Abstract
Nitrogen (N) is an essential requirement for kernel growth in maize (Zea mays); however, little is known about how N assimilates are metabolized in young earshoots during seed development. The objective of this study was to assess amino acid metabolism in cob and spikelet tissues during the critical 2 weeks following silking. Two maize hybrids were grown in the field for 2 years at two levels of supplemental N fertilizer (0 and 168 kg N/ha). The effects of the reproductive sink on cob N metabolism were examined by comparing pollinated to unpollinated earshoots. Earshoots were sampled at 2, 8, 14, and 18 d after silking; dissected into cob, spikelet, and/or pedicel and kernel fractions; then analyzed for amino acid profiles and key enzyme activities associated with amino acid metabolism. Major amino acids in the cob were glutamine (Gln), aspartic acid (Asp), asparagine (Asn), glutamate, and alanine. Gln concentrations dropped dramatically from 2 to 14 d after silking in both pollinated and unpollinated cobs, whereas all other measured amino acids accumulated over time in unpollinated spikelets and cobs, especially Asn. N supply had a variable effect on individual amino acid levels in young cobs and spikelets, with Asn being the most notably enhanced. We found that the cob performs significant enzymatic interconversions among Gln, alanine, Asp, and Asn during early reproductive development, which may precondition the N assimilate supply for sustained kernel growth. The measured amino acid profiles and enzymatic activities suggest that the Asn to Gln ratio in cobs may be part of a signal transduction pathway involving aspartate aminotransferase, Gln synthetase, and Asn synthetase to indicate plant N status for kernel development.
Though there are numerous studies concerning growth and development of maize (Zea mays) grain, there is a general lack of knowledge concerning the physiology of the cob tissues, to which kernels are attached. This is despite the fact that the cob tissues are the link between vegetative source and reproductive sink tissues. In addition, approximately one-half of the earshoot (minus husk and shank) is cob material at silking, with the cob proportion decreasing as the grain develops. Early studies on the physical characteristics of the earshoot and cob go back more than a century (Harshberger, 1893). More recent research has examined cob shape (Srinivas et al., 1991; Bhattacharya et al., 1996; Orr et al., 1997; Pearson, 2000) and the genetic control of cob color (Nguetta and Cross, 1997; Sidorenko et al., 1999). A few studies have also focused on sugars (BeMiller and Hoffmann, 1972), nitrogen (N; Crawford et al., 1982), minerals (Mozafar, 1990), and antioxidants (Cevallos and Cisneros, 2003) contained within maize earshoots.
A typical view of maize cobs is that they serve as a temporary storage organ and as a conveyor of nutrients to the developing kernels (Crawford et al., 1982). A larger metabolic role of the cob in kernel growth, however, can be inferred from the observation that an attached cob piece is necessary for continued growth of maize ovules in organ culture (Felker, 1992). Additionally, the ratio of cob to kernel sinks influences kernel growth, even under conditions of similar assimilate supplies (Jones et al., 1981). Therefore, the cob may interact with the assimilate supply to influence earshoot development and subsequent kernel growth.
It is clear that events early in earshoot development can have a pronounced effect on subsequent kernel growth, as the reproductive sink capacity of the maize plant is determined during the period from about 1 week before to 2 weeks after silking (Cantarero et al., 1999). For maize, this sink capacity is largely driven by final kernel number as a result of differences in kernel set or abortion (Schussler and Westgate, 1991; Cantarero et al., 1999). However, other sink differences can be attributed to changes in individual kernel size as a result of endosperm cell division rates, starch granule production, and enzymatic activities that peak during this critical period (Jacobs and Pearson, 1992; Lur and Setter, 1993; Cazetta et al., 1999). Many aspects of reproductive sink development are positively influenced by the N supply; thus, farmers routinely apply high levels of N fertilizer during maize cultivation to optimize grain yields.
Whereas endosperm and pedicel enzyme activities have been found to relate to subsequent kernel growth, we could not find any previous research that directly measured enzyme activities in the cob pertaining to N assimilation and amino acid movement through the young earshoot. Therefore, our objective was to determine the extent of N metabolism in young earshoots and the subsequent influence on pools of amino acid assimilates. To accomplish this objective, we altered the N assimilate supply (source) or prevented pollination (reproductive sink) to observe inherent N metabolism. Then, using metabolite profiling in conjunction with measuring activities of key N metabolism enzymes, we found that the young cob (2–18 d after silking [DAS]) actively metabolizes amino acids, possibly to regulate seed growth. Taken together, the changes in amino acid profiles and enzyme activities in the cob suggest that Aspartate aminotransferase (AspAT), Asn synthetase (AS), and Gln synthetase (GS) activities within this tissue act in concert to regulate the Gln to Asn ratio, which functions as a signal of vegetative N status for grain growth.
RESULTS
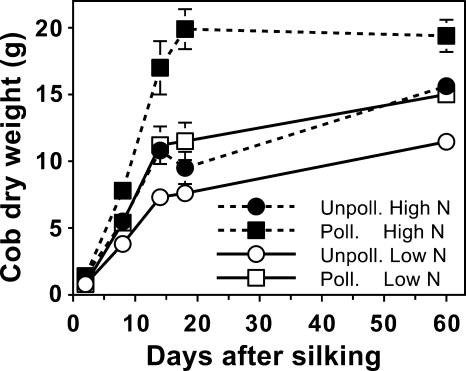
While both the N fertilizer level and the pollination status influenced the final cob growth, there was a period during early development (until approximately 18 DAS) when cobs of all treatments rapidly accumulated dry matter (Fig. 1). By 18 DAS, cobs of all the pollination and N treatment combinations acquired at least two-thirds of their final dry weight. Based on the mature cob weight, supplying adequate N or pollinating the earshoot resulted in independent and additive increases in cob growth of approximately 4 g.
Figure 1.
Seasonal growth of maize cobs as influenced by N supply and pollination status. Black symbols with dashed lines are for plants grown with an adequate N supply (High N), while white symbols with solid lines represent low N plants. Pollinated plants are represented by square symbols, and unpollinated plants by circles. Data are means ± se (n = 4).
To show the effects of inherent cob metabolism and N supply on cob composition, we selected two representative dates from our study and grouped the amino acids according to their synthetic pathways (Table I). Pollinated cobs grown at either N supply tended to have lower levels of amino acids than unpollinated cobs, especially at 14 DAS, presumably due to transport into developing kernels. From 8 to 14 DAS, the majority of amino acids either accumulated or remained constant in unpollinated cobs, with the exception of Gln and Met. Supplying adequate N also resulted in a higher sum total of free amino acid concentrations at 8 DAS (81% for pollinated versus 67% for unpollinated cobs). However, at 14 DAS, N supply only increased the amino acid concentration of unpollinated cobs.
Table I.
Influence of N supply and pollination status on the concentrations of individual free amino acids in the maize cob at two stages during early postsilking growth
| Amino Acid
|
8 DAS
|
14 DAS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Pollinated
|
Unpollinated
|
Pollinated
|
Unpollinated
|
|||||
| Low N | High N | Low N | High N | Low N | High N | Low N | High N | |
| μg/g dry weight | ||||||||
| Asp | 557 ± 66 | 1,047 ± 227 | 813 ± 137 | 1,271 ± 264 | 1,014 ± 241 | 779 ± 101 | 1,724 ± 276 | 2,296 ± 287 |
| Asn | 185 ± 15 | 634 ± 115 | 276 ± 33 | 965 ± 157 | 456 ± 177 | 369 ± 56 | 604 ± 61 | 1,552 ± 171 |
| Lys | 90 ± 13 | 102 ± 16 | 115 ± 25 | 165 ± 22 | 123 ± 42 | 133 ± 16 | 248 ± 36 | 464 ± 67 |
| Thr | 104 ± 17 | 126 ± 23 | 152 ± 30 | 174 ± 32 | 113 ± 21 | 105 ± 11 | 186 ± 21 | 267 ± 32 |
| Met | 20 ± 8 | 31 ± 9 | 35 ± 9 | 36 ± 13 | 3 ± 3 | 1 ± 1 | 5 ± 2 | 13 ± 6 |
| Ile | 18 ± 5 | 16 ± 4 | 28 ± 6 | 36 ± 13 | 47 ± 16 | 39 ± 8 | 111 ± 14 | 167 ± 21 |
| Glu | 334 ± 33 | 520 ± 89 | 477 ± 47 | 681 ± 85 | 381 ± 82 | 297 ± 48 | 705 ± 77 | 942 ± 155 |
| Gln | 1,029 ± 161 | 2,108 ± 227 | 1,609 ± 174 | 3,316 ± 335 | 399 ± 90 | 546 ± 86 | 547 ± 142 | 924 ± 156 |
| Arg | 220 ± 23 | 372 ± 40 | 474 ± 76 | 834 ± 119 | 236 ± 105 | 227 ± 54 | 479 ± 81 | 1,234 ± 197 |
| Ala | 457 ± 87 | 543 ± 89 | 664 ± 125 | 714 ± 132 | 448 ± 106 | 441 ± 45 | 705 ± 94 | 1,341 ± 165 |
| Val | 97 ± 17 | 124 ± 23 | 154 ± 25 | 218 ± 43 | 135 ± 30 | 114 ± 17 | 228 ± 26 | 379 ± 45 |
| Leu | 27 ± 4 | 31 ± 5 | 41 ± 5 | 65 ± 12 | 56 ± 21 | 47 ± 7 | 129 ± 20 | 262 ± 33 |
| Ser | 409 ± 61 | 540 ± 94 | 588 ± 84 | 633 ± 104 | 438 ± 101 | 423 ± 47 | 671 ± 80 | 1,142 ± 139 |
| Gly | 37 ± 6 | 58 ± 12 | 56 ± 8 | 87 ± 17 | 28 ± 9 | 38 ± 5 | 45 ± 6 | 105 ± 15 |
| Trp | 5 ± 2 | 4 ± 2 | 6 ± 2 | 22 ± 13 | 35 ± 28 | 8 ± 3 | 17 ± 6 | 28 ± 8 |
| Tyr | 100 ± 10 | 104 ± 10 | 117 ± 14 | 152 ± 19 | 91 ± 33 | 55 ± 10 | 195 ± 19 | 327 ± 44 |
| Phe | 30 ± 4 | 28 ± 5 | 40 ± 8 | 59 ± 14 | 14 ± 9 | 9 ± 4 | 49 ± 12 | 97 ± 15 |
| His | 61 ± 13 | 98 ± 21 | 114 ± 27 | 184 ± 38 | 134 ± 29 | 122 ± 13 | 193 ± 18 | 312 ± 35 |
| Sum | 3,780 ± 344 | 6,845 ± 822 | 5,759 ± 679 | 9,612 ± 1,236 | 4,151 ± 1,115 | 3,752 ± 448 | 6,842 ± 836 | 11,851 ± 1,433 |
Data are means ± se (n = 12).
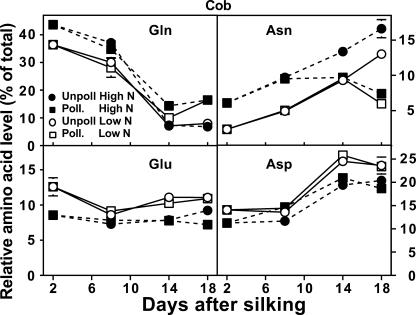
Gln was the most abundant amino acid in the cob at pollination, accounting for approximately 40% of the total free amino acids when grown at high N and 35% when grown with low N (Fig. 2). Regardless of pollination status or N supply, the relative level of Gln in cobs decreased markedly between 2 and 14 DAS. In contrast to Gln, Glu levels remained fairly constant over time, with a preference for greater proportion of Glu in cobs grown with low N.
Figure 2.
Influence of N supply and pollination status on the relative concentrations of individual free amino acids in the maize cob during early postsilking growth. Black symbols with dashed lines are for plants grown with an adequate N supply (High N), while white symbols with solid lines represent low N plants. Pollinated plants are represented by square symbols, and unpollinated plants by circles. Data are means ± se (n = 12).
The second most common amino acid in the cob was Asp, increasing from approximately 12% to 20% of the total amino acids between 2 to 18 DAS (Fig. 2). Similar to Glu, relative Asp levels were usually greater in cobs grown under low N, whereas levels of all the Asp-family amino acids (Asp, Asn, Lys, Thr, Ile, and Met) were relatively unaffected by N supply in pollinated cobs (Table I). However, in unpollinated cobs, concentrations of most of the Asp-family amino acids increased with high N, especially at 14 DAS. Asn was unique, with both high N and lack of pollination facilitating an increase in the relative Asn levels in cobs (Fig. 2). Additionally, most amino acids of the Ala (Ala, Val, and Leu) and Ser (Ser and Gly) groups doubled in relative concentration between 2 and 18 DAS for unpollinated cobs grown with high N (data not shown). Levels of the amino acids from the shikimate pathway (which includes Trp, Tyr, and Phe) and His also increased dramatically over time in high N unpollinated cobs. These increases ranged from 3.7-fold (His) to 5.3-fold (Phe; Table I).
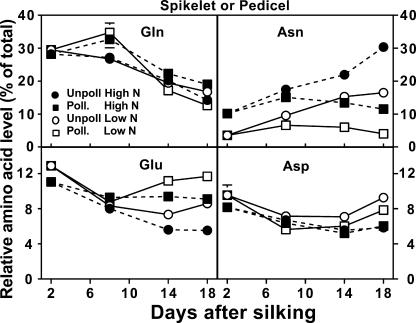
Free amino acid profiles of spikelet or pedicel fractions generally responded similarly as the cob to both N and pollination treatments, including the dramatic decrease in Gln from 2 to 18 DAS (Figs. 2 and 3). Notable differences of spikelet or pedicel tissues compared to the cob were that Asp levels remained fairly constant over time, while Asn accumulated to twice the relative level found in the cob by 18 DAS for unpollinated earshoots grown with high N.
Figure 3.
Influence of N supply and pollination status on the relative concentrations of individual free amino acids in the maize spikelet or pedicel fractions during early postsilking growth. Pedicel fractions are represented at 14 and 18 DAS for pollinated samples, while the spikelet fraction is represented for all other points. Black symbols with dashed lines are for plants grown with an adequate N supply (High N), while white symbols with solid lines represent low N plants. Pollinated plants are represented by square symbols, and unpollinated plants by circles. Data are means ± se (n = 12).
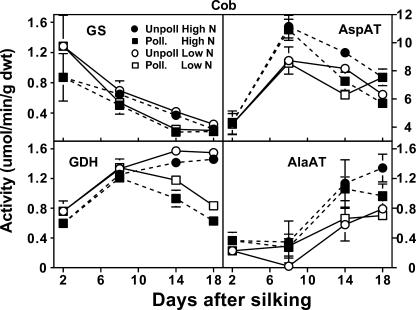
The observed differences in the amino acid compositions of the cob and spikelet or pedicel fractions led us to investigate whether specific enzyme activities were responsible for these physiological changes. Enzymes central to N metabolism were chosen that either form or utilize the major amino acids found in the cob (Gln, Asp, Glu, Ala, and Asn). The activity of GS in cobs decreased over time and was relatively unaffected by N supply or pollination status (Fig. 4). In contrast, AspAT displayed at least 6-fold greater activity in the cob than all the other measured enzymes. Between 2 and 8 DAS, cob AspAT activity increased rapidly in response to N supply, then by 14 DAS, remained higher in unpollinated cobs. Also responding to pollination status was Glu dehydrogenase (GDH) activity. The greatest increase in cob GDH activity was between 2 and 8 DAS (Fig. 4). After 8 DAS, cob GDH activity remained high in unpollinated cobs, while a decrease in activity was observed in pollinated cobs. Additionally, cob GDH activity tended to respond to N level, with low N producing greater GDH activity. In contrast to GDH, Ala aminotransferase (AlaAT) activity was not affected by pollination status, but did tend to respond to N supply.
Figure 4.
Influence of N supply and pollination status on changes in activities of four enzymes of amino acid metabolism in the maize cob during early postsilking growth. Black symbols with dashed lines are for plants grown with an adequate N supply (High N), while white symbols with solid lines represent low N plants. Pollinated plants are represented by square symbols, and unpollinated plants by circles. Data are means ± se (n = 8).
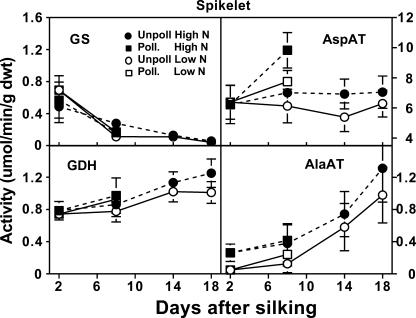
We also examined these enzyme activities in the spikelet fraction (Fig. 5), and similar to the cob, GS activity in the spikelets decreased over time, although at a more moderate rate. In contrast, spikelets that were pollinated and grown with high N increased in AspAT activity (54%) between 2 and 8 DAS. Activity of GDH increased slowly over the experimental period, with high N unpollinated spikelets having 59% greater GDH activity at 18 DAS than at 2 DAS. AlaAt activity increased in spikelets over time, exhibiting a 5-fold increase between 2 and 18 DAS. Similar to the cob, AlaAT activity was more likely to be higher in spikelets when grown with high N.
Figure 5.
Influence of N supply and pollination status on changes in activities of four enzymes of amino acid metabolism in the maize spikelets during early postsilking growth. Black symbols with dashed lines are for plants grown with an adequate N supply (High N), while white symbols with solid lines represent low N plants. Pollinated plants are represented by square symbols, and unpollinated plants by circles. Data are means ± se (n = 8).
In the pollinated earshoots, enzyme activities varied between the pedicel and the kernel fraction with the pedicel activities primarily being affected by N supply (Table II). The kernel displayed higher GS and AspAT activities than the pedicel at both 14 and 18 DAS, while AlaAT activity was less in the kernel than the pedicel at the latter sample time. Pedicel GS and GDH activity increased 100% and 25%, respectively, in response to N supply at 14 DAS. In the kernel, the only detected increase in activity due to N supply was found for AspAT at 18 DAS (20%), which was after the peak in activity observed at 14 DAS.
Table II.
Influence of N supply on enzyme activities of pedicel and kernel (seed minus pedicel) fractions of pollinated cobs during early postsilking growth
| Enzyme
|
DAS
|
Pedicel
|
Kernel
|
||
|---|---|---|---|---|---|
| N Supply
| |||||
| Low N | High N | Low N | High N | ||
| μmol min−1g−1 dwt | |||||
| GS | |||||
| 14 | 0.6 ± 0.1 | 1.2 ± 0.3 | 2.2 ± 0.6 | 2.2 ± 0.6 | |
| 18 | 0.3 ± 0.1 | 0.3 ± 0.1 | 1.1 ± 0.4 | 1.7 ± 0.7 | |
| GDH | |||||
| 14 | 1.6 ± 0.1 | 2.0 ± 0.2 | 1.6 ± 0.3 | 1.6 ± 0.2 | |
| 18 | 1.8 ± 0.1 | 2.1 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.1 | |
| AspAT | |||||
| 14 | 34.8 ± 6.9 | 45.0 ± 7.8 | 91.6 ± 1.3 | 96.9 ± 1.1 | |
| 18 | 43.8 ± 3.3 | 50.6 ± 5.6 | 70.0 ± 4.5 | 84.3 ± 8.4 | |
| AlaAT | |||||
| 14 | 3.7 ± 1.1 | 5.1 ± 1.4 | 3.3 ± 0.7 | 3.4 ± 0.7 | |
| 18 | 6.2 ± 1.3 | 7.6 ± 1.8 | 4.1 ± 1.6 | 4.0 ± 0.4 | |
Data are means ± se (n = 16).
DISCUSSION
Role of Cob Anatomy in Assimilate Movement
It is generally assumed that the maize cob acts initially as a temporary storage tissue that senesces early in reproductive growth, becoming only a physical structure for bearing the developing kernels (Crawford et al., 1982). Support for this idea can be derived from the work of BeMiller et al. (1969, 1973), who found a sharp apparent decline in total nucleic acids, DNA, and tRNA methylase activities within 4 DAS in cob pith tissue. Contrary to this early senescence belief, the cob accumulates dry matter and develops for at least the first 18 DAS (Fig. 1; Seka et al., 1995). At silking, the cob is soft, flexible, and not fully developed, and through the first 18 DAS continues its rapid expansion that initiated prior to silking (Fig. 1; Jacobs and Pearson, 1992). Pollination triggers a localized change in cob development directly under the pollinated ovule, and within 5 d of pollination, the outer cob parenchyma begins to harden with sclerenchyma (Lenz, 1948). Conversely, unpollinated cob areas remain soft because they do not develop sclerenchyma (J.R. Seebauer, unpublished data).
Two groups of vascular bundles extend along the cob, one toward the inner pith parenchyma and the other toward the outer cob (Lenz, 1948; Laubengayer, 1949). The outer vascular system supplies the glumes, and we theorize that this vascular system is destroyed during sclerenchyma differentiation, causing senescence of the glumes. Additionally, lignification of the cob could possibly promote maturation of the branched parenchyma surrounding the vascular bundles, which extend from near the cob pith to the grain and are thought to primarily support kernel growth. Evidence for the necessity of outer cob (and presumably, vascular bundle) integrity was found when cultured cob pieces were cut to permit only apoplastic movement of assimilates to the grain (Felker, 1992). Kernels where cob integrity was disrupted accumulated only a small portion of the supplied sugars compared to intact kernels, suggesting a symplastic role for this outer, hardened cob.
The complex anatomical structure of the cob and young earshoot makes it difficult to determine the supply of assimilates available to the kernels. For example, the aphid stylet technique (e.g. Lohaus et al., 1998) cannot be used to obtain cob phloem sap, since the husks, ovules, and glumes cover the cob surface. These glumes, and the small size of young ovules (i.e. before 20 d after pollination), also hinder using agar traps (e.g. Porter et al., 1985) to determine cob assimilate supplies. For these reasons, we used the amino acid levels in unpollinated earshoots as an indicator of the N assimilate supply, reasoning that amino acids would temporarily accumulate in the absence of a developing kernel sink.
Amino Acid Conversions within the Earshoot
Gln prevailed as the main amino acid in the cobs up to 8 DAS, with Asp becoming the major amino acid by 14 DAS (Fig. 2). For cobs grown with high N, Gln concentration at 2 DAS was over 3 times higher than Asp, similar to the ratio reported in the shank by Arruda and DaSilva (1979) at 7 d after pollination. Besides Gln and Asp, other abundant amino acids in the cob were Asn, Ala, and Ser (Table I), consistent with earlier reports of the cob vascular sap (Lyznik et al., 1982). Thus, metabolite profiling of cobs prior to or soon after pollination may be an effective approach to monitor assimilates supplied by source tissues.
Previous studies have demonstrated that amino acids or sugars supplied in vitro to cultured cobs and kernels are extensively converted to other compounds within the cob during grain fill (Shimamoto and Nelson, 1981; Misra and Oaks, 1985; Glawischnig et al., 2001), which indirectly implies the cob is indeed metabolically active. Our data confirm that the cob contains active enzymes of amino acid metabolism during the early postpollination phase (Fig. 4) that play an important role in amino acid transformation. Over the developmental period measured in this study, cobs accumulated amino acids and acted as a temporary storage tissue when pollination was prevented. Concentrations of the major amino acids responded the greatest to pollination status or N availability, with lesser responses typically observed further down each of the biosynthetic pathways, similar to findings of Noctor et al. (2002). This resulting profile may indicate that the primary functional role for amino acid metabolism in the cob is to perform interconversions that precondition the amino acid supply for use by developing kernels.
The relative concentration of Gln in the cob decreased the most dramatically over time compared to other amino acids, with a parallel decline in cob GS activity (Figs. 2 and 4). If the cob was merely a conduit for phloem assimilates, then it might be expected that incoming Gln from vegetative tissues would maintain steady-state Gln levels despite low GS activity. The decline in Gln can be accounted for by increases in other free amino acids, as a result of enzymes that use Gln as a substrate, most notably AS (EC 6.3.5.4). Despite repeated attempts, we were unable to directly measure AS activity, a difficulty encountered by others (Brouquisse et al., 1992; Chevalier et al., 1996; Lohaus et al., 1998). Thus, we can only infer that AS is active in young cobs, pedicels, and ovules based on the amino acid profiles. We base this inference on three lines of evidence: (1) AS has been documented to be the major enzymatic route for Asn synthesis in plants (Lam et al., 2003); (2) we found that Asn concentration increases in unpollinated ears, where developing seeds are absent (Table I); and (3) expressed sequence tags for at least one maize gene encoding AS are present in a cDNA library prepared from young maize earshoots, pedicles, and kernels (Zinselmeier et al., 2002). We have recently confirmed the mRNA expression of this particular maize AS gene via reverse transcription-PCR assays of mRNA isolated for the same developing cob tissue samples used in this study for metabolite profiling (S.P. Moose, J.R. Seebauer, and J. Church, unpublished data). The continued characterization of mRNA expression profiles of this and other maize AS genes during cob development is the subject of further study.
The accumulation of greater Asn in earshoots supplied with high N (Figs. 2 and 3) indicates that AS activity is almost certainly stimulated by increased N or by a more subtle decrease in C to N ratio than previously observed (Chevalier et al., 1996). The synthesis of Asn by AS also requires Asp, which can be produced by AspAT. The greatest activity of all measured enzymes throughout the earshoot was AspAT (Figs. 4 and 5; Table II). When grown under high N conditions, the activity of AspAT increased in all earshoot sections, similar to induction of endosperm AspAT activity in cultured kernels (Faleiros et al., 1996; Cazetta et al., 1999). In addition to providing Asp for AS activity, AspAT could also form Glu in the cob, similar to its role in pedicel-placento-chalazal tissues (Lyznik et al., 1985; Muhitch, 1993).
Besides GS and AspAT, numerous enzymes use Glu as a precursor, including AlaAT and GDH. This enzymatic activity may lead to high Glu turnover, producing the steady relative Glu levels observed in cobs and spikelets (Figs. 2 and 3). In a similar manner, GS in the pedicels (Table II) appears to metabolize the available Glu to stabilize the relative Gln concentration in both the spikelets and pedicels compared to the rapid decline of Gln found in the cobs (Fig. 3; Muhitch, 2003). In both cobs and pedicels, AlaAT activity increased with high N supply (Fig. 4; Table II), similar to the N enhancement of AlaAT activity reported in maize and rice seeds (Faleiros et al., 1996; Cazetta et al., 1999; Kikuchi et al., 1999). The resulting Ala produced is a major component of seed storage proteins, which are also typically at greater levels in seeds produced with high N fertility.
In developing kernels and cobs, GDH activity was either unaffected by N supply (Table II) or responded in a coordinate manner to relative Glu levels (Figs. 2 and 4), in contrast to an inverse relationship between GDH activity and Glu levels previously reported in endosperm (Hadzi-Taskovic Sukalovic, 1990). Activity of GDH increased up to 14 DAS and then remained high in unpollinated cobs (Fig. 4), which may be due to its role in degradation of the greater levels of free amino acids found in these tissues (Table I; Miflin and Habash, 2002). Higher total N supply down-regulated activity of GDH in cobs, but up-regulated GDH activity in pedicels at 14 DAS (Table II), demonstrating the compartmentalization nature of other N assimilating enzymes found in crop plants (Miflin and Habash, 2002).
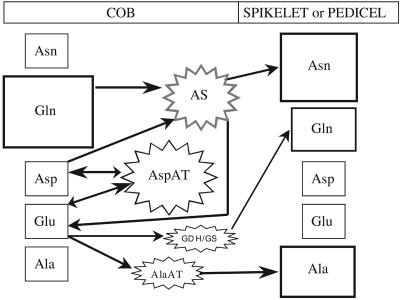
Based on the steady-state profiling and enzyme activities measured, we present as a summary in Figure 6 a schematic illustration of the proposed interconversions of major amino acids that occur during early kernel development. Gln is the major assimilate entering the cob at 2 DAS and is primarily metabolized into Asn by AS. AspAT is also an important enzyme in the cob, which catalyzes the reversible reaction between Glu and Asp and therefore acts to balance these amino acid levels (Miesak and Coruzzi, 2002). In our scheme, AspAT converts Glu to Asp regardless of N conditions. The high activity of cob AspAT supports the idea that Asp precursors are being supplied at high levels as substrate for AS (Fig. 5). We believe that AS is induced under high N conditions, thereby reducing the relative Asp levels while concurrently producing greater Asn. The resulting Asn is less metabolically active than Gln and is used for kernel growth, as shown by the lower relative Asn levels in pollinated cobs (Fig. 2). At the same time, the Glu formed by AS can be recycled back to Asp via AspAT. Additionally, Glu can be metabolized into Gln and Ala, making a variety of amino acids available for kernel growth and development. Thus, the coordinated activity of AS, AspAT, and GS appears to convert the incoming Gln primarily to Asn, thereby providing a more metabolically stable amino acid for transfer into the pedicel and developing sink.
Figure 6.
Schematic of a proposed metabolic pathway for the interconversions of the major amino acids in the maize cob. Relative assimilate concentrations are represented by size of the surrounding box and depict levels at 2 DAS for cobs and 18 DAS for spikelets of plants grown at high N and unpollinated. Average activities of measured and proposed (AS) N assimilation enzymes in the cob are represented by the relative size of the stars.
N Signaling and Kernel Development
We have demonstrated an enzyme-mediated interconversion of amino acids during early earshoot development that is more influenced by the N supply than by the pollination status. Nitrogen is necessary for the normal growth and development of maize kernels and is an essential component of enzymes, nucleic acids, and regulatory proteins. However, most studies examining kernel abortion and kernel set have focused on sugars and C supply (see Andersen et al., 2002, and refs. therein), even though a deficiency of N can also severely limit kernel development (Below et al., 2000). Unfortunately, it is not known how the cob and developing kernel sense the plant's N status and then determine the number of kernels to set and fill. Under high N conditions, AspAT and AlaAT (and presumably AS) activities increase in cobs and pedicels (Figs. 4 and 5), forming greater Asn, Ala, and other amino acids, which are subsequently available to support seed set and supply the N for kernel storage proteins (Lam et al., 1994; Miesak and Coruzzi, 2002; Lalonde et al., 2003). Interestingly, maize and rye cultivars with abnormally high concentrations of grain protein exhibit an altered profile of transported amino acids, which involves a much higher amount and proportion of N as Asn (Dembinski and Bany, 1991; Dembinski et al., 1996; Lohaus et al., 1998).
In Arabidopsis (Arabidopsis thaliana), the AS gene ASN2 and cytosolic GS1 are induced by sugars, whereas ASN1 is induced in the dark and by N (Lam et al., 1998; Oliveira and Coruzzi, 1999). The AS and GS enzymes, which form Asn and Gln, differ in susceptibility to phytochrome signaling and the C to N ratio depending on the specific isozyme, plant species and/or cultivar, and the plant part (Dembinski et al., 1996; Oliveira and Coruzzi, 1999; Lam et al., 2003). We propose the existence of a maize cob AS, possibly encoded by the gene first identified by Zinselmeier et al. (2002), which is induced by N supply and which plays an important role in amino acid metabolism. Other potential advantages of converting Gln to Asn in the maize cob are that Asn has a higher N to C ratio and is considered more metabolically stable for use in transport and storage (Joy and Ireland, 1990). We also believe that the conversion of amino N to Asn may be a signal of plant N status, as plants grown with high N had much higher molar ratios of Asn to Gln in all parts of the earshoot throughout most of its development (Table III). The only exception was pollinated cobs after 14 DAS (Table III), when the cob was likely no longer acting as a storage tissue (Crawford et al., 1982). Otherwise, Asn to Gln ratios in high N plants were 2-fold higher in cobs at 2 DAS, about 50% higher at 8 DAS, and always higher (average of 62%) in unpollinated cobs. The alteration in Asn to Gln ratio with N supply was even greater for pollinated spikelets, increasing more than 3-fold at 2 DAS and 2.5-fold at 8 DAS (Table III). Possibly a certain ratio or balance between Asn and Gln is crucial for optimal kernel growth and protein accumulation. Additional support for this idea can be inferred from an in vitro study where maize ovules grew better and accumulated more total protein when grown on media containing a greater Asn to Gln ratio (Rafalski and Dembinski, 1990).
Table III.
Influence of N supply on molar ratio of Asn to Gln in earshoot fractions during early postsilking growth as a function of N supply and pollination status
| Tissue
|
DAS
|
Pollinated
|
Unpollinated
|
||
|---|---|---|---|---|---|
| N Supply
| |||||
| Low N | High N | Low N | High N | ||
| Asn/Gln | |||||
| Cob | 2 | 0.08 ± 0.01 | 0.17 ± 0.03 | 0.08 ± 0.01 | 0.17 ± 0.03 |
| 8 | 0.24 ± 0.03 | 0.35 ± 0.05 | 0.20 ± 0.03 | 0.32 ± 0.04 | |
| 14 | 1.11 ± 0.18 | 0.75 ± 0.06 | 1.80 ± 0.26 | 2.36 ± 0.31 | |
| 18 | 0.55 ± 0.11 | 0.57 ± 0.06 | 2.10 ± 0.38 | 3.07 ± 0.48 | |
| Spikelet | 2 | 0.14 ± 0.01 | 0.45 ± 0.06 | 0.14 ± 0.01 | 0.45 ± 0.06 |
| 8 | 0.23 ± 0.03 | 0.58 ± 0.08 | 0.47 ± 0.07 | 0.81 ± 0.11 | |
| 14 | 1.00 ± 0.13 | 1.41 ± 0.19 | |||
| 18 | 1.20 ± 0.13 | 2.74 ± 0.33 | |||
| Pedicel | 14 | 0.39 ± 0.06 | 0.70 ± 0.09 | ||
| 18 | 0.41 ± 0.06 | 0.83 ± 0.16 | |||
| Kernel | 14 | 0.15 ± 0.02 | 0.31 ± 0.07 | ||
| 18 | 0.45 ± 0.04 | 0.52 ± 0.03 | |||
Data are means ± se (n = 12).
It is notable that the relative Asp levels accumulated to a greater extent than Asn levels in the cob, suggesting that AS activity may be limiting. Cobs also had 3 to 4 times greater relative Asp than did kernels; and kernels grown with low N had one-half the relative Asn of high N kernels (data not shown). This differential in amino acid profiles could imply that maize kernel growth is limited by AS activity in the cob, and thus increasing AS activity may enhance maize productivity. We hypothesize not only the absolute Asn supply but also the Asn to Gln ratio in the cob mediated by AspAT, AS, and GS to be key in identifying the N status of the earshoot for continued kernel development.
MATERIALS AND METHODS
Two single-cross commercial maize (Zea mays) hybrids (H6270248 and H6270249) were supplied by Renessen (St. Louis). These genotypes were planted on April 27, 2001, in Champaign, IL to achieve a stand density of 74,000 plants/ha. Treatments consisted of the two hybrids grown at two N levels either with or without pollination and were arranged as a 2 × 2 × 2 factorial in a randomized complete block design with four replications. An experimental unit consisted of four 5.3-m rows spaced 0.76 m apart. N treatments were applied at the V2 growth stage to make available either a minimal (0 supplemental; low N) or an adequate N supply (168 kg N/ha; high N) in the form of ammonium sulfate. To determine inherent cob metabolism, ears from each experimental unit were either prevented from pollinating (unpollinated) by covering with a waxed paper shoot bag (no. 217; Lawson Bags, Northfield, IL) or pollinated manually at 2 DAS using self or sib pollen. All other cultural practices were in accordance with local recommendations for high yield, and the area was irrigated once (just prior to pollination) to prevent water stress. The same procedures were followed in 2002 except that only one variety (H6270249) was used and the planting date was May 22.
Samplings and Analyses
Earshoots (three per treatment from four replicates) were sampled at 2, 8, 14, and 18 DAS. A separate sampling of cobs was made at physiological maturity (60 DAS) to determine final dry weight. For earshoots, the husk and shank were discarded and the remainder separated into cobs (whole, without spikelets), spikelets (including paired florets plus glumes and rachilla), pedicels (plus remaining glumes), or kernels (seed minus pedicels). Unpollinated ears were only separated into spikelet and cob fractions, whereas pollinated ears had the same two fractions for the 2- and 8-DAS samplings, with the further divisions of pedicels and kernels commencing at 14 DAS. For the 14- and 18-DAS sampling of pollinated ears, cob tissue samples were obtained from the middle section (approximately 15–30 ranks of spikelets from the base) of the ear. All other tissue samples were obtained and bulked from along the whole earshoot. At each sampling date, fractions were subdivided into two parts and immediately frozen in liquid N2. One subset was ground to a powder (with liquid N2 in a mortar), while the remainder was lyophilized before similar grinding. Both sets were stored at −80°C before analysis.
Free amino acids were analyzed by accurately weighing 50 mg of homogenous dry powder and extracting it for 1 h at room temperature with 1.5 mL of a 5% (w/v) TCA solution. The sample was clarified by centrifugation, and 1.5 μL of the supernatant was analyzed for free amino acids. Amino acid analysis was accomplished by precolumn o-phthalaldehyde derivatization of the sample followed by reverse phase separation on an Agilent 1100 HPLC (Agilent Technologies, Palo Alto, CA) with a cooled auto-sampler, fluorescence detector, and the Agilent HP Chemstation data station. Chromatography conditions are described by Henderson et al. (2000). Pro was not determined in these analyses.
GS (EC. 6.3.1.2) activity was assayed by measuring γ-glutamyl hydroxymate formation using nonlyophilized tissue (Muhitch, 1988). Frozen tissue was homogenized in extraction buffer containing 50 mm imidazole-HCl, pH 7, 20 mm MgSO4 7H2O, 1 mm dithiothreitol, 1 mm EDTA, 1 mm glutathione, 0.2% (v/v) β-mercaptoethanol, and 15% (v/v) ethylene glycol (Ericson, 1985). After precipitation with ammonium sulfate (using a saturated solution to obtain 65% final saturation), samples were centrifuged for 20 min at 20,000g at 4°C. The resulting pellet was resuspended in isolation buffer and desalted by passing through columns of Sephadex G-25 (Sigma-Aldrich, St. Louis). Enzyme activity was measured using a modification of the assay of O'Neal and Joy (1973). The final concentration of the assay components were 100 mm imidazole, pH 7, 180 mm l-Glu, 25 mm hydroxylamine-HCl, 50 mm MgCl2 6H2O. The reaction was initiated with 30 mm ATP. After an incubation for 20 min at 35°C, the reaction was terminated using a solution consisting of 370 mm FeCl3, 200 mm TCA, and 0.67 n HCl. The amount of γ-glutamyl hydroxymate formed was determined colorimetrically (at 540 nm) by comparison to a γ-glutamyl hydroxymate standard curve.
AspAT (EC. 2.6.1.1), AlaAT (EC. 2.6.1.2), and GDH (EC. 1.4.1.2) activities were measured in NADH coupled assays using crude tissue extracts (Cazetta et al., 1999). Lyophilized tissue was homogenized in an extraction buffer containing 50 mm HEPES, pH 7.5, 5 mm MgCl2 6H2O, 1 mm EDTA, 1 mm dithiothreitol, and 10% (v/v) ethylene glycol. Samples were then centrifuged for 20 min at 20,000g at 4°C. The resulting supernatant was subsequently used for the following assays which measured the change in A340 over time in a 1-mL cuvette at 30°C. The reaction mix for AspAT consisted of 50 mm Bicine, pH 8.5, 5 mm MgCl2, 30 mm Asp, 2.5 units malate dehydrogenase, 0.2 mm NADH, and sample extract. Ten millimolar 2-oxoglutarate (2OG) was added to initiate AspAT activity. Alanine aminotransferase activity was measured in a similar manner as AspAT with the assay buffer containing 50 mm HEPES, pH 7.2, 5 mm MgCl2, 40 mm Ala, 2.5 units lactate dehydrogenase, 0.2 mm NADH, 10 mm 2OG. GDH was measured in the aminating direction using a buffer containing 50 mm Bicine, pH 8.5, 5 mm MgCl2, 0.2 mm NADH, 10 mm 2OG and was initiated by adding 125 mm NH4Cl. Background activity was subtracted to obtain final results.
Data Analysis and Presentation
Pollination status and N supply had a much greater effect on the results than did the maize variety or the year. Thus, amino acid data are presented as an average of variety and year ± 1 se of the mean. Enzyme analysis was conducted on samples from both varieties in the first year only and is presented as the average of duplicate determinations of two separate weighings of each sample. Dry weights of cobs to estimate seasonal growth were measured in the second year only.
This work was supported in part by Renessen, LLC (grant no. 00–PRI–B/M–1171 to F.E.B. and S.P.M.). The study is part of project no. 15–0390 of the Agricultural Experiment Station, College of Agricultural, Consumer, and Environmental Sciences, University of Illinois at Urbana-Champaign.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043778.
References
- Andersen MN, Asch F, Wu Y, Jensen CR, Naested H, Morgensen VO, Koch KE (2002) Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol 130: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda P, DaSilva WJ (1979) Amino acid composition of vascular sap of maize ear peduncle. Phytochemistry 18: 409–410 [Google Scholar]
- Below FE, Cazetta JO, Seebauer JR (2000) Carbon/nitrogen interactions during ear and kernel development in maize. In ME Westgate, KJ Boote, eds, Physiology and Modeling Kernel Set in Maize, Vol 29. Crop Science Society of America and American Society of Agronomy, Madison, WI, pp 15–24
- BeMiller JN, Hoffmann WE (1972) Relationship of soluble carbohydrates to age of corn cob parenchyma tissue. Phytochemistry 11: 1321–1325 [Google Scholar]
- BeMiller JN, Hoffmann WE, Pappelis AJ (1973) Changes in tRNA methylase activity in senescing cob and stalk parenchyma tissue and the first developed leaf of corn (Zea mays). Mech Ageing Dev 2: 363–369 [DOI] [PubMed] [Google Scholar]
- BeMiller JN, Johnson DC, Pappelis AJ (1969) Relationship of nucleic acids to cell death in corn cob parenchyma tissue. Phytopathology 59: 989–991 [PubMed] [Google Scholar]
- Bhattacharya S, Chand N, Bhashyam MK, Srinivas T (1996) Influence of cob morphology and grain characteristics on oil content in maize (Zea mays L.). J Food Sci Technol 33: 302–307 [Google Scholar]
- Brouquisse R, James F, Pradet A, Raymond P (1992) Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved root tips. Planta 188: 384–395 [DOI] [PubMed] [Google Scholar]
- Cantarero MG, Cirilo AG, Andrade FH (1999) Night temperature at silking affects kernel set in maize. Crop Sci 39: 703–710 [Google Scholar]
- Cazetta JO, Seebauer JR, Below FE (1999) Sucrose and nitrogen supplies regulate growth of maize kernels. Ann Bot (Lond) 84: 747–754 [Google Scholar]
- Cevallos CBA, Cisneros ZL (2003) Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. J Agric Food Chem 51: 3313–3319 [DOI] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Just D, Raymond P (1996) Metabolic regulation of asparagine synthetase gene expression in maize (Zea mays L.) root tips. Plant J 9: 1–11 [DOI] [PubMed] [Google Scholar]
- Crawford TW, Rendig VV, Broadbent FE (1982) Sources, fluxes, and sinks of nitrogen during early reproductive growth of maize (Zea mays L.). Plant Physiol 70: 1654–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembinski E, Bany S (1991) The amino acid pool of high and low protein rye inbred lines Secale cereale l. J Plant Physiol 138: 494–496 [Google Scholar]
- Dembinski E, Wisniewska I, Raczynska-Bojanowska K (1996) The efficiency of protein synthesis in maize depends on the light regulation of the activities of the enzymes of nitrogen metabolism. J Plant Physiol 149: 466–468 [Google Scholar]
- Ericson M (1985) Purification and properties of glutamine synthetase from spinach leaves. Plant Physiol 79: 923–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faleiros RRS, Seebauer JR, Below FE (1996) Nutritionally induced changes in endosperm of shrunken-1 and brittle-2 maize kernels grown in vitro. Crop Sci 36: 947–954 [Google Scholar]
- Felker FC (1992) Participation of cob tissue in the uptake of medium components by maize kernels cultured in vitro. J Plant Physiol 139: 647–652 [Google Scholar]
- Glawischnig E, Gierl A, Tomas A, Bacher A, Eisenreich W (2001) Retrobiosynthetic nuclear magnetic resonance analysis of amino acid biosynthesis and intermediary metabolism. Metabolic flux in developing maize kernels. Plant Physiol 125: 1178–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzi-Taskovic Sukalovic V (1990) Properties of glutamate dehydrogenase from developing maize endosperm. Physiol Plant 80: 238–242 [Google Scholar]
- Harshberger JW (1893) Maize: a botanical and economic study. Contrib Bot Lab Univ Pa 1: 75–202 [Google Scholar]
- Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, Accurate, Sensitive and Reproducible Analysis of Amino Acids. Agilent Publication Number 5980-1193EN. Agilent Technologies, Palo Alto, CA
- Jacobs BC, Pearson CJ (1992) Pre-flowering growth and development of the inflorescences of maize. I. Primordia production and apical dome volume. J Exp Bot 43: 557–563 [Google Scholar]
- Jones RJ, Gengenbach BG, Cardwell VB (1981) Temperature effects on in vitro kernel development in maize. Crop Sci 21: 761–766 [Google Scholar]
- Joy KW, Ireland RJ (1990) Enzymes of asparagine metabolism. In PJ Lea, ed, Enzymes of Primary Metabolism. Methods in Plant Biochemistry, Vol 3. Academic Press, San Diego, pp 287–296
- Kikuchi H, Hirose S, Toki S, Akama K, Takaiwa F (1999) Molecular characterization of a gene for alanine aminotransferase from rice (Oryza sativa). Plant Mol Biol 39: 149–159 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26: 37–56 [Google Scholar]
- Lam HM, Hsieh MH, Coruzzi GM (1998) Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in Arabidopsis thaliana. Plant J 16: 345–353 [DOI] [PubMed] [Google Scholar]
- Lam HM, Peng SSY, Coruzzi GM (1994) Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol 106: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HM, Wong P, Chan HK, Yam KM, Chen L, Chow CM, Coruzzi GM (2003) Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol 132: 926–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubengayer RA (1949) The vascular anatomy of the eight-rowed ear and tassel of golden bantam sweet corn. Am J Bot 36: 236–244 [Google Scholar]
- Lenz LW (1948) Comparative histology of the female inflorescence of Zea mays L. Ann Mo Bot Gard 35: 353–377 [Google Scholar]
- Lohaus G, Büker M, Hubmann M, Soave C, Heldt H-W (1998) Transport of amino acids with special emphasis on the synthesis and transport of asparagine in the Illinois Low Protein and Illinois High Protein strains of maize. Planta 205: 181–188 [Google Scholar]
- Lur H-S, Setter TL (1993) Role of auxin in maize endosperm development. Timing of nuclear DNA endoreduplication, zein expression, and cytokinin. Plant Physiol 103: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyznik L, Rafalski A, Raczynska-Bojanowska K (1985) Amino acid metabolism in the pedicel-placenta-chalazal region of the developing maize kernel. Phytochemistry 24: 425–430 [Google Scholar]
- Lyznik LA, Zdrojewski W, Neumann M, Macewicz J, Raczynska-Bojanowska K (1982) A possible role of pedicel-placento-chalazal tissues in the amino acids supply to the developing maize endosperm. Maydica 27: 191–198 [Google Scholar]
- Miesak BH, Coruzzi GM (2002) Molecular and physiological analysis of Arabidopsis mutants defective in cytosolic or chloroplastic aspartate aminotransferase. Plant Physiol 129: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53: 979–987 [DOI] [PubMed] [Google Scholar]
- Misra S, Oaks A (1985) Glutamine metabolism in corn kernels cultured in vitro. Plant Physiol 77: 520–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafar A (1990) Kernel abortion and distribution of mineral elements along the maize ear. Agron J 82: 511–514 [Google Scholar]
- Muhitch MJ (1988) Glutamine synthetase activity of the endosperm, embryo, and pedicel-placento-chalazal regions of developing maize (Zea mays) kernels. Physiol Plant 74: 176–180 [Google Scholar]
- Muhitch MJ (1993) In vitro metabolism of L-aspartate by maize kernels. Phytochemistry 32: 1125–1130 [Google Scholar]
- Muhitch MJ (2003) Distribution of the glutamine synthetase isozyme GSp1 in maize (Zea mays). J Plant Physiol 160: 601–605 [DOI] [PubMed] [Google Scholar]
- Nguetta ASP, Cross HZ (1997) Correlated responses in ear and plant traits in maize synthetics selected for R-nj color expression. Crop Sci 37: 739–744 [Google Scholar]
- Noctor G, Novitskaya L, Lea PJ, Foyer CH (2002) Co-ordination of leaf minor amino acid contents in crop species: significance and interpretation. J Exp Bot 53: 939–945 [DOI] [PubMed] [Google Scholar]
- Oliveira IC, Coruzzi GM (1999) Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol 121: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D, Joy KW (1973) Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys 159: 113–122 [DOI] [PubMed] [Google Scholar]
- Orr AR, Haas G, Sundberg MD (1997) Organogenesis of fascicled ear mutant inflorescences in maize (Poaceae). Am J Bot 84: 723–734 [PubMed] [Google Scholar]
- Pearson CH (2000) How blunt ear syndrome of corn is affected by hybrid and irrigation at Fruita, Colorado 2000. Western Colorado Research Center 2000 Annual Report. http://www.colostate.edu/programs/wcrc/annrpt/00/Pearson_BluntEarSyndrome (March 30, 2004)
- Porter GA, Knievel DP, Shannon JC (1985) Sugar efflux from maize (Zea mays L.) pedicel tissue. Plant Physiol 77: 524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski A, Dembinski E (1990) Genotypic differences in ammonia metabolism of the developing maize kernel. Acta Physiol Plant 12: 331–339 [Google Scholar]
- Schussler JR, Westgate ME (1991) Maize kernel set at low water potential. II. Sensitivity to reduced assimilates at pollination. Crop Sci 31: 1196–1203 [Google Scholar]
- Seka D, Cross HZ, Mcclean PE (1995) Maize kernel development in-vitro sucrose concentration, xenia and maternal effects. Crop Sci 35: 74–79 [Google Scholar]
- Shimamoto K, Nelson OE (1981) Movement of 14-C-compounds from maternal tissue into maize seeds grown in vitro. Plant Physiol 67: 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko L, Li X, Tagliani L, Bowen B, Peterson T (1999) Characterization of the regulatory elements of the maize P-rr gene by transient expression assays. Plant Mol Biol 39: 11–19 [DOI] [PubMed] [Google Scholar]
- Srinivas T, Bhashyam MK, Chand N, Bhattacharya S, Murthy SS, Narasimha HV (1991) Relationship of corn characters with grain morphology in maize (Zea mays, Poaceae). Econ Bot 45: 503–510 [Google Scholar]
- Zinselmeier C, Sun Y, Helentjaris T, Beatty M, Yang S, Smith H, Habben J (2002) The use of gene expression profiling to dissect the stress sensitivity of reproductive development in maize. Field Crops Res 75: 111–121 [Google Scholar]