Abstract
UDP-GlcA 4-epimerase (UGlcAE) catalyzes the epimerization of UDP-α-d-glucuronic acid (UDP-GlcA) to UDP-α-d-galacturonic acid (UDP-GalA). UDP-GalA is a precursor for the synthesis of numerous cell-surface polysaccharides in bacteria and plants. Using a biochemical screen, a gene encoding AtUGlcAE1 in Arabidopsis (Arabidopsis thaliana) was identified and the recombinant enzyme biochemically characterized. The gene belongs to a small gene family composed of six isoforms. All members of the UGlcAE gene family encode a putative type-II membrane protein and have two domains: a variable N-terminal region approximately 120 amino acids long composed of a predicted cytosolic, transmembrane, and stem domain, followed by a large conserved C-terminal catalytic region approximately 300 amino acids long composed of a highly conserved catalytic domain found in a large protein family of epimerase/dehydratases. The recombinant epimerase has a predicted molecular mass of approximately 43 kD, although size-exclusion chromatography suggests that it may exist as a dimer (approximately 88 kD). AtUGlcAE1 forms UDP-GalA with an equilibrium constant value of approximately 1.9 and has an apparent Km value of 720 μm for UDP-GlcA. The enzyme has maximum activity at pH 7.5 and is active between 20°C and 55°C. Arabidopsis AtUGlcAE1 is not inhibited by UDP-Glc, UDP-Gal, or UMP. However, the enzyme is inhibited by UDP-Xyl and UDP-Ara, suggesting that these nucleotide sugars have a role in regulating the synthesis of pectin. The cloning of the AtUGlcAE1 gene will increase our ability to investigate the molecular factors that regulate pectin biosynthesis in plants. The availability of a functional recombinant UDP-GlcA 4-epimerase will be of considerable value for the facile generation of UDP-d-GalA in the amounts required for detailed studies of pectin biosynthesis.
GalA is a major sugar residue of plant pectic polysaccharides (Mohnen, 2002) and a minor component of some plant arabinogalactan proteins (Darvill et al., 1980; Yates et al., 1996). GalA is also found in various cell-surface polysaccharides of different Gram-negative bacteria, including human pathogenic bacteria Klebsiella pneumoniae (for review, see Holst et al., 1998), Shigella spp. (Feng et al., 2004), and Vibrio cholera (Adeyeye et al., 2003); plant pathogenic bacteria Agrobacterium larrymoorei (Molinaro et al., 2003) and Erwinia chrysanthemi spp. (Gray et al., 2000); plant symbiotic rhizobacteria (Forsberg and Carlson, 1998); aerobic bacteria from the deep sea (Raguénès et al., 2003); and the hyperthermophilic bacterium Aquifex pyrophilus (Plötz et al., 2000). The existence of GalA is not restricted to Gram-negative bacteria being present in the capsular polysaccharide of the Gram-positive human pathogen type-I Streptococcus pneumoniae (Garcia et al., 1997; Stroop et al., 2002; for review, see Jann and Westphal, 1975), as well as in certain polysaccharides of the Cyanobacterium spp. (Gloaguen et al., 1995).
Genetic and biochemical evidence demonstrates that the synthesis of GalA-containing polymers requires UDP-α-d-galacturonic acid (UDP-GalA). A recent review by Mohnen (2002) predicted that synthesis of the major pectin polymers homogalacturonan, rhamnogalacturonan I, and rhamnogalacturonan II and the minor polymers xylogalacturonan and apiose-galacturonan requires more than 10 distinct enzymes (galacturonosyltransferases), each incorporating GalA from its activated nucleotide-sugar form, UDP-GalA, to a unique polymer. In 1958, Neufeld et al. reported the first isolation of a specific nucleotide-sugar 4-epimerase activity that converts UDP-α-d-glucuronic acid (UDP-GlcA) to UDP-GalA (Neufeld et al., 1958). Subsequently, the activity was isolated from Cyanobacterium anabaena flos-aquae (Ankel and Tischer, 1969; Gaunt et al., 1974) and from different plants (Feingold et al., 1960; Dalessandro and Northcote, 1977a, 1977b; Liljebjelke et al., 1995; Orellana and Mohnen, 1999). A genetic screen of a polysaccharide-deficient S. pneumoniae strain identified the cap1J gene (Munoz et al., 1997) as encoding the active UDP-GlcA epimerase (Munoz et al., 1999). We have developed methods to identify the function of various cDNAs encoding putative nucleotide-sugar epimerases. Here, we report the isolation of a cDNA from Arabidopsis (Arabidopsis thaliana) that encodes a protein sharing high sequence similarity to the cap1J protein, and provide the biochemical proof for the identification of a plant UDP-GlcA epimerase that we named AtUGlcAE1 (for Arabidopsis UDP-GlcA 4-epimerase 1).
RESULTS
Cloning and Characterization of AtUGlcAE from Arabidopsis
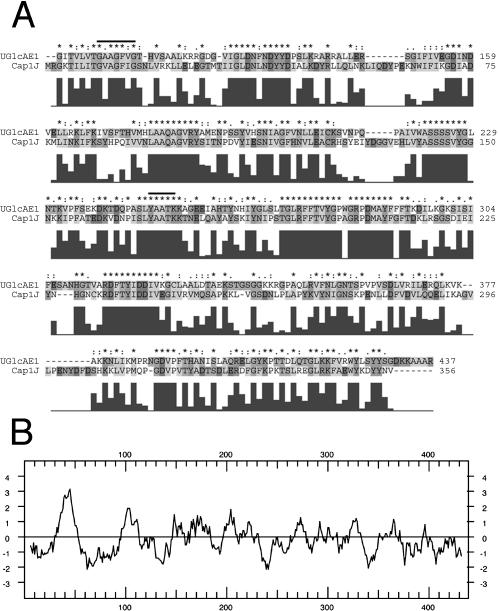
With the functional cloning of the prokaryotic, human, and plant UDP-Gal epimerases (Lemaire and Muller-Hill, 1986; Daude et al., 1995; Dormann and Benning, 1996) and the putative UDP-GlcA epimerase (Garcia et al., 1993; Munoz et al., 1997), it became apparent that most, if not all, nucleotide-sugar 4-epimerases belong to a large family of proteins that have conserved catalytic motifs (Thoden et al., 1997). These motifs include the GxxGxxG (x = any amino acid) sequence within the N-terminal domain that is likely involved in the binding of β-NAD+, and a catalytic triad consisting of S/T and YxxxK (Weirenga et al., 1986). These conserved domains are common in nucleotide-sugar 4-epimerases from eukaryotes and prokaryotes. Given the size of the gene family among all organisms, it remains a challenge to predict the substrate specificity of a particular gene product based on the gene/protein sequence alone. As a result, we have developed an HPLC-based biochemical screen to identify the function of these putative epimerases. The amino acid sequence of the cap1J (Munoz et al., 1997) was useful to initially identify candidate gene homologs from the various Arabidopsis expressed sequence tag cDNA database (dbEST) projects and available genomic data. Using sequence data and biochemical reports that UDP-GlcA epimerases from several plants are membrane associated (Feingold et al., 1960), we cloned several gene candidates from an Arabidopsis cDNA library (Table I). We identified three distinct putative UGlcAE cDNAs each encoding a protein having two regions: (1) an N-terminal region (approximately 120 amino acids long) that shares no sequence similarity with known proteins and contains a hydrophobic domain (Fig. 1B) predicted to have a type-II membrane orientation; and (2) a large region (approximately 300 amino acids long) that is evolutionarily conserved, sharing more than 64% amino acid sequence similarity to the cap1J prokaryote UDP-GlcA epimerase (Munoz et al., 1999), and contains the catalytic epimerase domains (Fig. 1A). To determine the function of the cap1J-like Arabidopsis homologs, we overexpressed one of them in Escherichia coli and studied the properties of the recombinant protein.
Table I.
Molecular data for the three isoforms of the Arabidopsis AtUGlcAE gene family
| AtUGlcAE | Amino Acid | Molecular Mass | Loci | %a Similarity/Identity | Typeb |
|---|---|---|---|---|---|
| kD | |||||
| AtUGlcAE1 | 437 | 48.5 | At2g45310 | 100/100 | A |
| AtUGlcAE2 | 460 | 47.4 | At3g23820 | 78/89 | B |
| AtUGlcAE3 | 429 | 50.5 | At4g30440 | 78/88 | C |
The amino acid sequence identity/similarity comparison (Altschul et al., 1997) was performed using amino acids spanning from 97 to 437 of AtUGlcAE1.
The type is defined arbitrarily to indicate that the 3 isoforms described have a variable amino acid region spanning from amino acid 1 to approximately 100.
Figure 1.
Amino acid sequence alignment and characterization of Arabidopsis AtUGlcAE1 A, Comparison of the conserved amino acid sequence between AtUGlcAE1 (GenBank accession no. AAT06796) and bacterial cap1J (accession no. 1870164) protein. Protein sequence alignment was performed using ClustalX (version 1.83; **Jeanmougin et al., 1998). The putative conserved GX2GX2G motif and the putative catalytic triad YX3K (Weirenga et al., 1986) are marked with a line. The histograms beneath the sequence indicate the degree of amino acid identity/similarity between the two proteins as determined by ClustalX. B, Hydropathy plot of AtUGlcAE1.
AtUGlcAE1 Encodes Active Recombinant UGlcAE
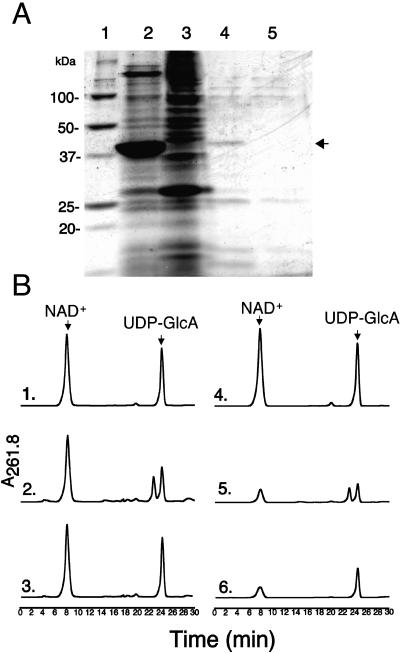
Recombinant cap1J UDP-GlcA epimerase was shown to be difficult to express and purify, and only partial purification was achieved due to loss of activity (Munoz et al., 1999). We were unable to recover active recombinant enzymes when full-length AtUGlcAE1 and AtUGlcAE2 proteins were expressed in E. coli, presumably due to the hydrophobic domain that results in un- or misfolded protein in inclusion bodies within E. coli. We have experienced similar problems when trying to actively express other recombinant plant proteins that contain a putative trans-membrane domain (Harper and Bar-Peled, 2002). Recombinant AtUGlcAE1Δ1–64, lacking the putative transmembrane domain, on the other hand, could be expressed in E. coli (Fig. 2A, lane 2) and was active (Fig. 2B, subsection 2) and relatively stable when stored as a crude extract. E. coli cells expressing the empty control vector (Fig. 2A, lane 3) had no UDP-GlcA epimerase activity, indicating that the observed epimerase activity was exclusively due to the expression of the recombinant plant gene (Fig. 2B, subsection 3). Recombinant Arabidopsis AtUGlcAE2, lacking its putative membrane domain (amino acids 1–69), was also expressed in E. coli, but the activity was too low to permit further characterization.
Figure 2.
Isolation, chromatography, and initial characterization of recombinant AtUGlcAE1Δ1–64. A, SDS-PAGE of total protein isolated from E. coli cells expressing AtUGlcAE1Δ1–64 (lane 2) or vector control (lane 3). Proteins from cells expressing recombinant AtUGlcAE1Δ1–64 were separated by chromatography (see “Materials and Methods”), and the active fractions eluted from the SourceQ column (fractions 60–65) were combined, desalted, and separated on SDS-PAGE (lane 4). As a control, total proteins from an E. coli cell expressing vector alone were separated under the same chromatographic conditions and the combined desalted fractions (60–65) eluting from SourceQ column were separated on SDS-PAGE as well (lane 5). The arrow indicates the position of the partially purified recombinant AtUGlcAE1Δ1–64. B, Aliquot of each protein sample (total or from SourceQ column) was incubated with β-NAD+ and UDP-GlcA, and the enzymatic reactions were separated on ion-exchange HPLC column. The arrows in subsections 1 and 4 indicate migration of standards β-NAD+ and UDP-GlcA. Activity of total protein isolated from cell expressing recombinant AtUGlcAE1Δ1–64 (subsection 2) or vector alone (subsection 3) is shown. The activity of partially purified recombinant AtUGlcAE1Δ1–64 (subsection 5) or control vector alone (subsection 6), fractionated on SourceQ column, was determined with 0.2 mm NAD and 0.5 mm UDP-GlcA.
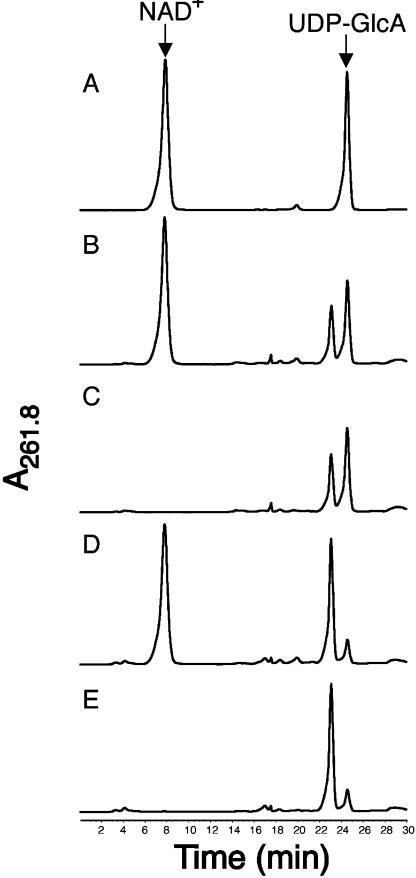
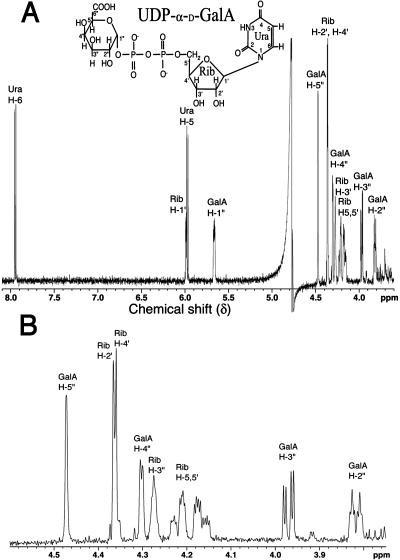
The active 43-kD AtUGlcAE1Δ1–64, expressed in E. coli, was partially purified by anion-exchange chromatography (Fig. 2, A, lane 4, and B, subsection 5). Purification of the active recombinant protein on a Ni column could not be used because the imidazole used to elute such a column completely inhibits AtUGlcAE1Δ1–64 activity. AtUGlcAE1Δ1–64, readily converts UDP-GlcA to a product that elutes from HPLC column at a retention time of 22.8 min (Fig. 3, subsection 2). This conversion did not require exogenous NAD+ (Fig. 3, subsection 3). To determine the identity of the product produced by the recombinant protein, the product eluting from the HPLC column was collected and analyzed by proton 1H-NMR. The 500-mHz 1H-NMR spectrum (Fig. 4, A and B) confirmed that the enzymatic product is UDP-GalA. The spectrum contains a doublet-of-doublet signal at 5.6 ppm with a coupling constant, 3J1″,P, of 7 Hz, diagnostic for the H-1″ proton of an α-d-GalA residue that is linked to the phosphate group of uridine. The coupling constant 3J1″,2″ equal to 3.7 Hz value is diagnostic for coupling between protons H-1″ and H-2″ in UDP-GalA. Other specific chemical shifts and coupling constant values (Table II) diagnostic for GalA are the large 10.2-Hz coupling constant between the trans-configuration of protons 2″ and 3″ and the short 3.2-Hz signal corresponding to coupling constants between the cis-configuration of protons 3″ and 4″. The uridine proton assignments in the 1H-NMR spectra are characteristic of those for uridine in other UDP-sugars (Harper and Bar-Peled, 2002; Watt et al., 2004), including UDP-Xyl and UDP-rhamnose. Having confirmed that the enzyme product of AtUGlcAE1Δ1–64 is UDP-GalA, we tested the ability of the recombinant enzyme to convert UDP-GalA to UDP-GlcA. The data shown in Figure 3, subsection 4, demonstrates that AtUGlcAE1Δ1–64 is in fact a reversible 4-epimerase capable of converting UDP-GalA to UDP-GlcA. This conversion also did not require exogenous NAD+ (Fig. 3, subsection 5). To determine the equilibrium constant for the interconversion, the enzyme was incubated with UDP-GlcA for various times, and the UDP-GalA/UDP-GlcA ratio was determined. The data demonstrate that recombinant AtUGlcAE1Δ1–64 favors the formation of UDP-GalA over UDP-GlcA with an equilibrium constant of approximately 1.9 (Table IV).
Figure 3.
Recombinant AtUGlcAE1Δ1–64 is an active UDP-GlcA/UDP-GalA 4 epimerase. The HPLC separation of standards (NAD+ and UDP-GlcA, indicated by arrow in A) and of UDP-GlcA epimerase enzymatic products (B–E) is shown. Recombinant AtUGlcAE1Δ1–64 was incubated with UDP-GlcA and 1 mm NAD+ (B), with UDP-GlcA but without NAD+ (C), with UDP-GalA and 1 mm NAD+ (D), or with UDP-GalA but without NAD+ (E).
Figure 4.
The 1H-NMR spectrum of the product generated by reacting UDP-GlcA with recombinant AtUGlcAE1Δ1–64. A, One-dimensional 500-mHz NMR spectrum of AtUGlcAE1Δ1–64 product (UDP-α-d-GalA) is shown between 3.5 to 8 ppm. Location for each proton residue on the spectrum is indicated: the position of protons on the uracil (Ura) ring are indicated by H, the ribose (Rib) protons by H′, and the GalA protons are in H″. B, An expanded region between 3.8 and 4.6 ppm is shown.
Table II.
Proton chemical shifts and coupling constants of UDP-GalA formed from UDP-GlcA by recombinant AtUGlcAE1Δ1–64
| Proton | H1 | H2 | H3 | H4 | H5 | H6 |
|---|---|---|---|---|---|---|
| GalA | ||||||
| Chemical shifts, δ (ppma) | 5.664 | 3.818 | 3.970 | 4.302 | 4.472 | – |
| J coupling constants (Hz) | J1″,P 7 J1″,2″ 3.5 | J2″,3″ 10.2 J 2″,P 3 | J3″,4″ 3.2 | J4″,5″ < 1 | – | – |
| Rib | ||||||
| Chemical shifts, δ (ppma) | 5.984 | 4.36 | 4.272 | 4.36 | 4.266–4.22 | – |
| J coupling constants (Hz) | J 1′,2′ 3.6 | – | J5,5′ 12 | – | ||
| Uracil | ||||||
| Chemical shifts, δ (ppma) | – | – | – | – | 5.972 | 7.948 |
| J coupling constants (Hz) | – | – | – | – | J5,6 8.1 | – |
Chemical shifts are in ppm relative to internal acetone signal set at 2.218 ppm. GalA proton-proton coupling constants in hertz are indicated as well as the 3J1″,P coupling values between phosphate and the H-1″ proton of GalA. The chemical shift values for uracil protons (H) and ribose protons (H′) are the same as for other UDP-sugars.
Table IV.
Enzymatic properties and characterization of recombinant AtUGlcAE1Δ1–64
| Optimal pHa | Optimal Temperatureb | Kmc | Kcat | Catalytic Efficiency | Equilibrium Constantd | Mass of Active Proteine (Denatured) |
|---|---|---|---|---|---|---|
| °C | mm | s−1 | m−1 s−1 | kD | ||
| 7.4–7.6 | 30–42 | 0.72 | 24 | 3.2 × 104 | 1.9 | 88 (43.5) |
At the pH value indicated, the relative activity in phosphate buffer is 100%; in Tris-HCl, −80%; in Bis-Tris-HCl, −75%.
Optimal temperature assays were conducted in phosphate buffer.
The reciprocal initial velocity was plotted against UDP-GlcA concentration according to Lineweaver and Burk to calculate Km value.
The conversion ratio between UDP-GalA to UDP-GlcA was determined over a period of 2 h.
The molecular mass of the active AtUGlcAEΔ1–64 eluted from Superdex 75 gel-filtration column (17.8 min) was estimated based on extrapolation (R2 = 0.996) from relative time of standard protein marker.
Further testing demonstrated that the AtUGlcAE1Δ1–64 is specific for UDP-uronic acids, and none of the other nucleotide sugars examined (UDP-Glc, UDP-Gal, UDP-Ara, UDP-Xyl, CDP-Glc, and GDP-Man) are substrates for AtUGlcAE1Δ1–64. Based on these product analyses and substrate-specificity studies, we have functionally cloned a plant gene whose product is involved in 4-epimerization of UDP-GlcA. We accordingly suggest naming the gene AtUGlcAE1, for Arabidopsis UDP-GlcA epimerase 1.
Enzymatic Characterization of AtUGlcAE1Δ1–64
Recombinant AtUGlcAE1Δ1–64 does not require metal ions for activity, and the enzyme maintains maximum activity in the presence of EDTA (Table III). Glycerol, at 20% (v/v), was found to increase activity significantly, while dimethyl sulfoxide has no effect. High concentration of monovalent salts (KCl and NaCl, > 300 mm), on the other hand, inhibit the activity and cause complete inactivation of the enzyme when stored at −20°C for 2 weeks. However, storing the enzyme with glycerol sustains full AtUGlcAE1Δ1–64 activity. The enzyme is active over a relatively broad pH range with activity observed between pH 6.8 and 8.2, and has maximal activity at a pH of 7.4 to 7.6 (Table IV) in phosphate buffer. The activity is completely abolished at pH values lower than 4 and above 9.5 in phosphate buffer (data not shown). AtUGlcAE1Δ1–64 is active between 25°C and 55°C, with maximal activity between 30°C and 42°C (Table IV).
Table III.
Effect of various additives on AtUGlcaE1Δ1–64 activity
| Additive and Concentration in the Assay | Relative AtUGlcAE1Δ1–64 Activity |
|---|---|
| %a | |
| Water | 100 |
| KCl (200 mm) | 64 |
| NaCl (300 mm) | 36 |
| EDTA (1 mm) | 98 |
| DTT (2 mm) | 91 |
| MgCl2 (2 mm) | 96 |
| Glycerol (20%, v/v) | 146 |
| Dimethyl sulfoxide (4%) | 100 |
AtUGlcAE1Δ1–64 was separately mixed with each additive for 30 min on ice. One millimolar UDP-GlcA was added, and the reactions were incubated for 30 min at 30°C. The amounts of UDP-GalA were determined by HPLC. Data are the average relative amounts of UDP-GalA produced compared to the control water (no additives). Each value is the mean of duplicate reactions, and the values varied by no more than 5%.
Under the assay conditions described, the reaction rate (calculated as the amount of UDP-GalA produced from UDP-GlcA) is linear with time for up to 50 min. Kinetic studies were carried out for 15 min, and the apparent Km value for UDP-GlcA was 0.72 mm. The Kcat of the recombinant enzyme was 24 (s−1) with a catalytic efficiency [Kcat/Km] value of 3.2 × 104 (m−1 s−1; Table IV).
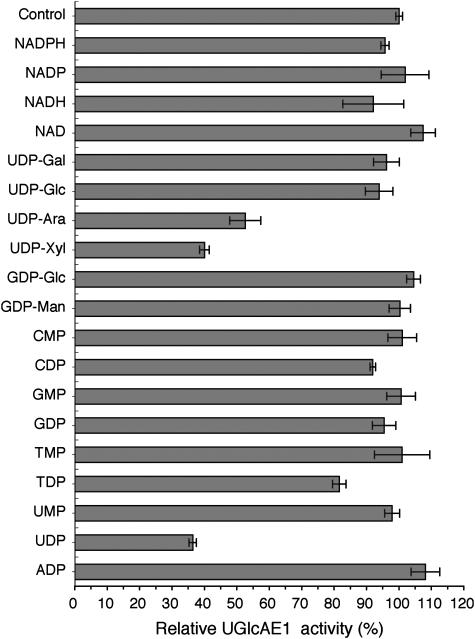
To further compare the AtUGlcAE1Δ1–64 to UDP-GlcA epimerases isolated from other species, the activity of AtUGlcAE1Δ1–64 was tested with various nucleotides and nucleotide sugars (Fig. 5). Unlike the strong inhibition observed with UDP-Glc and UDP-Gal for the Algal (Gaunt et al., 1974) and the Streptococcal (Munoz et al., 1999) UDP-GlcA epimerases, these nucleotide sugars had no effect on the activity of the Arabidopsis enzyme (Fig. 5). However, UDP-Xyl and UDP both strongly inhibited AtUGlcAE1Δ1–64 by 60% (Fig. 5), while UMP had negligible effect on the activity of the enzyme. The 4-epimer of UDP-Xyl, UDP-Ara, also inhibited the activity of AtUGlcAE1Δ1–64.
Figure 5.
The effects of nucleotides and nucleotide sugars on the activity of the recombinant AtUGlcaE1Δ1–64. UDP-GlcA (1 mm) was reacted for 30 min with recombinant AtUGlcAE1Δ1–64 in the absence or presence of different nucleotides and nucleotide sugars (2 mm). Note that 100% activity corresponds to 30 nmol of UDP-GalA produced. The data are the average relative amount of UDP-GalA produced compared to the control from two experiments, and each value is the mean of triplicate reactions.
The Recombinant AtUGlcAE1Δ1–64 May Function as a Dimer
Gaunt et al. (1974) estimated the Mr of the native Algal UDP-GlcA epimerase to be 54,000. The product of the recombinant Streptococcal cap1J gene (encoding a polypeptide of approximately 40 kD) was estimated by gel filtration to have a Mr of 80,000 (Munoz et al., 1999), suggesting that the prokaryotic enzyme is a dimer. The recombinant Arabidopsis AtUGlcAE1Δ1–64 has a Mr of 43,500. To determine the Mr of the active AtUGlcAE1Δ1–64, the recombinant enzyme was separated by size-exclusion chromatography using a Superdex 75 gel-filtration column. The elution position of the AtUGlcAE1Δ1–64 activity peak was calculated to correspond to a mass of about 88 kD (Table IV), suggesting that the Arabidopsis enzyme, like the prokaryote cap1J, is active as a dimer.
All Three Isoforms of AtUGlcAE Are Transcribed in Arabidopsis
The expression of gene-specific AtUGlcAE transcript was determined by reverse transcription (RT)-PCR. Transcripts of AtUGlcAE1, 2, and 3 were observed in all Arabidopsis tissues examined (Fig. 6). However, the relative amount of transcript encoding AtUGlcAE1 was lower in leaf and stem tissues when compared to AtUGlcAE2 and 3.
Figure 6.
Gene expression of three AtUGlcAE isoforms in Arabidopsis. Total RNA was extracted from root, flower, stem, and leaf and used for a RT-PCR reaction with gene-specific primers. The lane labeled 1 in each section is the AtUGlcAE3 (At4g30440) transcript, lane 2 is the AtUGlcAE1 (At2g45310) transcript, and lane 3 indicates AtUGlcAE2 (At3g23820). UXS3 (Harper and Bar-Peled, 2002) was used as a control (labeled as lane 4).
DISCUSSION
GalA accounts for between 20% and 35% of the glycosyl residues present in plant primary cell walls, principally as a major component of pectic polysaccharides. Thus, enzymes involved in the formation of the activated form of GalA are likely to have important roles in regulating pectin biosynthesis (Orellana and Mohnen, 1999; Mohnen, 2002).
Our study reports the functional identification of a distinct Arabidopsis gene (At2g45310) that encodes a 4-epimerase (AtUGlcAE1) that interconverts UDP-GlcA and UDP-GalA, as determined by HPLC and NMR (Fig. 3; Table II). We have shown that recombinant AtUGlcAE1 favors the formation of UDP-GalA because at equilibrium AtUGlcAE1 has a conversion ratio of 1 to approximately 2 (UDP-GlcA:UDP-GalA; Table IV). We suggest that UDP-GalA is less susceptible than UDP-GlcA to epimerization at C-4 because a hydrogen bond may form between the axial hydroxyl at C-4″ and the C-6″ carboxyl group of GalA. This hydrogen bond will reduce the net negative charge of UDP-GalA and will therefore stabilize the molecule when compared with UDP-GlcA. Support for the net charge differences between UDP-GalA and the more ionic UDP-GlcA is evident by the fact that the UDP-GlcA is retained longer on an anion-exchange column, when compared with UDP-GalA (Fig. 3).
The hydropathy plot of the N-terminal region of AtUGlcAE1 (Fig. 1B) predicts that the protein has a single trans-membrane domain. In addition to AtUGlcAE1, we isolated two additional cDNAs (AtUGlcAE2 and 3) that share sequence similarity with AtUGlcAE1. All three cloned cDNAs (AtUGlcAE1, 2, and 3; At2g45310, At3g23820, and At4g30440 respectively; Table I) encode proteins that have distinct N-terminal amino acid regions (amino acids approximately 1–100), and each includes a putative trans-membrane domain, which supports the biochemical data for the existence of multiple UGlcAE isoforms in plants. We further speculate that the different UDP-GlcA epimerases in Arabidopsis may correspond to the digitonin-soluble and digitonin-insoluble isoforms reported in other plants (Feingold et al., 1960).
A total of six genes with similarity to the Streptococcal cap1J gene exist in the Arabidopsis genome (Reiter and Vanzin, 2001). While this work was being revised for publication, the activities of two Arabidopsis UDP-GlcA epimerases distinct from the epimerase described here were reported, although detailed chemical analysis of the product was not performed (Molhoj et al., 2004; Usadel et al., 2004). The NMR data provided in this report clearly and without ambiguity indicate that AtUGlcAE1 is a true reversible UDP-GlcA epimerase that favors the formation of UDP-GalA. Furthermore, as AtUGlcAE1 (At2g45310) shares a high amino acid sequence similarity to At4g30440 (GAE1; Molhoj et al., 2004) and At4g12250 (GAE6; Usadel et al., 2004), as well as to the other three uncharacterized member of this gene family, it is highly likely that all six isoforms are UDP-GlcA/UDP-GalA 4-epimerases. However, these isoforms have distinct enzymatic properties: AtUGlcAE1 (At2g45310) described in this report, for example, is inhibited by UDP, UDP-Xyl, and UDP-Ara, while the At4g30440 protein (GAE1) was reported to be inhibited by only UDP-Xyl. Recombinant AtUGlcAE1 (At2g45310) has a Km of 0.72 mm and UDP-GlcA to UDP-GalA conversion ratio of 1.9, whereas recombinant At4g30440 protein (GAE1) and At4g12250 (GAE6) protein were reported to have lower Km values (0.19 mm and 0.23 mm, respectively) and a lower conversion ratio (1.3) of UDP-GlcA to UDP-GalA (Molhoj et al., 2004). These significant biochemical differences could indicate that the distinct isoforms of UDP-GlcAE in Arabidopsis have different biological roles.
It has been suggested that control of the flux of nucleotide sugars could play a role in the regulation of polysaccharide synthesis (Dalessandro and Northcote, 1977a, 1997b). The mechanisms that control flux have yet to be determined. Recent studies provide evidence that allosteric inhibition of nucleotide-sugar interconverting enzymes could be one such control mechanism. For example, UDP-Xyl strongly inhibits UDP-Glc dehydrogenase (Hinterberg et al., 2002; Bar-Peled et al., 2004) and several isoforms of UDP-GlcA decarboxylase (Harper and Bar-Peled, 2002; Suzuki et al., 2003). We have shown here that UDP-Xyl and UDP-Ara inhibit the recombinant AtUGlcAE1 (Fig. 5), which suggests that these nucleotide sugars could regulate the amount of UDP-GalA that is available for pectin biosynthesis. Whether activity of all of the AtUGlcAE isoforms is affected by these (or other) nucleotide sugars remains to be determined.
MATERIALS AND METHODS
Cloning and Expression Analysis of Arabidopsis UGlcAE
Arabidopsis (Arabidopsis thaliana) dbEST were searched to identify cDNAs that encode amino acid sequences with similarity to the Streptococcus pneumoniae UGlcAE (cap1J). Several ESTs (r90663, t20825, t21711, h76650, and ai100746) and genomic clones (F6n15.16, att4ca, f17i23, and aab82632) that showed similarity to the cap1J bacterial gene products were identified and used to isolate cDNA clones and to design primers to obtain the corresponding full-length Arabidopsis cDNA by RT-PCR. Total RNA from Arabidopsis ecotype Columbia plants was isolated using Trizol reagent (Gibco-BRL, Gaithersburg, MD; Chomczynski, 1993). RNA was reverse transcribed into cDNA at 42°C for 60 min using 1 μm oligo(dT) primer (5′TTCTAGAATTCAGCGGCCGCTT15-TTV) in a 20-μL total reaction consisting of 50 mm Tris-HCl, pH 8.4, 75 mm KCl, 3 mm MgCl2, 0.2 mm of each deoxynucleotide triphosphate (dNTP), 10 mm dithiothreitol (DTT), and 200 units SuperScript II− RNaseH− reverse transcriptase (Gibco-BRL). Following the reaction, 2 units of RNaseH (Gibco-BRL) were added. An aliquot (2 μL) of the resulting reverse-transcribed products was used as a template for PCR using 1 unit of high- fidelity Platinum Taq DNA polymerase mixed (Gibco-BRL) with GB-D proofreading DNA polymerase and Platinum antibody, 0.2 μm of the sense primer (120#1S1-26 5′CATATGTCACGTCTTGACGACATACCTTC), 0.2 μm of the antisense primer (120#2AS/1294-1317 5′GCGGCCGCTCATCTAGCGGCGGCTTTTTTGTCG), and 0.2 mm of each dNTP (Roche, Basel) in buffer containing 60 mm Tris-SO4, pH 8.9, 18 mm ammonium-sulfate, and 1 mm MgSO4. The RT-PCR reaction product (1,325 bp) was separated by agarose-gel electrophoresis, purified, and cloned into pCR2.1-TOPO plasmid (Invitrogen, Carlsbad, CA). The cloned RT-PCR product in vector pCR2.1:121.1 was sequenced and the nucleotide sequence submitted to GenBank (accession no. AY594693, AtUGlcAE1). For expression in Escherichia coli, PCR reaction was carried for 15 cycles with sense 122#3S/196-221 5′CATATGTCTCGCCGTTCCTCCGAACAACAC and antisense 120#2AS/1294-1317 primers. The 1,217-bp PCR product consisting of a truncated coding region lacking the putative trans-membrane region (Δ1–64) was subcloned into pCR2.1 to generate pCR2.1:122.2 and sequenced. The NdeI-NotI fragment from pCR2.1:122.2, consisting of the coding region spanning from amino acid 65 to 438 of AtUGlcAE1, was subcloned into pET28b E. coli expression vector (Novagen, Madison, WI). The resulting clone, pET28b:122.2#3, was constructed to give an in-frame N-terminal 6His-AtUGlcAE1 gene fusion.
For AtUGlcAE1, 2, and 3 expression studies in Arabidopsis Columbia, total RNA was isolated from flowers, fully expanded rosette leaves, and stems of 6-week-old plants and from roots of 4-week-old plants grown in liquid media as described (Bar-Peled and Raikhel, 1997). The RNA was reverse transcribed into cDNA in 20-μL reactions using 200 units of SuperScript II reverse transcriptase (Invitrogen) and 1 μm oligo(dT) primer using the manufacturer's recommended buffer, 10 mm DTT, and 0.2 mm of each dNTP. One-twentieth of each of the reverse-transcribed products was used as a template for PCR reactions using 0.5 units Taq DNA polymerase (Roche), the manufacturer's buffer, 0.2 mm dNTP, 1.5 mm MgCl2, and 0.2 μm AtUGlcAE1 gene-specific sense and antisense primers (see above). The transcripts of AtUXS3 (UDP-GlcA decarboxylase) and AtUGlcAE3 described by Harper and Bar-Peled (2002) and AtUGlcAE2 were used as internal RT-PCR controls. PCR conditions were: 1 cycle at 95°C for 2 min; and 30 cycles (95°C, 30 s; 54°C, 30 s; 70°C, 1.5 min) and a final extension at 70°C for 5 min. One-tenth of each sample and the DNA MW marker (1 KB plus; Invitrogen) were resolved on a Tris-acetate EDTA-1% agarose gel and visualized by staining with ethidium bromide.
Protein Expression, Purification, and Mass Determination
Fifteen milliliters of an overnight culture of E. coli carrying the pET28b:122.2#3 (AtUGlcAE1) or control vector were used to inoculate 0.5 L of Luria-Bertani liquid broth supplemented with 50 μg/mL kanamycin and 30 μg/mL chloramphenicol. Cells were grown at 37°C while shaking (200 rpm) until a cell density of A600 reached 0.6 to 0.8. Gene expression was induced by the addition of isopropylthio-β-galactoside to a final concentration of 0.5 mm. After 3 h of growth at approximately 25°C, the cells were collected by centrifugation (6,000g at 4°C for 10 min). The cells were washed with cold water, resuspended in 20 mL of extraction buffer (20 mm Tris-HCl, pH 7.6, 10% glycerol, 1 mm EDTA), and supplemented with fresh 1 mm DTT and 0.5 mm phenylmethylsulfonyl fluoride. The cells were cooled in an iced-water bath and ruptured by 24 sonication intervals (10-s pulse followed by 20-s rest) using a microtip probe and a Fisher 550 sonicator (Fisher Scientific, Loughborough, Leicestershire, UK) set at a power of 4.5. The suspension was centrifuged (20,000g, 30 min, 4°C), and 1 mL of the supernatant was injected at a flow rate of 1 mL min−1 onto an anion-exchange column (0.5 cm × 5 cm, packed with Source Q15; Pharmacia, Piscataway, NJ) that was preequilibrated with cold buffer A (50 mm sodium phosphate, pH 7.6). After the supernatant was loaded, the column was washed with buffer A until the UV (A280) baseline was stabilized (30 min). The bound proteins were then eluted using a linear 30-min salt gradient from 0 to 0.5 m NaCl in the same buffer, at a flow rate of 0.5 mL min−1, followed by a 5-min gradient from 0.5 to 0.8 m NaCl at the same flow rate. Proteins were detected by monitoring the A280 of the column effluent. The active fractions were desalted and concentrated using a YM-10 Centricon centrifuge filter (Amicon, Bedford, MA) and stored at −20°C. Gel-filtration chromatography was used to estimate the Mr of the native recombinant AtUGlcAE1Δ1–64. AtUGlcAE1Δ1–64 (0.25 mL) was loaded at a flow rate of 0.5 mL min−1 onto a Superdex75 column (HR10X30; Pharmacia) previously equilibrated with 0.1 m sodium phosphate, pH 7.6, 4% (v/v) glycerol. Proteins were eluted with the same buffer at a flow rate of 0.5 mL min−1. Fractions (0.25, 0.5 mL) were collected for analysis. The gel-filtration column was calibrated using protein markers (aldolase, 158 kD; bovine serum albumin, 67 kD; ovalbumin, 43 kD; chymotrypsinogen A, 25 kD; myoglobin, 17.6 kD; ribonuclease A, 13.7 kD [Amersham Biosciences, Piscataway, NJ]). The A210 of the eluant was monitored to determine the elution times of each protein marker. Chromatography steps were performed at room temperature, and fractions containing AtUGlcAE1Δ1–64 activity were analyzed and stored at −20°C. Proteins were separated by 0.1% SDS-12% PAGE (Bar-Peled et al., 1991) alongside Mr markers (Bio-Rad, Hercules, CA) and visualized by staining with Coomassie blue or SimplyBlue (Invitrogen).
Enzyme Assay, HPLC, and NMR Product Analysis
UDP-GlcA epimerase activity was initiated by adding UDP-GlcA (1 mm) to a reaction mix (50 μL) consisting of 0.1 m sodium phosphate, pH 7.6, 20% (v/v) glycerol, 0.2 mm β-NAD+, 1 μg/mL AtUGlcAE1Δ1–64. The assay was carried out for 30 min at 37°C (unless otherwise mentioned), and the reaction was terminated by the addition of 50 μL of chloroform. The mixture was vortexed and centrifuged at 16,000g for 5 min at room temperature. The aqueous phase was retained, and the organic phase extracted with 80 μL of water. The aqueous phases were combined and injected via HPLC at 1 mL min−1 onto an anion-exchange column (Source15Q [Pharmacia] packed in 250- × 4.6-mm column) previously equilibrated with 2 mm ammonium format. After a 5-min wash, the sample was eluted with a linear 2 to 600 mm ammonium-formate gradient formed over 25 min (Bar-Peled et al., 2001). Nucleotides and nucleotide sugars were detected by their UV absorbance using a Waters (Milford, MA) photodiode array detector. The maximum absorbance for uridine, UDP-GlcA, and UDP-GalA was 261.8 nm in 0.5 to 0.6 m ammonium formate. The column was calibrated with authentic nucleotides and nucleotide sugars (Sigma, St. Louis). For NMR analysis, UV-absorbing peaks eluting from the HPLC column were collected, diluted five times with water, and lyophilized to remove the ammonium formate. The residue was dissolved in water, relyophilized twice, and exchanged twice with 99.96% deuterium oxide. Proton NMR spectroscopy was performed at 25°C on a Varian (Palo Alto, CA) Inova spectrometer operating at 500 MHz. The chemical shifts (δ) are reported in ppm relative to external acetone (2.224 ppm).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AAT06796.
Acknowledgments
We wish to thank Dr. John Glushka of the Complex Carbohydrate Research Center for his expertise and discussion regarding NMR data, and Dr. Michael Hahn for his constructive comments on this manuscript.
This work was supported in part by the U.S. Department of Agriculture (grant no. 2002–35318–12620 to M.B.-P.) and by the U.S. Department of Energy (Center for Plant and Microbial Complex Carbohydrates grant no. DE–FG05–93ER20097).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.052365.
References
- Adeyeye J, Azurmendi HF, Stroop CJ, Sozhamannan S, Williams AL, Adetumbi AM, Johnson JA, Bush CA (2003) Conformation of the hexasaccharide repeating subunit from the Vibrio cholerae O139 capsular polysaccharide. Biochemistry 42: 3979–3988 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankel H, Tischer RG (1969) UDP-D-glucuronate 4-epimerase in blue-green algae. Biochim Biophys Acta 178: 415–419 [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Griffith CL, Doering TL (2001) Functional cloning and characterization of a UDP-glucuronic acid decarboxylase: The pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc Natl Acad Sci USA 98: 12003–12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M, Griffith CL, Ory JJ, Doering TL (2004) Biosynthesis of UDP-GlcA, a key metabolite for capsular polysaccharide synthesis in the pathogenic fungus Cryptococcus neoformans. Biochem J 381: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M, Lewinsohn E, Fluhr R, Gressel J (1991) UDP-rhamnose:flavanone-7-O-glucoside-2″-O-rhamnosyltransferase. Purification and characterization of an enzyme catalyzing the production of bitter compounds in citrus. J Biol Chem 266: 20953–20959 [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV (1997) An efficient method for cloning in-frame fusion protein genes. Anal Biochem 250: 262–264 [DOI] [PubMed] [Google Scholar]
- Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15: 536–537 [PubMed] [Google Scholar]
- Dalessandro G, Northcote DH (1977. a) Possible control sites of polysaccharides synthesis during cell growth and wall expansion of pea seedlings (Pisum sativum L.). Planta 134: 39–44 [DOI] [PubMed] [Google Scholar]
- Dalessandro G, Northcote DH (1977. b) Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in sycamore and poplar. Biochem J 162: 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill AG, Albersheim P, Delmer DP (1980) The primary cell walls of flowering plants. In NE Tobert, ed, The Biochemistry of Plants: A Comprehensive Treatise, Vol 1. Academic Press, New York, pp 92–162
- Daude N, Gallaher TK, Zeschnigk M, Starzinski-Powitz A, Petry KG, Haworth IS, Reichardt JK (1995) Molecular cloning, characterization, and mapping of a full-length cDNA encoding human UDP-galactose 4′-epimerase. Biochem Mol Med 56: 1–7 [DOI] [PubMed] [Google Scholar]
- Dormann P, Benning C (1996) Functional expression of uridine 5′-diphospho 4-epimerase (E.C. 5.1.3.2) from Arabidopsis thaliana in Saccharomyces cerevisiae and Escherichia coli. Arch Biochem Biophys 327: 27–34 [DOI] [PubMed] [Google Scholar]
- Feingold DS, Neufeld EF, Hassid WZ (1960) The 4-epimerization and decarboxylation of uridine diphosphate d-glucuronic acid by extracts from Phaseolus aureus seedlings. J Biol Chem 235: 910–913 [PubMed] [Google Scholar]
- Feng L, Tao J, Guo H, Xu J, Li Y, Rezwan F, Reeves P, Wang L (2004) Structure of the Shigella dysenteriae 7 O antigen gene cluster and identification of its antigen specific genes. Microb Pathog 36: 109–111 [DOI] [PubMed] [Google Scholar]
- Forsberg LS, Carlson RW (1998) The structures of the lipopolysaccharides from Rhizobium etli strains CE358 and CE359. The complete structure of the core region of R. etli lipopolysaccharides. J Biol Chem 273: 2747–2757 [DOI] [PubMed] [Google Scholar]
- Garcia E, Garcia P, Lopez R (1993) Cloning and sequencing of a gene involved in the synthesis of the capsular polysaccharide of Streptococcus pneumoniae type 3. Mol Gen Genet 239: 188–195 [DOI] [PubMed] [Google Scholar]
- Garcia E, Arrecubieta C, Munoz R, Mollerach M, Lopez R (1997) A functional analysis of the Streptococcus pneumoniae genes involved in the synthesis of type 1 and type 3 capsular polysaccharides. Microb Drug Resist 3: 73–87 [DOI] [PubMed] [Google Scholar]
- Gaunt MA, Maitra US, Ankel H (1974) Uridine diphosphate galacturonate 4-epimerase from the blue-green alga Anabaena flos-aquae. J Biol Chem 249: 2366–2372 [PubMed] [Google Scholar]
- Gloaguen V, Wieruszeski JM, Strecker G, Hoffmann L, Morvan H (1995) Identification by NMR spectroscopy of oligosaccharides obtained by acidolysis of the capsular polysaccharides of a thermal biomass. Int J Biol Macromol 17: 387–393 [DOI] [PubMed] [Google Scholar]
- Gray JS, Yang BY, Montgomery R (2000) Extracellular polysaccharide of Erwinia chrysanthemi A350 and ribotyping of Erwinia chrysanthemi spp. Carbohydr Res 324: 255–267 [DOI] [PubMed] [Google Scholar]
- Harper AD, Bar-Peled M (2002) Biosynthesis of UDP-xylose. Cloning and characterization of a novel Arabidopsis gene family, UXS, encoding soluble and putative membrane-bound UDP-glucuronic acid decarboxylase isoforms. Plant Physiol 130: 2188–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberg B, Klos C, Tenhaken R (2002) Recombinant UDP-glucose dehydrogenase from soybean. Plant Physiol Biochem 12: 1011–1017 [Google Scholar]
- Holst O, Susskind M, Grimmecke D, Brade L, Brade H (1998) Core structures of enterobacterial lipopolysaccharides. Prog Clin Biol Res 397: 23–35 [PubMed] [Google Scholar]
- Jann K, Westphal O (1975) Microbial polysaccharides. In M Sela, ed, The Antigens, Vol 3. Academic Press, New York, pp 1–125
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23: 403–405 [DOI] [PubMed] [Google Scholar]
- Lemaire HG, Muller-Hill B (1986) Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res 14: 7705–7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljebjelke K, Adolphson R, Baker K, Doong RL, Mohnen D (1995) Enzymatic synthesis and purification of uridine diphosphate [14C]galacturonic acid: a substrate for pectin biosynthesis. Anal Biochem 225: 296–304 [DOI] [PubMed] [Google Scholar]
- Mohnen D (2002) Biosynthesis of pectins. In GB Seymour, JP Knox, eds, Pectins and Their Manipulation. Blackwell Publishing and CRC Press, Oxford, pp 52–98
- Molhoj M, Verma R, Reiter WD (2004) The biosynthesis of d-galacturonate in plants. Functional cloning and characterization of a membrane-anchored UDP-d-Glucuronate 4-epimerase from Arabidopsis. Plant Physiol 135: 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro A, De Castro C, Lanzetta R, Parrilli M, Raio A, Zoina A (2003) Structural elucidation of a novel core oligosaccharide backbone of the lipopolysaccharide from the new bacterial species Agrobacterium larrymoorei. Carbohydr Res 338: 2721–2730 [DOI] [PubMed] [Google Scholar]
- Munoz R, Lopez R, de Frutos M, Garcia E (1999) First molecular characterization of a uridine diphosphate galacturonate 4-epimerase: an enzyme required for capsular biosynthesis in Streptococcus pneumoniae type 1. Mol Microbiol 31: 703–713 [DOI] [PubMed] [Google Scholar]
- Munoz R, Mollerach M, Lopez R, Garcia E (1997) Molecular organization of the genes required for the synthesis of type 1 capsular polysaccharide of Streptococcus pneumoniae: formation of binary encapsulated pneumococci and identification of cryptic dTDP-rhamnose biosynthesis genes. Mol Microbiol 25: 79–92 [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Feingold DS, Hassid WZ (1958) Enzymatic conversion of uridine diphosphate d-glucuronic acid to uridine diphosphate galacturonic acid, uridine diphosphate xylose, and uridine diphosphate arabinose. J Am Chem Soc 80: 4430–4431 [Google Scholar]
- Orellana A, Mohnen D (1999) Enzymatic synthesis and purification of [3H] uridine diphosphate galacturonic acid for use in studying Golgi-localized transporters. Anal Biochem 272: 224–231 [DOI] [PubMed] [Google Scholar]
- Plotz BM, Lindner B, Stetter KO, Holst O (2000) Characterization of a novel lipid A containing D-galacturonic acid that replaces phosphate residues. The structure of the lipid a of the lipopolysaccharide from the hyperthermophilic bacterium Aquifex pyrophilus. J Biol Chem 275: 11222–11228 [DOI] [PubMed] [Google Scholar]
- Raguenes G, Cambon-Bonavita MA, Lohier JF, Boisset C, Guezennec J (2003) A novel, highly viscous polysaccharide excreted by an alteromonas isolated from a deep-sea hydrothermal vent shrimp. Curr Microbiol 46: 448–452 [DOI] [PubMed] [Google Scholar]
- Reiter WD, Vanzin GF (2001) Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Mol Biol 47: 95–113 [PubMed] [Google Scholar]
- Stroop CJ, Xu Q, Retzlaff M, Abeygunawardana C, Bush CA (2002) Structural analysis and chemical depolymerization of the capsular polysaccharide of Streptococcus pneumoniae type 1. Carbohydr Res 337: 335–344 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Suzuki Y, Kitamura S (2003) Cloning and expression of a UDP-glucuronic acid decarboxylase gene in rice. J Exp Bot 54: 1997–1999 [DOI] [PubMed] [Google Scholar]
- Thoden JB, Hegeman AD, Wesenberg G, Chapeau MC, Frey PA, Holden HM (1997) Structural analysis of UDP-sugar binding to UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 36: 6294–6304 [DOI] [PubMed] [Google Scholar]
- Usadel B, Schluter U, Molhoj M, Gipmans M, Verma R, Kossmann J, Reiter WD, Pauly M (2004) Identification and characterization of a UDP-d-glucuronate 4-epimerase in Arabidopsis. FEBS Lett 569: 327–331 [DOI] [PubMed] [Google Scholar]
- Watt G, Leoff C, Harper AD, Bar-Peled M (2004) A bifunctional 3,5-epimerase/4-keto reductase for nucleotide-rhamnose synthesis in Arabidopsis. Plant Physiol 134: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirenga RK, Terpstra P, Hol WGJ (1986) Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol 187: 101–107 [DOI] [PubMed] [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139 [DOI] [PubMed] [Google Scholar]