016_supplement (357.4KB, pdf)
Disclosures
G. Austen 00073-2016_Austen (1.2MB, pdf)
A. Fowler 00073-2016_Fowler (1.2MB, pdf)
P.R. Kowey 00073-2016_Kowey (1.2MB, pdf)
L.P. McGarvey 00073-2016_McGarvey (1.2MB, pdf)
N. Metzdorf 00073-2016_Metzdorf (1.2MB, pdf)
A. Mueller 00073-2016_Mueller (1.2MB, pdf)
R.A. Wise 00073-2016_Wise (1.2MB, pdf)
Acknowledgements
The authors wish to thank the other TIOSPIR Steering Committee members (Antonio Anzueto (University of Texas Health Science Center, and South Texas Veterans Health Care System, San Antonio, TX, USA), Peter M.A. Calverley (Institute of Ageing and Chronic Disease, Liverpool, UK), Ronald Dahl (Odense University Hospital, Odense, Denmark) and Daniel Dusser (Hôpital Cochin, Paris, France)) for their input into the analyses.
Editorial and writing assistance was provided by Sarah J. Petit at PAREXEL (funded by Boehringer Ingelheim). The TIOSPIR trial and this subsequent post hoc analysis were funded by Boehringer Ingelheim.
This study was previously presented as a poster at the European Respiratory Society International Congress 2015, Amsterdam, the Netherlands.
All authors had full access to the data and contributed to the conception and design of this study, and to the analysis and interpretation of the data. All authors were involved in drafting the manuscript or revising it critically for important intellectual content, have read and approved the final manuscript, and assume full responsibility for the integrity of the submission as a whole.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Clinical trial: This study is registered at www.clinicaltrials.gov with identifier number NCT01126437.
Support statement: The TIOSPIR study was funded by Boehringer Ingelheim. Boehringer Ingelheim participated in the design of the study, the collection and analysis of the data, and preparation of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: Disclosures can be found alongside this article at openres.ersjournals.com
References
- 1.Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 2.Boehringer Ingelheim. Summary of Product Characteristics (SPC): Spiriva Respimat 2.5 microgram inhalation solution. EMC. www.medicines.org.uk/emc/medicine/20134/SPC Date last accessed: April 13, 2016. Date last updated: March 22, 2013.
- 3.Wise RA, Anzueto A, Calverley P, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial – design and rationale. Respir Res 2013; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 5.Celli B, Decramer M, Leimer I, et al. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010; 137: 20–30. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AC, Knapman PA, Krikler DM, et al. Community study of the causes of “natural” sudden death. BMJ 1988; 297: 1453–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnier MJ, Blom MT, Bardai A, et al. Increased risk of sudden cardiac arrest in obstructive pulmonary disease: a case–control study. PLoS ONE 2013; 8: e65638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahousse L, Niemeijer MN, van den Berg ME, et al. Chronic obstructive pulmonary disease and sudden cardiac death: the Rotterdam Study. Eur Heart J 2015; 36: 1754–1761. [DOI] [PubMed] [Google Scholar]
- 9.McGarvey LP, Magder S, Burkhart D, et al. Cause-specific mortality adjudication in the UPLIFT® COPD trial: findings and recommendations. Respir Med 2012; 106: 515–521. [DOI] [PubMed] [Google Scholar]
- 10.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Medical Dictionary for Regulatory Activities (MedDRA). www.meddra.org Date last accessed: February 22, 2016. Date last updated: September 2016.
- 11.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death – executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J 2006; 27: 2099–2140. [DOI] [PubMed] [Google Scholar]
- 12.ACC/AHA 2016 Prevention of Sudden Cardiac Death Measures: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. www.cardiosource.org Date last accessed: July 28, 2016. Date last updated: January 29, 2016.
- 13.International Classification of Diseases (ICD-10). Current version (2016) R96. World Health Organization, 2016. http://apps.who.int/classifications/icd10/browse/2016/en#/R95-R99
- 14.McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 2007; 62: 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surawicz B. Prognosis of ventricular arrhythmias in relation to sudden cardiac death: therapeutic implications. J Am Coll Cardiol 1987; 10: 435–447. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Cupples LA, D'Agostino RB. Sudden death risk in overt coronary heart disease: the Framingham Study. Am Heart J 1987; 113: 799–804. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Gagnon DR, Cupples LA. Epidemiology of sudden coronary death: population at risk. Can J Cardiol 1990; 6: 439–444. [PubMed] [Google Scholar]
- 18.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990's: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol 1997; 30: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 19.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation 2012; 125: 620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manfredini R, Portaluppi F, Grandi E, et al. Out-of-hospital sudden death referring to an emergency department. J Clin Epidemiol 1996; 49: 865–868. [DOI] [PubMed] [Google Scholar]
- 21.Pratt CM, Greenway PS, Schoenfeld MH, et al. Exploration of the precision of classifying sudden cardiac death. Implications for the interpretation of clinical trials. Circulation 1996; 93: 519–524. [DOI] [PubMed] [Google Scholar]
- 22.Rosman HS, Goldstein S, Landis JR, et al. Clinical characteristics and survival experience of out-of-hospital cardiac arrest victims without coronary heart disease. Eur Heart J 1988; 9: 17–23. [PubMed] [Google Scholar]
- 23.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005; 128: 2640–2646. [DOI] [PubMed] [Google Scholar]
- 24.Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J 2003; 22: 809–814. [DOI] [PubMed] [Google Scholar]
- 25.Drummond MB, Wise RA, John M, et al. Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD 2010; 7: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldacre MJ, Roberts SE, Griffith M. Multiple-cause coding of death from myocardial infarction: population-based study of trends in death certificate data. J Public Health Med 2003; 25: 69–71. [DOI] [PubMed] [Google Scholar]
- 27.Hohlfeld JM, Furtwaengler A, Konen-Bergmann M, et al. Cardiac safety of tiotropium in patients with COPD: a combined analysis of Holter-ECG data from four randomised clinical trials. Int J Clin Pract 2015; 69: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashkin DP, Leimer I, Metzdorf N, et al. Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res 2015; 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00073-2016_supplement (357.4KB, pdf)
G. Austen 00073-2016_Austen (1.2MB, pdf)
A. Fowler 00073-2016_Fowler (1.2MB, pdf)
P.R. Kowey 00073-2016_Kowey (1.2MB, pdf)
L.P. McGarvey 00073-2016_McGarvey (1.2MB, pdf)
N. Metzdorf 00073-2016_Metzdorf (1.2MB, pdf)
A. Mueller 00073-2016_Mueller (1.2MB, pdf)
R.A. Wise 00073-2016_Wise (1.2MB, pdf)