Abstract
Most enzymes in the central pathway of carotenoid biosynthesis in plants have been identified and studied at the molecular level. However, the specificity and role of cis-trans-isomerization of carotenoids, which occurs in vivo during carotene biosynthesis, remained unresolved. We have previously cloned from tomato (Solanum lycopersicum) the CrtISO gene, which encodes a carotene cis-trans-isomerase. To study the biochemical properties of the enzyme, we developed an enzymatic in vitro assay in which a purified tomato CRTISO polypeptide overexpressed in Escherichia coli cells is active in the presence of an E. coli lysate that includes membranes. We show that CRTISO is an authentic carotene isomerase. Its catalytic activity of cis-to-trans isomerization requires redox-active components, suggesting that isomerization is achieved by a reversible redox reaction acting at specific double bonds. Our data demonstrate that CRTISO isomerizes adjacent cis-double bonds at C7 and C9 pairwise into the trans-configuration, but is incapable of isomerizing single cis-double bonds at C9 and C9′. We conclude that CRTISO functions in the carotenoid biosynthesis pathway in parallel with ζ-carotene desaturation, by converting 7,9,9′-tri-cis-neurosporene to 9′-cis-neurosporene and 7′9′-di-cis-lycopene into all-trans-lycopene. These results establish that in plants carotene desaturation to lycopene proceeds via cis-carotene intermediates.
Carotenoids comprise a large group of terpenoid pigments synthesized by all plants algae and cyanobacteria as well as by several nonphotosynthetic bacteria and fungi. Carotenoids fulfill indispensable functions in photosynthesis (Frank et al., 1999), and in plants they also provide colors to flowers and fruits. Dietary carotenoids are essential precursors to vitamin A, and they are implicated in reducing the occurrence of certain cancers and cardiovascular diseases, possibly by serving as antioxidants and free radical scavengers (for review, see Demmig-Adams and Adams, 2002; Fraser and Bramley, 2004).
Our understanding of the carotenoid biosynthetic pathway in plants has been advanced greatly in the past decade, mainly due to cloning of many of the genes for enzymes involved in the pathway. The first carotenoid in the pathway is phytoene, a product of condensation of two geranylgeranyl diphosphate (GGDP) molecules. The colorless phytoene undergoes four consecutive dehydrogenation reactions that introduce four double bonds in conjugation, giving rise to the red polyene chromophore of lycopene, the precursor of a diverse group of cyclic carotenoids. In plants, two desaturases, phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS), carry out these reactions, whereas in bacteria all four dehydrogenation reactions are carried out by a single gene product, named CRTI (for review, see Hirschberg, 2001). Most plant carotenoids carry trans-configured double bonds. However, specific cis-isomers do exist, for example, 9-cis-neoxanthin in the light-harvesting complex (Liu et al., 2004; Snyder et al., 2004) and 9-cis-epoxyxanthophylls that serve as substrates in the biogenesis of the plant hormone abscisic acid (Schwartz et al., 1997; Qin and Zeevaart, 1999). A well-studied example of occurrence of cis-carotenes is the tomato (Solanum lycopersicum) mutant tangerine. In the orange-colored fruit of this mutant, 7,9,7′,9′-tetra-cis-lycopene (prolycopene) replaces the all-trans-lycopene, which is normally synthesized in wild-type tomatoes (Zechmeister et al., 1941; Clough and Pattenden, 1979). Flowers, etiolated seedlings, and apical shoot meristems of tangerine accumulate cis-carotenes instead of the typical xanthophyll profile (Isaacson et al., 2002). We have previously cloned the tangerine locus, CrtISO, and discovered that mutations in this gene abolish in tangerine a specific cis-trans-isomerization of carotenoids, causing the accumulation of prolycopene and other cis-isomers of its upstream precursors. Functional expression of CrtISO cDNA in Escherichia coli engineered to accumulate the plant complement of cis-carotenes indicated that CRTISO is a carotene isomerase involved in the biosynthesis of all trans-lycopene (Isaacson et al., 2002). Similar data on CRTISO were obtained in Arabidopsis (Arabidopsis thaliana) following map-based cloning of the locus ccr2 (Park et al., 2002). An ortholog of CrtISO was also found in the cyanobacterium Synechocystis sp. PCC 6803 (Breitenbach et al., 2001; Masamoto et al., 2001).
This study was carried out in order to characterize CRTISO biochemically. For this purpose, we developed an in vitro assay that relied on CRTISO from tomato expressed in E. coli. Activity of cis-to-trans isomerization was analyzed with different carotenoid isomers substrates under various reaction conditions. The results determined that the intermediates of the carotenoid biosynthesis pathway from phytoene to neurosporene are cis-configured. The CRTISO enzyme requires active cell membranes, and its activity is driven by a membrane-bound redox chain.
RESULTS
Functional Expression of CRTISO in E. coli Cells
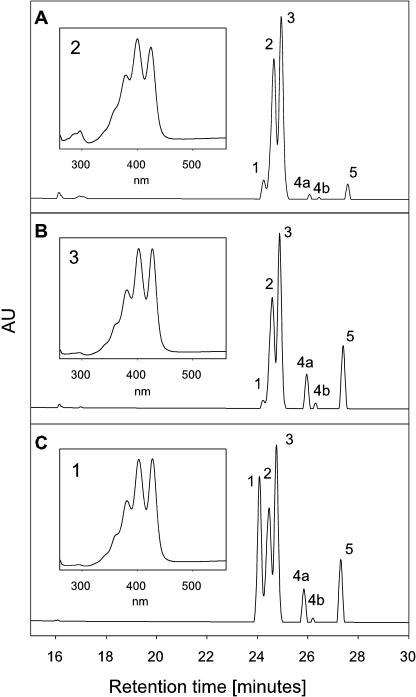
E. coli cells carrying plasmid pAC-Zeta accumulate ζ-carotene (Isaacson et al., 2002). This carotene intermediate typically occurs in three isomeric forms that can be resolved by HPLC system 2 (Fig. 1). To identify the ζ-carotene species accumulated in the bacteria, we used synthetic all-trans-ζ-carotene as a reference. It was found that the E. coli cells accumulated two types of cis-isomers and only trace amounts of the all-trans-isomer. Based on published data (Clough and Pattenden, 1983; Beyer et al., 1989), we assigned peak 2 in Figure 1 as 9,15,9′-tri-cis-ζ-carotene and peak 3 as 9,9′-di-cis-ζ-carotene. This identification is corroborated by the typical absorption at 297 nm of the 9,15,9′-tri-cis-ζ-carotene and by the fact that the ratio of the peaks 2 and 3 changed from 3:2 in dark-grown bacteria to 2:3 when the bacteria were grown under illumination. This phenomenon is attributed to photoisomerization of the C15 cis-double bond to trans (Beyer et al., 1989). Complementation of CRTISO in E. coli cells that carried plasmids pAC-Zeta and pCRTISO did not change the pattern of ζ-carotene isomers, thus demonstrating that cis-ζ-carotenes are not substrates of CRTISO. It is important to note that in the same E. coli cells, CRTISO is active in isomerizing 7,9,9′-tri-cis-neurosporene and prolycopene (see below). It is concluded that isomerization of carotenes by CRTISO to form trans-intermediates takes place during or after the desaturation of ζ-carotene and not during the phytoene desaturation. For unknown reason, a small increase of phytoene and phytofluene was always observed in cells that expressed CRTISO. This phenomenon could be due to interference of CRTISO with the activity of phytoene desaturase or because of an indirect effect of overexpressing an additional protein in the bacteria.
Figure 1.
HPLC analysis of carotenes extracted from E. coli cells accumulating cis-ζ-carotene in the absence (A) and presence (B) of CRTISO. To identify the isomers, the sample in B was mixed with a synthetic all-trans-ζ-carotene and analyzed by HPLC (C). MaxPlot chromatograms showing each peak at its λmax are presented. Peak 1, All-trans-ζ-carotene; peak 2, 9,15,9′-tri-cis-ζ-carotene; peak 3, 9,9′-di-cis-ζ-carotene; peaks 4a and 4b are cis-isomers of phytofluene; peak 5, 15-cis-phytoene. Absorption spectra of specific peaks are presented to the boxes. 9,15,9′-Tri-cis-ζ-carotene is distinguished from the 9,9′-cis-isomer by the typical absorbance at 297.5 nm.
Substrate Specificity of CRTISO
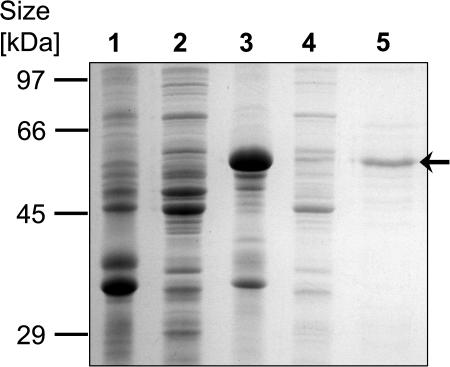
E. coli cells carrying pCRTISO654 accumulated a polypeptide of an apparent molecular mass of approximately 60 kD, matching the predicted size of the mature CRTISO polypeptide from tomato after cleavage of the predicted transit peptide (Fig. 2). These cells were disintegrated by a French press, and the lysate was centrifuged at 13,000g. Based on SDS-PAGE of polypeptides from the pellet and supernatant, an estimated 5% to 10% of the recombinant protein was in the supernatant while the rest was in inclusion bodies (Fig. 2). The supernatant of the lysate served as a crude enzyme preparation used in vitro to assay the enzymatic activity of CRTISO. The supernatant contained plasma membranes showing active respiration upon NADH addition, as measured with an oxygen electrode (data not shown). Equivalent supernatants of E. coli cells transformed with the empty vector pQE60 were used as controls in all assays.
Figure 2.
Expression of CRTISO in E. coli. Proteins were separated by SDS-PAGE and stained with Coomassie blue. Lane 1, Insoluble fraction (pellet) of a lysate from E. coli carrying pQE60; lane 2, soluble fraction (supernatant) of E. coli carrying pQE60; lane 3, insoluble fraction of E. coli carrying pCrtISO654; lane 4, soluble fraction of E. coli carrying pCrtISO654; lane 5, column-purified CRTISO polypeptide.
The mixture of cis-carotenes extracted from ripe fruits of the tomato mutant tangerine (LA3183) was used as a substrate in the initial experiments. These carotenoids consisted of 30% to 50% 7,9,7′,9′-tetra-cis-lycopene (prolycopene), approximately 2% 7,9-di-cis-lycopene, 6% to 11% 7,9,9′-tri-cis-neurosporene, 20% to 30% 9,9′-di-cis-ζ-carotene, 9,15,9′-cis-ζ-carotene, and approximately 8% to 10% 9-cis-phytofluene. This composition is consistent with the data given by Clough and Pattenden (1983), who used NMR to determine the various carotenoids. cis-Carotenes applied to the assay in the form of CHAPS micelles or in small amounts of acetone were isomerized very poorly or not at all. When the cis-carotenes were applied to the assay in liposomal membranes made of either E. coli lipid extracts or dimyristoyl phosphatidyl choline (DMPC), most of the prolycopene was isomerized by CRTISO into di-cis-lycopene and only a small portion into all-trans-lycopene (data not shown). Therefore, we used poly-cis-carotene substrate solubilized in 0.25% (w/w) Triton X-100 routinely.
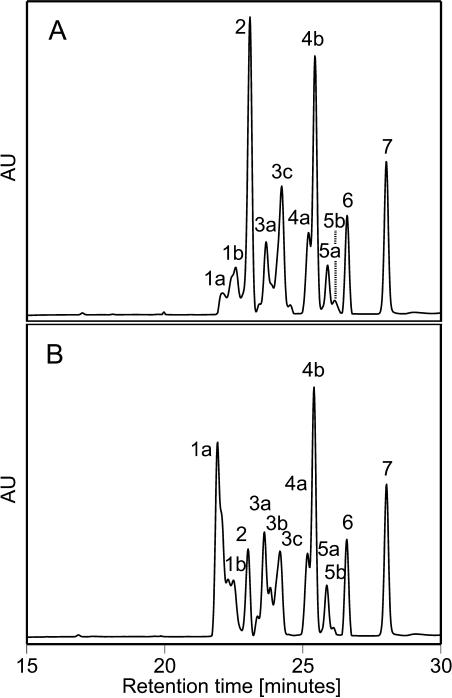
Incubation of bacterial supernatant containing the CRTISO with cis-carotenes in the dark at 28°C for up to 2 h resulted in a substantial cis-to-trans isomerization of carotenes (Fig. 3). Time-course analysis of carotenoid conversion showed that prolycopene decreased concomitantly with an increase in di-cis- and all-trans-lycopene (Fig. 4A). Similarly, 7,9,9′-tri-cis-neurosporene decreased concomitantly with an increase of two other isomers of neurosporene, none of which was all-trans-neurosporene, which was identified by running a standard. It is worth noting that similar to the complementation study, the relative proportion of cis-isomers of ζ-carotene, phytofluene, and phytoene did not change significantly during the incubation. No conversion of cis-carotenes was observed in the control assays (data not shown). To examine whether 7,9,9′-tri-cis-neurosporene alone is a substrate of CRTISO, this proneurosporene was purified from tangerine fruits by HPLC system 1 and applied to the in vitro assay (Fig. 4B). Several lines of evidence suggest that the main product of the isomerization of 7,9,9′-tri-cis-neurosporene is 9-cis-neurosporene (peak 3a in Fig. 3). (1) An all-trans-neurosporene standard ruled out this species as a product. (2) In the HPLC analysis used, a longer retention time is correlated with increasing number of cis-double bonds in the molecule of a specific carotenoid. The neurosporene isomer produced by CRTISO was eluted in the HPLC immediately after the all-trans-neurosporene standard and before the two other isomers, one of which was clearly identified as 7,9,9′-tri-cis-neurosporene. This fits an isomer with a single cis-double bond. (3) A 7-cis-neurosporene or 7,9-di-cis-neurosporene are excluded because, as was determined with 9,9′-di-cis-ζ-carotene, the cis-double bond in position 9 is not a substrate for CRTISO. Taken together, these results indicate that CRTISO does not recognize a single cis-double bond at C9 or C9′ but utilizes substrates containing 7,9 (or 7′,9′)-cis-pairs of adjacent double bonds.
Figure 3.
HPLC analysis of cis-carotene substrates and products in a CRTISO in vitro assay. A, Carotenes at the beginning of the reaction (time 0 min); B, after 90 min. Peak 1a, trans-Lycopene; peak 1b, di-cis-lycopene; peak 2, prolycopene; peak 3a, neurosporene, isomer 1; peak 3b, neurosporene, isomer 2; peak 3c, 7,9,9′-cis-isomer of neurosporene; peak 4a, 9,15,9′-cis-ζ-carotene; peak 4b, 9,9′-cis-ζ-carotene isomer; peak 5a and 5b are β-carotene isomers; peak 6, phytofluene; peak 7, phytoene.
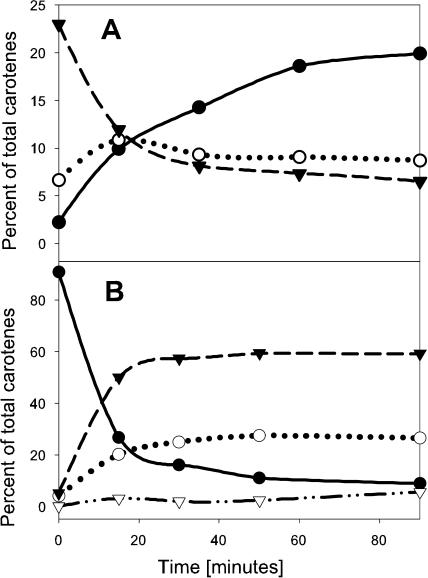
Figure 4.
A, Time course of prolycopene isomerization in vitro. A mixture of cis-carotenes from tangerine, at a final concentration of 180 μg mL−1, was used as a substrate in the assay with lysate of E. coli that expressed CRTISO. Aliquots were subjected to HPLC analysis at the indicated time points. Dashed line, Prolycopene; dotted line, 7,9-di-cis-lycopene; solid line, trans-lycopene. B, Reaction carried out with purified 7,9,9′-tri-cis-neurosporene as a substrate. Solid line, 7,9, 9′-Tri-cis-neurosporene; dashed line, neurosporene isomer 1; dotted line, neurosporene isomer 2; dotted-dashed line, trans-neurosporene.
Biochemical Analysis of CRTISO Activity in Vitro
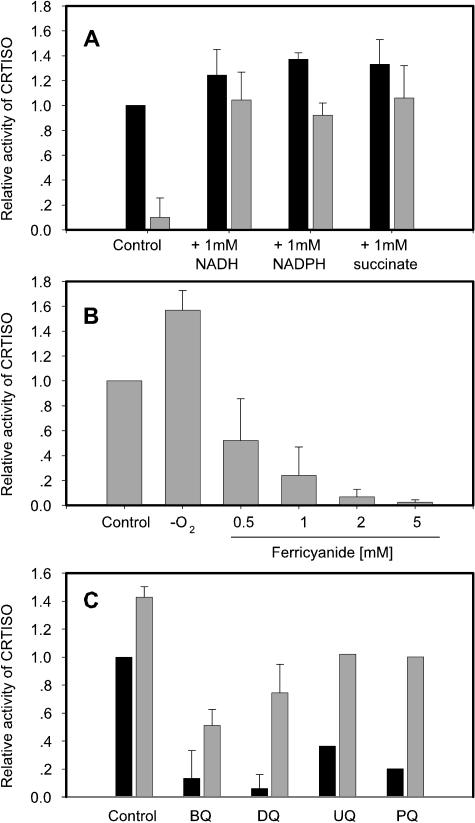
A purified His-tagged form of CRTISO was enzymatically inactive with poly-cis-carotenes when present in Triton X-100 micelles or in liposomes prepared from E. coli lipids or DMPC. However, CRTISO-mediated isomerization occurred only when supernatant of E. coli lysate obtained from cells expressing the empty vector (pQE60) was added to the assay system instead of the liposomes or Triton X-100 micelles. This indicated that a component in the bacterial supernatant was essential for CRTISO activity in vitro. Dialysis of the E. coli cells lysate prior to its addition to the in vitro assay abolished this activity, indicating that a dialyzable component in the bacterial lysate supernatant was essential (Fig. 5A). We considered the possibility that the isomerization reaction proceeds via a reversible hydrogen extraction. Such a process could involve an oxidoreduction process that depends on activity of components of the respiratory electron transfer chain of the bacterial membranes still present in the lysate. To test for this possibility, soluble electron donors or acceptors of various nature and/or redox potentials were added to the dialyzed cell lysate referred to as the basal assay system. Among these, electron donors such as NADH, NADPH, or succinate restored the activity to the level of the nondialyzed supernatant and even increased to some extent the activity of the enzyme when assayed using the nondialyzed lysate (Fig. 5A).
Figure 5.
Effect of redox components on the activity in vitro of CRTISO. Time-course experiments were run and plotted as in Figure 4A in the presence of the different effectors. For each condition, the area under the curves was calculated. CRTISO activity in the control condition was taken as 1.0. A, Requirement for respiratory redox intermediates; black bars represent nondialyzed E. coli lysate (supernatant) and gray bars, dialyzed preparations. B, Influence of anaerobic conditions and ferricyanide. C, Effect of various oxidized quinones on CRTISO activity was measured in the absence (black bars) or presence (gray bars) of 1 mm NADH. BQ, benzoquinone; DQ, duroquinone; UQ, ubiquinone; PQ, plastoquinone (1 mm each).
The promoting effect on CRTISO activity of succinate can be explained in terms of membrane participation and an active succinate:ubiquinone oxidoreductase (succinate dehydrogenase) being present and points to the involvement of ubihydroquinone (UQH2). The CRTISO reaction mechanism possibly depends on active redox chains, such as the respiratory redox chain or parts thereof downstream of UQH2, which are present in E. coli membranes and which are added with the supernatant. To investigate this hypothesis, we applied anaerobic conditions to the assay by using an enzymatic oxygen trap leading to an accumulation of reduced redox-active components in the membrane. Anaerobic conditions enhanced the cis-to-trans isomerization activity of CRTISO (Fig. 5B). This result points to the involvement of a membrane-bound electron donor and excludes the possibility of participation of molecular oxygen in the reaction mechanism. It is in agreement with these findings that the addition of ferricyanide (1–5 mm) as an oxidizing agent inhibited the reaction (Fig. 5B).
To test for the hypothesis of UQH2 involvement, we examined several quinones in their reduced form. Addition of hydroquinones such as benzoquinone, duroquinone, plastoquinone, or ubiquinone had no effect on the reaction (data not shown). However addition of the above quinones in their oxidized state inhibited the reaction (Fig. 5C). This inhibition points to the participation of hydroquinones and is further corroborated by the fact that this inhibition could be overcome by adding NADH (Fig. 5C).
Antimycin A is a known specific inhibitor of cytochrome b, which acts as an electron acceptor downstream of UQH2 (Izzo et al., 1978). In the presence of antimycin A, cytochrome b can be reduced but not oxidized. No significant effect on isomerization in vitro was observed with antimycin A (data not shown), which excludes the participation of complex III (ubiquinone cytochrome c-oxidoreductase) and IV (cytochrome c-oxygen oxidoreductase) in the phenomenon observed.
Overall, these results suggest that presence of a reduced component(s) of the electron transfer chain is required for the isomerization activity of CRTISO in the basal assay system. The reduced cofactor, acting as an intermediate carrier between succinate dehydrogenase of complex II or NADH ubiquinone oxidoreductase of complex I and cytochrome b of complex III, most probably UQH2, is involved in CRTISO cis-to-trans isomerization. This cofactor undergoing oxidoreduction is poised at a finely tuned redox potential that serves as intermediate in the isomerization process, possibly via a reversible hydrogen donation/extraction at a specific cis-oriented double bonds.
DISCUSSION
Carotenoid Biosynthesis in Plants Follows the cis-Desaturation Pathway
Most carotenoids in plants occur in the all-trans-configuration. The carotene intermediates through the four desaturation reactions from phytoene to lycopene have been considered to be in the all-trans-configuration (Spurgeon and Porter, 1980; Britton, 1988; Hugueney et al., 1992). However, occurrence of cis-carotenes was reported in mutants of tomato (tangerine; Zechmeister et al., 1941), Scenedesmus (Humbeck, 1990), and Euglena (Cunningham and Schiff, 1985), in experiments with purified membranes from daffodil (Narcissus pseudonarcissus) chromoplasts (Beyer et al., 1989) and in E. coli cells expressing plant-type biosynthetic enzymes (Bartley et al., 1999; Isaacson et al., 2002; Matthews et al., 2003). Consequently, a poly-cis-pathway of carotene desaturation has been often regarded as being abnormal or as an in vitro artifact. This cis-versus-trans pathway conundrum has remained unsolved for nearly six decades. Characterization of CRTISO that is described here approves the view that cis-configured carotenes that are found in tangerine represent the legitimate intermediates that normally exist in the plant carotenoid biosynthesis pathway.
It is a well-established fact that the central C15-15′double bond in phytoene is cis-configured (Britton, 1998). The first desaturation reactions of phytoene catalyzed by PDS can take place with 15-cis-phytoene, producing trans-double bonds at C11 and C11′. This is shown by the existence of 15-cis-phytofluene and 15-cis-ζ-carotene (Kushwaha et al., 1970; Powls and Britton, 1977; Beyer et al., 1994). However, during ZDS-mediated desaturation, this double bond is found to be trans-configured. Moreover, as demonstrated in this work (Fig. 1), the cis-double bond at C15 is not a substrate for isomerization by CRTISO. It was shown in vitro that ZDS activity was regained only after isomerizing this cis-double bond to trans (Beyer et al., 1989). Since the 15 cis-double bond is known to be very photo labile, this step can be enhanced by light in vitro. How this is achieved in vivo remains unclear. It is evident from the data we present here that CRTISO does not play this part because it leaves the isomeric states of cis-ζ-carotene species unaffected. Thus, the 15-cis-double bond is distinct from other cis-double bonds in carotenes that are discussed here.
The double bonds at positions 9 and 9′ are not a product of the desaturation sequence but originate from the prenyl transferase reactions that produce GGDP. These double bonds exist in trans in the phytoene found in wild-type as well as in tangerine tomatoes (Clough and Pattenden, 1979, 1983; Britton, 1998). Therefore, there is currently no support for the possibility that the cis-double bonds at positions 9 and 9′ in prolycopene are derived from cis-configured GGDP. Based on NMR analysis of fruit carotenes from the mutant tangerine, Clough and Pattenden (1979, 1983) suggested that during the introduction of the trans-double bonds at position 11 (in phytoene) and 11′ (in phytofluene) by PDS, the double bonds at positions 9 and 9′ undergo specific trans-to-cis isomerization (Clough and Pattenden, 1979, 1983). Accumulation of cis-carotenes in the mutant C-6 D of the alga Scenedesmus obliquus grown in the dark was explained by impairment of the synthesis of all-trans-ζ-carotene, possibly due to a mutation in the phytoene desaturase (Sandmann, 1991).
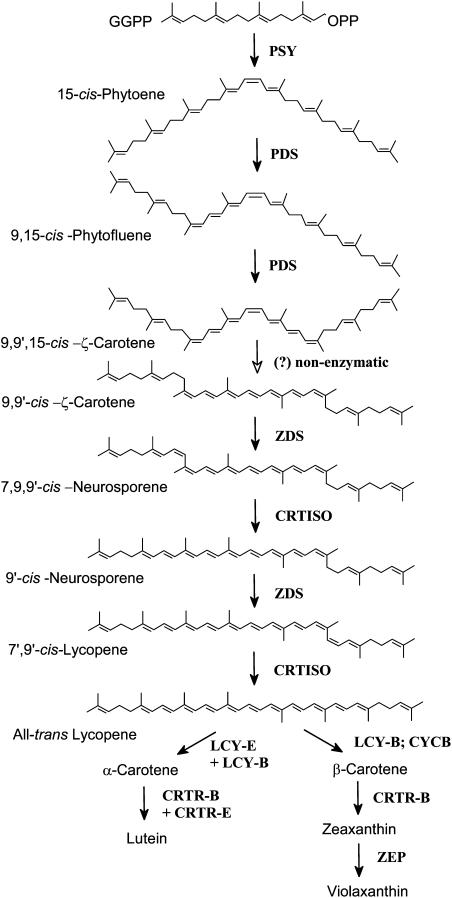
By contrast, our data support the poly-cis-pathway to be the default mechanism of carotene desaturation in plants and cyanobacteria. It is evident that CRTISO was unable to isomerize the cis-isomers of ζ-carotene in the in vitro assay, while it was actively converting tri-cis-neurosporene (proneurosporene) and tetra-cis-lycopene (prolycopene). This is in agreement with CRTISO recognizing only cis-double bonds at positions 9 or 9′ in conjunction with adjacent cis-double bonds at 7 or 7′. This is further substantiated by the fact that, in our assays, CRTISO converted prolycopene into all-trans-lycopene, through the 7,9-di-cis-lycopene intermediate, as has been suggested previously (Beyer et al., 1991). Additional evidence for a cis-desaturation pathway is the fact that the plant-type carotene desaturases produce in E. coli only cis-isomers of ζ-carotene as well as prolycopene (Bartley et al., 1999). Furthermore, all the ζ-carotene detected in trace amounts during fruit ripening in the wild-type tomato, and in large amounts in fruits of a mutant that is impaired in ζ-carotene desaturation, occurs in the 9,9′-cis- and the 9,15,9′-cis-configuration (data not shown). Finally, ZDS is capable of using 9′,9-di-cis-ζ-carotene as a substrate to produce prolycopene via 7,9,9′-tri-cis-neurosporene (Breitenbach et al., 1999). Since 7,9,9′-tri-cis-neurosporene is efficiently isomerized in vitro by CRTISO, most probably to 9′-cis-neurosporene, we reason that in vivo this proneurosporene isomer is most likely the authentic substrate for isomerization in the normal desaturation pathway. This inference is supported by the fact that we have not been able to detect any prolycopene in wild-type plants under normal conditions while an isomer of di-cis-lycopene could be detected in trace amounts. Therefore, we conclude that CRTISO acts in conjunction with or immediately after ZDS during the desaturation of ζ-carotene to all-trans-lycopene (Fig. 6).
Figure 6.
Proposed pathway of carotenoid biosynthesis in plants. CRTISO, Carotene isomerase; CRTR-B, β-ring hydroxylase, CRTR-E, ɛ-ring hydroxylase; GGPP, geranylgeranyl diphosphate; LCY-B, lycopene β-cyclase; LCY-E, lycopene ɛ-cyclase; PDS, phytoene desaturase; PSY, phytoene synthase; ZDS, ζ-carotene desaturase; ZEP, zeaxanthin epoxidase.
CRTISO Activity Requires a Redox Mechanism
The findings that reducing reagents such as NADH, NADPH, and succinate accelerate the isomerization reaction in vitro and that oxidizing reagents such as ferricyanide, oxygen, or different oxidized quinones are inhibitory suggest the involvement of the bacterial membrane's respiratory chain in the process. More specifically, complex I, the NADH ubiquinone oxidoreductase, and complex II, the succinate ubiquinone oxidoreductase, affect the reaction, probably via the quinone pool. These results suggest that, although no net electron transfer occurs in the isomerization reaction, a redox driving force is needed. The isomerization reaction may include a transient reduction of the cis-double bond followed by reoxidation yielding a trans-configuration of the newly formed double bond. The fact that CRTISO harbors a conserved dinucleotide-binding domain at its N terminus supports this possibility. A dinucleotide-binding domain is highly conserved among many of the carotenoid biosynthesis enzymes. PDS from pepper was found to bind flavin adenine dinucleotide (FAD) (Hugueney et al., 1992), and PDS from daffodil was found to be active only when FAD was added to the medium during its membrane association (Al-Babili et al., 1996). Lycopene β-cyclase was shown to be active in vitro only in the presence of NADH or NADPH (Schnurr et al., 1996), although NADPH does not transfer its hydrogen during the cyclization reaction to the carotene (Hornero-Mendez and Britton, 2002). We have not been able to detect any bound flavin in the His-tagged purified CRTISO. The nature of the redox-active CRTISO dinucleotide cofactor remains to be elucidated. However, it cannot be excluded that its binding occurs only transiently during catalysis. The electrons that participate in the reaction might be transferred back to this cofactor and fed into the electron transfer chain.
The interaction of CRTISO with the electron chain seems rather unspecific. This is because it was assayed with E. coli membranes, while it must be assumed that the photosynthetic electron transport is involved in plants. This is somewhat comparable to PDS functioning in E. coli (Bartley et al., 1999; this study) that employs quinones and depends on active redox chains (Mayer et al., 1990; Nievelstein et al., 1995). Here again, the photosynthetic electron transport is thought to provide the driving force that is replaced by an alternative redox chain in nongreen plastids. Consistent with this line of reasoning, the involvement of an alternative terminal oxidase was recently shown in Arabidopsis (Carol et al., 1999; Wu et al., 1999; Carol and Kuntz, 2001; Joet et al., 2002) and tomato (Josse et al., 2000). Just like PDS, CRTISO appears to be able to mechanistically link to respiratory as well as photosynthetic redox chains. This again points to the involvement of a benzoquinone as the common redox component in respiration as well as in photosynthesis. In contrast to carotene desaturation, however, there is no involvement of oxygen in carotene isomerization, as we show here, supporting earlier evidence (Beyer et al., 1991).
Phytoene Desaturation in Plants and Bacteria
In plants and cyanobacteria, phytoene desaturation to all-trans-lycopene is catalyzed by three enzymes, PDS, ZDS, and CRTISO, whereas in bacteria a single enzyme, CRTI, carries out this function. The bacterial CRTI and plant CRTISO share sequence similarity and probably evolved from a common ancestor (Giuliano et al., 2002; Isaacson et al., 2002; Park et al., 2002). However, CRTISO does not possess any desaturase activity. Although both CRTI and PDS were shown to bind FAD (Al-Babili et al., 1996; Dailey and Dailey, 1998), the mechanism of dehydrogenation of phytoene is evidently different. One aspect relates to the sequence of dehydrogenation in the two enzymes. In plants the desaturation of phytoene by PDS is symmetrical at C11 and C11′, to produce a symmetric cis-isomer of ζ-carotene (7,8,7′,8′-tetrahydrolycopene), whereas CRTI-type phytoene desaturases apparently dehydrogenate phytoene in one-half of the molecule first to produce an asymmetric isomer of ζ-carotene (7,8,11,12-tetrahydrolycopene). Support for this possibility comes from the detection of 7,8,11,12-tetrahydrolycopene in bacteria (Marshall and Wilmoth, 1981) and the ability of CRTI enzymes to convert phytoene into other asymmetric end products, such as neurosporene (Raisig et al., 1996; Armstrong, 1997) and 3,4-didehydrolycopene (Hausmann and Sandmann, 2000).
The PDS:ZDS:CRTISO desaturation of phytoene appeared in evolution first in cyanobacteria. Since nonoxygenic photosynthetic bacteria that possess CRTI preceded cyanobacteria in evolution, the emergence of a three-enzyme desaturation pathway may be associated with the development of oxygenic photosynthesis. The reason for this is yet unknown. One possibility is a biochemical and/or biophysical constraint that necessitates a different enzymatic mechanism of carotenoid desaturation in cyanobacteria and plants in which poly-cis-carotene intermediates are only by-products. Alternatively, the reason for the emergence of a different desaturation pathway is a requirement to maintain the carotenoid intermediates in a cis-configuration. Support for the latter hypothesis comes from the finding that cis-isomers of the early biosynthetic intermediates are more stable than the corresponding trans-isomers (Paneth et al., 1992).
MATERIALS AND METHODS
Expression of CRTISO in Escherichia coli
Plasmid pAC-Zeta, which carries the genes crtB and crtE from Erwinia herbicola and crtP from Synechococcus PCC7942, has been described (Cunningham et al., 1994). All strains of E. coli contained plasmid pGB-Ipi that was constructed by inserting the cDNA of Ipi from Haematococcus pluvialis (Cunningham and Gantt, 2001; kindly provided by F.X. Cunningham, University of Maryland, College Park, MD) into the HindIII site of the plasmid vector pGB2 (Churchward et al., 1984). Plasmid pCRTISO, which carries the cDNA of CrtISO from tomato (Solanum lycopersicum), has been described (Isaacson et al., 2002). E. coli cells of the strain XL1-Blue carrying plasmid pGB-Ipi were cotransformed with plasmids pAC-Zeta and pCRTISO and selected on solid Luria-Bertani medium containing 1.5% agar and the appropriate antibiotics: spectinomycin (50 mg/L), ampicillin (100 mg/L), and chloramphenicol (50 mg/L). Cells were incubated overnight at 37°C in the dark and then further incubated for additional 48 h at room temperature either in the dark or under dim light of 10 to 30 μmol m−2·s−1 photon flux.
Plasmid pCRTISO654 was constructed by subcloning a 1,604 bp-PCR-amplified fragment from the tomato cDNA of CrtISO (Isaacson et al., 2002). The primers used for amplification were 5′-gacaaaaccatggagagctat-3′ (forward); and 5′-gatatccatggctagtgtcct-3′ (reverse). All primers contained mismatches to create NcoI restriction sites. The PCR fragments were subcloned into the NcoI site of the expression vector pQE60 (Qiagen, Valencia, CA). The recombinant polypeptide expressed from pCRTISO654 represents the expected mature form of CRTISO without the predicted transit peptide. The recombinant protein contained a 6× His tag at its C terminus. E. coli cells carrying plasmids pCRTISO654 or pQE60 were grown in Luria-Bertani medium containing ampicillin (100 mg/L) at 37°C to OD550 = 0.5. Isopropyl thio-β-d-galactoside was then added (0.5 mm) for the duration of 8 to 10 h at 30°C to induce expression of the recombinant genes. Bacterial cells were harvested, resuspended in one-tenth volume of cold incubation buffer (100 mm Tris, 10 mm MgCl2, pH 7.4), and ruptured twice using a French press. The lysate was centrifuged at 13,000g for 15 min at 4°C. The clear supernatant was collected and kept on ice, either for the in vitro assay or for protein purification by the Talon kit (BD Biosciences, Palo Alto, CA). The pure recombinant CRTISO protein was eluted by 0.1× volume imidazole elution buffer (20 mm Tris, 100 mm NaCl, 100 mm imidazole, pH 7).
Carotenoid Analysis by HPLC
Carotenoids were separated by HPLC using a Waters system and a Spherisorb ODS2 C18 (5 μm, 4.6 × 250 mm) reversed-phase column (Waters, Milford, MA). In HPLC system 1, acetonitrile was used as the eluent at a constant flow rate of 1.6 mL/min. HPLC system 2 was a gradient. Using acetonitrile:water (9:1; A) and ethylacetate (B), at a constant flow rate of 1.6 mL/min the gradient was: 100% to 80% A during 8 min; 80% to 65% A during 4 min, followed by 65% to 45% A during 14 min and a final segment at 100% B. Carotenoids were analyzed with a Waters 996 photodiode array detector as described previously (Ronen et al., 2000). Carotenes were identified by their absorption spectra and retention time, and in some cases by comparison with authentic reference substances (see Table I for details). A standard of all-trans-neurosporene was obtained by extraction from E. coli cells carrying plasmid pAC-NEUR (Cunningham et al., 1994). A standard of trans-ζ-carotene was purchased from CaroteNature GmbH (Lupsingen, Switzerland). The pattern of cis-carotenes extracted from fruit of the tomato mutant tangerine was essentially identical to data given by Clough and Pattenden (Clough and Pattenden, 1979, 1983). Quantification was performed by integrating the peak areas using the Millennium chromatography software (Waters).
Table I.
Absorption spectra of carotenoids in the HPLC analysis
| Peak No. in Chromatogram in Figure 3 | Carotenoid | λ max nma | % III/IIab |
|---|---|---|---|
| 1a | trans-Lycopene | 449, 474, 503 | 63 |
| 1b | Di-cis-lycopene | 450, 474 | 0 |
| 2 | Prolycopene | 440 | 0 |
| 3a | Neurosporene isomer 1 | 419, 440, 470 | 83 |
| 3b | Neurosporene isomer 2 | 417, 440, 469 | 56 |
| 3c | 7,9,9′-cis-Neurosporene | 411, 435, 463 | 0 |
| 4a | 9,15,9′-cis-ζ-Carotene | (297), 380, 401, 425 | 76 |
| 4b | 9,9′-cis-ζ-Carotene | 380, 401, 425 | 95 |
| 5a | β-Carotene isomer 1 | 455, 484 | 13 |
| 5b | β-Carotene isomer 2 | 450, 476 | 0 |
| 6 | Phytofluene | 331, 350, 367 | 62 |
| 7 | Phytoene | 288 | 0 |
| Standard | trans-Lycopene | 449, 474, 503 | 62 |
| Standard | trans-Neurosporene | 420, 445, 474 | 90 |
| Standard | trans-ζ-Carotene | 380, 401, 425 | 95 |
| Standard | trans-β-Carotene | 430, 455, 484 | 21 |
Absorbance spectra as detected in the HPLC running solvent: a gradient of acetonitrile:water (9:1) and ethylacetate (HPLC system 2).
% III/II, Fine structure of the absorption spectra expressed as the relative heights of the longest wavelength peak (III) to the middle peak (II). Zero means lack of peak III.
Extraction of cis-Carotenes from tangerine Fruit
Fruits of the tangerine tomato mutant were ground in chloroform:MeOH (2:1, v/v). The chloroform phase was collected and carotenes were separated by thin-layer chromatography (Silica gel 60 F254; Merck, Rahway, NJ) with petroleum ether:diethyl ether:acetone, 4:1:1. The carotene fraction migrating with the solvent front was scraped off, eluted with acetone, and dried under flow of nitrogen in the dark. This total carotene fraction, when used as substrates, was dissolved in 1% Triton X-100 in sonication buffer (20 mm Tris-HCl, pH 8, 100 mm NaCl) by sonication and further diluted in sonication buffer to a final concentration of 0.25% (v/v; carotene concentration of approximately 180 μg mL−1). In a similar way, carotenes were solubilized in a solution of 1 mm CHAPS. Individual cis-carotene species were isolated form the total carotene fraction by using HPLC system 1. Fractions containing selected cis-carotenes were collected, dried under a stream of nitrogen, and dissolved in 0.25% Triton X-100 as described above.
Carotene Extraction from Bacterial Lysate
Supernatants of bacterial lysate were mixed with acetone to a concentration of 80% and centrifuged at 4,000g for 5 min to pellet bacterial debris. The supernatant was collected and 1/3 the volume of petroleum ether was added. The organic phase was collected, dried, and dissolved in 65 μL of acetone and applied to HPLC analysis.
In Vitro Isomerization Assay
Thirty microliters of the cis-carotene preparation in 0.25% Triton X-100 (approximately 180 μg mL−1) were added per milliliter of bacterial extract. Samples were incubated at 30°C in the dark. At various times, 1-mL aliquots were extracted with 4 mL of acetone. Anaerobic conditions were applied in some experiments using an enzymatic oxygen trap according to Lam and Malkin (1982) consisting of catalase, 45 μg mL−1; Glc oxidase, 300 μg mL−1; and Glc, 1,500 μg mL−1.
Liposome Preparation
E. coli lipids were extracted with chloroform:MeOH (2:1, v/v). Carotenes from tangerine tomato fruit dissolved in chloroform were added. The organic phase was collected, dried under a stream of nitrogen, and dissolved in incubation buffer by sonication. The same was done with DMPC (Sigma, St. Louis) dissolved in chloroform. The final lipid concentration was 5 mg mL−1 in incubation buffer.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AF416727.
Acknowledgments
We thank Moshe Amiton for his assistance in experimentation, and Dr. Francis X. Cunningham from the University of Maryland for providing plasmids pGB-Ipi and pAC-Zeta.
This work was supported by The Israel Science Foundation and by the European Commission (contract “ProVitA”; QLK3–CT2000–0809). Work in the laboratories of J.H. and I.O. is carried out under the auspices of the Avron Even-Ari Minerva Center.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.052092.
References
- Al-Babili S, von Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601–612 [DOI] [PubMed] [Google Scholar]
- Armstrong GA (1997) Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu Rev Microbiol 51: 629–659 [DOI] [PubMed] [Google Scholar]
- Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259: 396–403 [DOI] [PubMed] [Google Scholar]
- Beyer P, Kroncke U, Nievelstein V (1991) On the mechanism of lycopene isomerase cyclase reaction in Narcissus pseudonarcissus L. chromoplasts. J Biol Chem 266: 17072–17078 [PubMed] [Google Scholar]
- Beyer P, Mayer M, Kleinig H (1989) Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184: 141–150 [DOI] [PubMed] [Google Scholar]
- Beyer P, Nievelstein V, Albabili S, Bonk M, Kleinig H (1994) Biochemical aspects of carotene desaturation and cyclization in chromoplast membranes from Narcissus pseudonarcissus. Pure Appl Chem 66: 1047–1056 [Google Scholar]
- Breitenbach J, Kuntz M, Takaichi S, Sandmann G (1999) Catalytic properties of an expressed and purified higher plant type ζ-carotene desaturase from Capsicum annuum. Eur J Biochem 265: 376–383 [DOI] [PubMed] [Google Scholar]
- Breitenbach J, Vioque A, Sandmann G (2001) Gene sll0033 from Synechocystis 6803 encodes a carotene isomerase involved in the biosynthesis of all-E lycopene. Z Naturforsch [C] 56: 915–917 [DOI] [PubMed] [Google Scholar]
- Britton G (1988) Biosynthesis of carotenoids. In TW Goodwin, ed, Plant Pigments. Academic Press, London, pp 133–180
- Britton G (1998) Overview of carotenoid biosynthesis. In G Britton, S Liaaen-Jensen, H Pfander, eds, Carotenoids, Vol 3: Biosynthesis. Birkenhauser, Basel, pp 13–147
- Carol P, Kuntz M (2001) A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci 6: 31–36 [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G, Belin D, Nagamine Y (1984) A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31: 165–171 [DOI] [PubMed] [Google Scholar]
- Clough JM, Pattenden G (1979) Naturally occurring poly-cis carotenoids. Stereochemistry of poly-cis lycopene and in congeners in ‘tangerine’ tomato fruits. J Chem Soc Chem Commun 14: 616–619 [Google Scholar]
- Clough JM, Pattenden G (1983) Stereochemical assignment of prolycopene and other poly-Z-isomeric carotenoids in fruits of the tangerine tomato Lycopersicon esculentum var. ‘Tangella’. J Chem Soc Perkin Trans I 1: 3011–3018 [Google Scholar]
- Cunningham FX Jr, Gantt E (2001) One ring or two? Determination of ring number in carotenoids by lycopene epsilon-cyclases. Proc Natl Acad Sci USA 98: 2905–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FX Jr, Schiff JA (1985) Photoisomerization of zeta-carotene stereoisomers in cells of Euglena gracilis mutant W3BUL and in solution. Photochem Photobiol 42: 295–307 [DOI] [PubMed] [Google Scholar]
- Cunningham FX Jr, Sun ZR, Chamovitz D, Hirschberg J, Gantt E (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6:1107–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey TA, Dailey HA (1998) Identification of an FAD superfamily containing protoporphyrinogen oxidases, monoamine oxidases, and phytoene desaturase. Expression and characterization of phytoene desaturase of Myxococcus xanthus. J Biol Chem 273: 13658–13662 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW III (2002) Antioxidants in photosynthesis and human nutrition. Science 298: 2149–2153 [DOI] [PubMed] [Google Scholar]
- Frank HA, Young A, Britton G, Cogdell RJ (1999) The Photochemistry of Carotenoids. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43: 228–265 [DOI] [PubMed] [Google Scholar]
- Giuliano G, Giliberto L, Rosati C (2002) Carotenoid isomerase: a tale of light and isomers. Trends Plant Sci 7: 427–429 [DOI] [PubMed] [Google Scholar]
- Hausmann A, Sandmann G (2000) A single five-step desaturase is involved in the carotenoid biosynthesis pathway to beta-carotene and torulene in Neurospora crassa. Fungal Genet Biol 30: 147–153 [DOI] [PubMed] [Google Scholar]
- Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 [DOI] [PubMed] [Google Scholar]
- Hornero-Mendez D, Britton G (2002) Involvement of NADPH in the cyclization reaction of carotenoid biosynthesis. FEBS Lett 515: 133–136 [DOI] [PubMed] [Google Scholar]
- Hugueney P, Romer S, Kuntz M, Camara B (1992) Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and ζ-carotene in Capsicum chromoplasts. Eur J Biochem 209: 399–407 [DOI] [PubMed] [Google Scholar]
- Humbeck K (1990) Light-dependent carotenoid biosynthesis in mutant C-6D of Scenedesmus obliquus. Photochem Photobiol 51: 113–118 [Google Scholar]
- Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo G, Guerrieri F, Papa S (1978) On the mechanism of inhibition of the respiratory chain by 2-heptyl-4-hydroxyquinoline-N-oxide. FEBS Lett 93: 320–322 [DOI] [PubMed] [Google Scholar]
- Joet T, Genty B, Josse EM, Kuntz M, Cournac L, Peltier G (2002) Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem 277: 31623–31630 [DOI] [PubMed] [Google Scholar]
- Josse EM, Simkin AJ, Gaffe J, Laboure AM, Kuntz M, Carol P (2000) A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol 123: 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha SC, Suzue G, Subbarayan C, Porter JW (1970) The conversion of phytoene-14C to acyclic, monocyclic, and dicyclic carotenes and the conversion of lycopene-15,15′-3H to mono- and dicyclic carotenes by soluble enzyme systems obtained from plastids of tomato fruits. J Biol Chem 245: 4708–4717 [PubMed] [Google Scholar]
- Lam E, Malkin R (1982) Ferredoxin-mediated reduction of cytochrome b-563 in a chloroplast cytochrome b-563/f complex. FEBS Lett 141: 98–101 [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Marshall JH, Wilmoth GJ (1981) Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol 147: 900–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Wada H, Kaneko T, Takaichi S (2001) Identification of a gene required for cis-to-trans carotene isomerization in carotenogenesis of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 42: 1398–1402 [DOI] [PubMed] [Google Scholar]
- Matthews PD, Luo R, Wurtzel ET (2003) Maize phytoene desaturase and ζ-carotene desaturase catalyse a poly-Z desaturation pathway: implications for genetic engineering of carotenoid content among cereal crops. J Exp Bot 54: 2215–2230 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Beyer P, Kleinig H (1990) Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem 191: 359–363 [DOI] [PubMed] [Google Scholar]
- Nievelstein V, Vandekerckhove J, Tadros MH, Lintig JV, Nitschke W, Beyer P (1995) Carotene desaturation is linked to a respiratory redox pathway in Narcissus pseudonarcissus chromoplast membranes. Involvement of a 23-kDa oxygen-evolving-complex-like protein. Eur J Biochem 233: 864–872 [DOI] [PubMed] [Google Scholar]
- Paneth P, Madhavan S, Oleary MH (1992) Significance of the cis-trans isomerization of early intermediates in the carotene biosynthetic-pathway. J Phys Org Chem 5: 783–786 [Google Scholar]
- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powls R, Britton G (1977) A series of mutant strains of Scenedesmus obliquus with abnormal carotenoid compositions. Arch Microbiol 133: 275–280 [DOI] [PubMed] [Google Scholar]
- Qin XQ, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96: 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisig A, Bartley G, Scolnik P, Sandmann G (1996) Purification in an active state and properties of the 3-step phytoene desaturase from Rhodobacter capsulatus overexpressed in Escherichia coli. J Biochem (Tokyo) 119: 559–564 [DOI] [PubMed] [Google Scholar]
- Ronen G, Carmel-Goren L, Zamir D, Hirschberg J (2000) An alternative pathway to beta-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97: 11102–11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G (1991) Light-dependent switch from formation of poly-cis carotenes to all-trans carotenoids in the Scenedesmus mutant C-6D. Arch Microbiol 155: 229–233 [Google Scholar]
- Schnurr G, Misawa N, Sandmann G (1996) Expression, purification and properties of lycopene cyclase from Erwinia uredovora. Biochem J 315: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Snyder AM, Clark BM, Robert B, Ruban AV, Bungard RA (2004) Carotenoid specificity of light-harvesting complex II binding sites. Occurrence of 9-cis-violaxanthin in the neoxanthin-binding site in the parasitic angiosperm Cuscuta reflexa. J Biol Chem 279: 5162–5168 [DOI] [PubMed] [Google Scholar]
- Spurgeon SL, Porter JW (1980) Biosynthesis of carotenoids. In JW Porter, SL Spurgeon, eds, Biochemistry of Isoprenoid Compounds. Wiley, New York, pp 1–122
- Wu DY, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The immutans variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmeister L, LeRosen AL, Went F, Pauling L (1941) Prolycopene, a naturally occurring stereoisomer of lycopene. Proc Natl Acad Sci USA 27: 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]