Abstract
Interleukin-1β (IL-1β) is a key proinflammatory cytokine that drives antimicrobial immune responses. IL-1β is aberrantly activated in autoimmune diseases, and IL-1β inhibitors are used as therapeutic agents to treat patients with certain autoimmune disorders. Review of postmarketing surveillance of patients receiving IL-1β inhibitors found a disproportionate reporting of invasive infections by group A Streptococcus (GAS). IL-1β inhibition increased mouse susceptibility to GAS infection, but IL-1β was produced independent of canonical inflammasomes. Newly synthesized IL-1β has an amino-terminal prodomain that blocks signaling activity, which is usually proteolytically removed by caspase-1, a protease activated within the inflammasome structure. In place of host caspases, the secreted GAS cysteine protease SpeB generated mature IL-1β. During invasive infection, GAS isolates may acquire pathoadaptive mutations eliminating SpeB expression to evade detection by IL-1β. Pharmacological IL-1β inhibition alleviates this selective pressure, allowing invasive infection by nonpathoadapted GAS. Thus, IL-1β is a sensor that directly detects pathogen-associated proteolysis through an independent pathway operating in parallel with host inflammasomes. Because IL-1β function is maintained across species, yet cleavage by caspases does not appear to be, detection of microbial proteases may represent an ancestral system of innate immune regulation.
INTRODUCTION
Interleukin-1β (IL-1β) is an important alarm to infection that stimulates intrinsic, innate, and adaptive immune pathways. Conversely, excessive or chronic IL-1β activation is implicated in the pathogenesis of inflammatory diseases such as rheumatoid arthritis, type 1 diabetes, celiac disease, gout, and systemic lupus erythematosus (1). Accordingly, IL-1β inhibitor therapy has therapeutic benefit in these diseases and hereditary inflammasome disorders including cryopyrin-associated periodic syndromes and familial Mediterranean fever (2). In contrast to these pathological consequences, inflammasome function is protective during most infections; IL-1β coordinates an immune response that can antagonize pathogen colonization, replication, invasion, and dissemination (3).
Group A Streptococcus (GAS) is a ubiquitous human pathogen, colonizing 10 to 20% of individuals at any given time and causing ~700 million cases of pharyngitis and superficial skin infections yearly (4). These infections are classically pyogenic; the interaction of GAS with the host is highly inflammatory and is accompanied by substantial neutrophil influx and pus formation. The role of IL-1β in the inflammatory response to GAS has not been defined, but IL-1 receptor knockout (IL-1R1−/−) mice are markedly more susceptible to infection by the pathogen (5). Typically, IL-1β is regulated by posttranslational cleavage of an N-terminal prodomain by the protease caspase-1 within the inflammasome complex (3). GAS can induce IL-1β maturation through the NACHT, LRR, and PYD family pyrin domain-containing 3 (NLRP3) inflammasome in vitro (6, 7), yet in vivo NLRP3 is required neither for IL-1β release nor for innate immune resistance to the bacterium (6–8), leaving the mechanism for IL-1β generation unclear (9).
Here, we present an alternative mechanism for activation of IL-1β that reconciles these disparate findings. We show that a GAS protease, SpeB, directly processes IL-1β during infection and generates a mature, inflammatory cytokine that can promote macrophage bactericidal functions. Robust activation of IL-1β in this fashion triggers a hyperinflammatory state that restricts GAS invasion. In murine models and human patients, invasive GAS may develop SpeB-repressing pathoadaptive mutations, which aid the bacterium in escaping IL-1β restriction. A requirement of this mutation to reduce innate immune “visibility” provides a barrier to invasive infection that is lost if IL-1β signaling is therapeutically inhibited.
RESULTS
Although numerous experimental models identified marked susceptibility of IL-1β-deficient mice to infection (9), clinical trials supporting the U.S. Food and Drug Administration (FDA) approval of IL-1β inhibitors revealed only modest infectious complications (10). Our review of postmarketing surveillance from the FDA Adverse Event Reporting System [FAERS (11)] confirmed this low infection rate with one striking exception: severe invasive infections produced by GAS. Patients receiving an IL-1β inhibitor are reported to experience invasive GAS disease at an ~330-fold increased rate, including at least 13 hospitalizations (and 3 fatalities) in patients with necrotizing fasciitis (NF), sepsis, or tissue abscess (Fig. 1A) (4). This extraordinarily high reporting rate of GAS infectious complications was not found for other common immunosuppressant therapies used in rheumatoid arthritis (Fig. 1A). Furthermore, GAS NF infections in anakinra-treated individuals were associated with greater mortality than similar infections in patients receiving alternative immunosuppressant drugs (Fig. 1B) or the overall expected mortality rate (4).
Fig. 1. IL-1 inhibition is associated with severe invasive GAS infection.

(A) Proportional reporting risk of bacterial species overrepresented in infections associated with the IL-1 signaling inhibitor anakinra (M. tb., Mycobacterium tuberculosis) and the types of GAS infection associated with IL-1 inhibition (APSGN, acute poststreptococcal glomerulonephritis). Proportional reporting of streptococcal NF infection in individuals taking IL-1 inhibitors compared with incidents associated with other classes of anti-inflammatories prescribed for the same conditions, including tumor necrosis factor (TNF), IL-6, CD20 (rituximab), disease- modifying antirheumatic drugs (DMARDs) such as methotrexate, and nonsteroidal anti-inflammatory drugs (NSAIDs). Several of these drugs have been described in single case reports to be associated with streptococcal NF (40–44) except for anakinra, although it is the most overreported in the FAERS. (B) Reported outcomes of streptococcal NF infections associated with anakinra compared to all other drugs.
The rarity of NF infection makes the assignment of the underlying risk factors difficult. Severe GAS infectious complications reported in patients receiving IL-1β inhibitors suggested a pivotal role for IL-1β in controlling streptococcal NF. Mice treated with the FDA‐approved IL‐1β inhibitor anakinra were deficient in IL-1β signaling and more permissive for GAS replication and dissemination (Fig. 2A), consistent with our previous observations in IL‐1R−/− mice (5). Anakinra likewise inhibited IL-1 signaling, but not IL-1β maturation, in GAS-infected macrophages (Fig. 2B). Autocrine IL-1β signaling can promote macrophage cell activation and immune functions (3, 7); correspondingly, anakinra also reduced macrophage killing of GAS (Fig. 2C). To exclude off-target drug effects, we compared anakinra treatment to host deficiency in IL-1R, as well as caspase-1, the key regulator of inflammasome-mediated IL-1β maturation and release. Although IL-1R−/− cells and mice were similarly impaired in GAS killing, caspase-1−/− cells and mice were not (Fig. 2, C and D). Caspase-1-independent IL-1 signaling was observed during infections by GAS, but not with similar Gram-positive bacterial pathogens group B Streptococcus (GBS), group C Streptococcus (GCS), methicillin-resistant Staphylococcus aureus (MRSA), or during incubation with the sterile inflammatory stimulus adenosine triphosphate (ATP) (fig. S1). These data indicate that anakinra treatment leads to GAS susceptibility by blocking IL-1β signaling but that caspase-1 is not involved.
Fig. 2. IL-1 restricts invasive GAS independent of inflammasome regulation.

(A) Quantification of GAS in the lesions or the blood of anakinra-treated or control mice (n = 5) 72 hours after intradermal infection (P = 0.016 and P = 0.026), measurement of lesion sizes (n = 5, P = 0.0004), and quantification of lesion (n = 5) cytokines by ELISA (P = 0.0264, P = 0.0281, and P = 0.1631). ND, none detected; ns, not significant. (B) Total and active IL-1β production by GAS-infected BMM (n = 3) 2 hours after infection via immunoblot or IL-1R reporter assay (P < 0.0001). RU, relative unit. (C) GAS survival in mock- or anakinra-treated C57Bl/6, IL-1R−/− or Casp-1/11−/− BMMs (n = 4; P = 0.012, P = 0.006, and P = 0.262). (D) Seventy-two hours after intradermal GAS infection into anakinra- or mock-treated C57BL/6, IL-1R−/−, or Casp1/11−/− mice (n = 5), lesions were excised and CFU were quantified (P = 0.0004, P = 0.081, P = 0.046, and P = 0.029). (E) BMMs treated with NLRP3 inhibitor MCC950, caspase-1 inhibitor VX-765, or the IL-1 inhibitor anakinra for 1 hour and then treated for 2 hours with ATP (NLRP3 inflammasome inducer), MSU (monosodium urate crystals; NLRP3 inflammasome inducer), and dA:dT [poly(dA:dT); AIM2 inflammasome inducer) or infected with GAS were examined for IL-1 signaling via reporter assay (n = 4). Data are means ± SEM and are representative of at least three experiments; statistical significance is determined by Student’s t test.
Two emergent immunotherapies target caspase-1 [VX-765, (12)] or NLRP3 [MCC950, (13)] rather than IL-1β. Both drugs inhibited IL-1β signaling in response to ATP and crystalline monosodium urate, sterile NLRP3 agonists involved in autoinflammatory diseases; VX-765 additionally inhibited the response to absent in melanoma 2 (AIM2) agonist, poly(dA:dT) [poly(deoxyadenylic-deoxythymidylic) acid sodium salt] (Fig. 2E). However, host-protective IL-1β signaling was maintained during GAS infections in the presence of these direct inflammasome inhibitors (Fig. 2E). Moreover, therapeutic administration of the NLRP3 inhibitor MCC950 did not increase the in vivo proliferation of GAS (fig. S2), a finding consistent with previous infectious challenge studies in NLRP3−/− mice that did not exhibit a hypersusceptible phenotype (6, 7). These findings suggest that strategies for blocking endogenous IL‐1β maturation may not carry the same risk for GAS infection as those blocking its receptor signaling.
IL-1β signaling requires proteolytic processing of immature pro-IL-1β (9), but the lack of caspase-1 involvement indicates another source of the IL-1β-converting protease. Caspase-1-independent IL-1β signaling during GAS infection was prevented by pretreatment of the microorganism with broad-spectrum protease inhibitors, suggesting that a bacterial protease was acting as a surrogate of host caspase (Fig. 3A). Examining the three major secreted proteases of GAS we found that cysteine protease SpeB was necessary and sufficient to induce IL-1 signaling (Fig. 3A). SpeB colocalized with IL-1β within cells (fig. S3) and induced the release of cleaved IL-1β (~p17) into the supernatant, independent of caspase-1 (Fig. 3B).
Fig. 3. GAS protease SpeB cleaves and activates IL-1β.

(A) IL-1 signaling from infected BMM detected after 2 hours of incubation with GAS using IL-1R reporter cells. Where indicated, bacteria (GAS) or macrophages (BMM) were pretreated for 1 hour with protease inhibitor cocktail (PI) (n = 3; P = 0.002 and P = 0.040). BMMs infected with the indicated GAS strains (n = 3) were examined by IL-1R reporter assay for IL-1 signaling (P ≤ 0.0001, P = 0.427, and P = 0.937) or ELISA specific for each cytokine (P = 0.043 and P = 0.038). (B) Immunoblot of total and active IL-1β production by GAS-infected C57BL/6 or Casp1/11−/− BMM 2 hours after infection. WT, wild type. (C) Casp1/11−/− macrophages (n = 3) mock or pretreated with YVAD-fmk (caspase-1 inhibitor), IETD-fmk (caspase-8 inhibitor), DEVD-fmk (caspase-3/6/7 inhibitor), E-64 (broad-spectrum cysteine protease inhibitor), or BCAN [N-(benzyloxycarbonyl)-2-aminoacetonitrile; SpeB inhibitor] were infected with GAS for 2 hours, and then, supernatants were examined by IL-1R reporter assay (P = 0.266, P = 0.232, P = 0.380, P = 0.0005, and P = 0.0005). (D) Examination of IL-1 signaling activity by IL-1R reporter assay from HEK293T cells 8 hours after transfection with p-proil1b (n = 3) or cotransfection with pSpeB (P = 0.002) compared to cotransfected pSpeBΔ (C192S catalytic mutation), pCasp1, or pCasp7. (E) Diagram of reporter assay for normalized IL-1–converting enzyme analysis. Two hours after transfection with the indicated PAMPs, DAMPs, and proteases, proteolysis was measured by FRET, and supernatants were analyzed for conversion of pro–IL-1β into a signaling-competent form. Am, Ametrine; To, Tomato; Ex, excitation; Em, emission. (F) Silver-stained gel of recombinant human pro–IL-1β incubated with SpeB and biological activation of pro–IL-1β (n = 4) at the indicated time points (P = 0.61 and P = 0.07; <0.0001 remainder). The asterisk indicates the band of mature IL-1β sequenced for cleavage site identification. Rx, reaction. (G) Cleavage of internally quenched fluorescent peptides with the indicated sequences was monitored after incubation with SpeB, caspase-1, or neutrophil elastase (NE) (n = 3; P = 0.0001, P = 0.002, P = 0.012, P = 0.0001, and P = 0.0005). Data are means ± SEM and are representative of at least three experiments. Statistical significance was determined by Student’s t test. RFU, relative fluorescence unit.
Confirming that SpeB is sufficient for the activation of IL-1β, caspase inhibitors YVAD-fmk, IETD-fmk, and DEVD-fmk did not alter IL-1 signaling in these cells, whereas cysteine-protease inhibitors that target SpeB, E64 (14), and N-(benzyloxycarbonyl)-2-amino-acetonitrile (15) did (Fig. 3C). Furthermore, cotransfection of human embryonic kidney (HEK) 293 cells with vectors expressing pro-IL-1β and various proteases showed that SpeB, such as the positive control caspase-1, was sufficient to establish IL-1β signaling in the cells (Fig. 3D). Catalytically inactive SpeB or the apoptosis regulator caspase-7 did not result in IL-1β signaling, indicative of protease substrate specificity to this activation. Active caspase-8 can cleave IL-1β (16), but caspase-8 activation was not observed during infection (fig. S4), and would be inhibited by IETD-fmk (Fig. 3C). Caspase-1-like activity was decreased, not increased, by SpeB (fig. S5). Notably, SpeB degrades the inflammasome-activating GAS virulence factors streptolysin O (SLO) and SpyA (fig. S5) (17), explaining the decreased activation of the canonical inflammasome in its presence. Nonetheless, cell death was induced in the presence of SpeB, even in the absence of caspase-1 (fig. S5), providing a mechanism for the release of IL-1β (18). Together, these studies exclude several alternative explanations for in vitro maturation of IL-1β and point to direct activation by SpeB.
Similar to caspases, SpeB targets numerous proteins for cleavage including those involved in the extracellular matrix, antibodies, cell receptors and cytokines, including IL-1β (19); SpeB can also cleave several of GAS’ own surface-associated and secreted virulence factors (17, 20). These data indicate that SpeB has broad substrate specificity. Because enzyme saturation during in vitro assays can falsely identify protease substrates, we first developed a cellular reporter system to analyze specific pro-IL-1β cleavage. Relative proteolysis was measured using Förster resonance energy transfer (FRET) through a construct containing a protease-labile linker separating the dual fluorescent proteins (21). Concurrently, pro-IL-1β conversion was measured with IL-1R reporter cells (Fig. 3E). In caspase-1/11− cells, pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) activating the caspase-11 [lipopolysaccharide (LPS)], NLRC4 [flagellin (FliC)], AIM2 [poly(dA:dT)], or NLRP3 (ATP, SLO, or exotoxin A) inflammasomes did not induce IL-1 signaling (Fig. 3E). In contrast to these inflammasome agonists, SpeB activated IL-1β with similar relative specificity as the known IL-1β convertases caspase-1 (22, 23) and neutrophil elastase (Fig. 3E) (24).
To further examine pro-IL-1β processing by SpeB, we co-incubated recombinant human pro-IL-1β with purified, native SpeB. In these reactions, we observed accumulation of a major cleavage product approximating the size of mature IL-1β generated by caspase-1 (22, 23) and concurrent with the generation of an active IL-1R agonist (Fig. 3F). Peptide sequencing by Edman degradation revealed SpeB cleaved after Phe105, 11 amino acids that are N-terminal to the Asp116 site of caspase-1 cleavage. To corroborate these cleavages, we created a set of internally quenched peptides to quantify proteolysis of this region of pro-IL-1β (Fig. 3G). Caspase-1 and neutrophil elastase both efficiently cleaved the EAYVHDAPV peptide containing the Asp116 and Val114 sites each enzyme is known to cleave (Fig. 3G) (22–24). SpeB had no activity toward this peptide but, instead, efficiently cleaved the more distal peptide IFFDTWDNE that contained the Phe105 identified by Edman degradation that was not previously known to mediate IL-1β activation (Fig. 3G).
Because IL-1β, but not its canonical inflammasome regulators, suppressed GAS replication, we examined whether SpeB was responsible for IL-1β maturation during infection. Compared to wild-type GAS, an isogenic SpeB− mutant induced less IL-1β, and its proliferation during mouse necrotizing skin infections was not potentiated by anakinra treatment (Fig. 4A). Caspase-1 knockout partially rescued the virulence potential of SpeB− mutant GAS, suggesting that canonical inflammasome responses can function and provide some protection in the absence of SpeB (Fig. 4B). However, because this rescue was not concurrent with differences in IL-1β, a role for other inflammasome-regulated processes such as pyroptosis is indicated.
Fig. 4. IL-1β activation by SpeB limits GAS invasive potential.
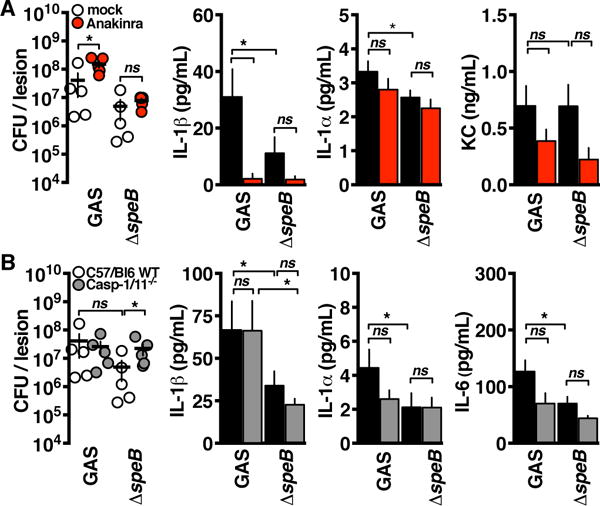
(A) Seventy-two hours after intradermal infection by the GAS strains indicated into anakinra-treated or control C57BL/6 mice (n = 5), lesions were excised, CFU were quantified (P = 0.03 and P = 0.57), and cytokines were quantified by ELISA (*P < 0.05). KC, keratinocyte chemoattractant. (B) Seventy-two hours after intradermal infection by the GAS strains indicated into C57BL/6 or caspase-1/11−/− mice (n = 5), lesions were excised, CFU were quantified (P = 0.42 and P = 0.03), and cytokines were quantified by ELISA (*P < 0.05). Data are means ± SEM (n = 5) and are representative of at least three experiments. Statistical significance was determined by Student’s t test.
Clinical isolates from GAS invasive infections often have mutations in the covR/S two-component regulator (25, 26) that abolish SpeB expression (17) and confer hypervirulence (27). Because invasive infection could be restricted by SpeB activation of IL-1β, we hypothesized that IL-1β can select for this pathoadaptive mutation. GAS isolated from mice treated with anakinra to inhibit IL-1β signaling had not pathoadaptively switched and maintained their SpeB expression (Fig. 5A). In vitro, macrophages are sufficient to select for SpeB− GAS (Fig. 5B), and this required IL-1β, but not the inflammasome or other cytokines tested (Fig. 5C).
Fig. 5. IL-1β selects for bacterial protease inactivation.
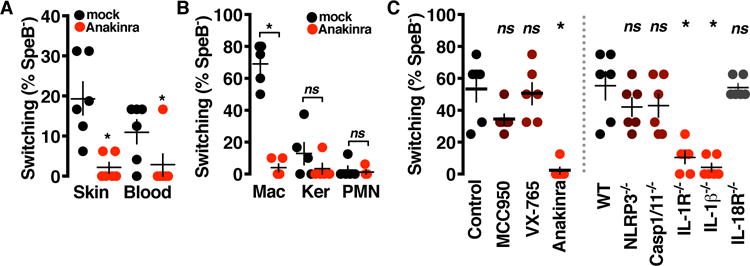
(A) GAS isolates recovered 72 hours after intradermal infection (n = 5 samples of 8 isolates each) examined for switching by testing for expression of SpeB by casein hydrolysis assay (P < 0.0001 and P = 0.034). (B) GAS (n = 5 samples of 8 isolates each) recovered from infected macrophages (Mac), keratinocytes (Ker), and neutrophils (PMN) 4 hours after infection examined for SpeB expression by casein hydrolysis assay (P < 0.0001, P = 0.25, and P = 0.67). (C) BMM treated 1 hour with inflammasome-targeting drugs or mutant for the indicated inflammasome components infected with GAS 4 hours and the recovered CFU (n = 5 samples of 8 isolates each) examined for SpeB expression by casein hydrolysis assay (*P < 0.005). Data are means ± SEM and are representative of at least three experiments. Statistical significance was determined by Student’s t test.
An animal-passaged (AP) GAS strain that had already undergone SpeB inactivation by covR/S mutation was unable to induce IL-1β or IL-1α independently of caspase-1 (Fig. 6A). This resulted in scant IL-1 signaling from caspase-1−/− bone marrow-derived (BMMs) (Fig. 6B) and greatly reduced and delayed IL-1 signaling kinetics in wild-type BMMs (fig. S6). This IL-1 signaling deficiency rendered these cells less able to control GAS infection (Fig. 6C), highlighting the importance of IL-1β generated in response to the microbial protease SpeB during GAS skin infections (Fig. 7A).
Fig. 6. CovRS−/SpeB− GAS hypoactivate IL-1β.

(A and B) IL-1 signaling in BMM infected with AP GAS deficient in SpeB determined by ELISA (P = 0.034 and P = 0.003) and bioactive IL-1 reporter assay (*P < 0.05). (C) GAS replication with the cells was concurrently monitored by dilution plating (*P < 0.05). Data are means ± SEM (n = 4) and are representative of at least three experiments. Statistical significance was determined by Student’s t test.
Fig. 7. Models of IL-1β activation.

(A) Diagram of IL-1β signaling during GAS infection. IL-1β is processed by both caspase-1 through canonical inflammasome pathways and directly by SpeB via an alternative pathway to result in pathogen-restrictive inflammation. The covR/S− pathoadaptation that occurs during invasive infection represses SpeB to prevent alternative activation of IL-1β and allow evasion of IL-1β-induced immune mechanisms. mIL-1b, mature IL-1β. (B) Alignment of the activation region of IL-1β in vertebrates with potentially caspase-targeted aspartic acids highlighted, and compared to the conservation of related signaling and inflammasome proteins. PRR, pattern recognition receptor; D. melanogaster, Drosophila melanogaster; NLRC, nuclear oligomerization domain-like protein subfamily C; PHYIN, pyrin and HIN domain; ASC, apoptotic speck-like protein containing a caspase recruitment domain.
Several proteases other than caspase-1 cleave IL-1β, suggesting extra-inflammasomal protease detection might be a conserved pathway for IL-1β activation. Activation by these other proteases may have ancestral evolutionary origins, because although less complex vertebrates such as fish encode for both IL-1β and the machinery of the inflammasome, such inflammasomes do not always activate IL-1β (28). Aspartic acid, the key amino acid targeted by caspase family proteases for cleavage, is notably absent within the prodomains of many of IL-1β orthologs of several species (Fig. 7B). Thus, proteases other than caspase-1 are likely to commonly mediate IL-1β conversion, suggesting a model wherein IL-1β evolved to sense aberrant proteolysis, as during pathogenic infection.
DISCUSSION
Although the inflammasome typically plays a central role in the maturation of IL-1β, human IL-1β can be activated by other proteases. Here, we show that the infectious pathogen GAS induces noncanonical IL‐1β activation in host macrophages and that this occurs via direct maturation of the cytokine by the bacterium’s own secreted cysteine protease SpeB. Our data support the idea that IL-1β is a sensor of intracellular proteolytic activity whose origins might predate the evolution of caspase-dependent processing of IL-1β. Although the inflammasome is typically more important for immunity than IL-1β during acute bacterial infection, it becomes less relevant in response to a pathogen that itself directly activates IL-1β. Activation of the canonical inflammasome by GAS virulence factors such as SLO (6) and SpyA (7) occurs in parallel but has a lesser contribution to innate immunity (Fig. 2, C and D), likely due to the potential inactivation of these protein toxins by SpeB (fig. S5). Through the process of covR/S mutation and loss of SpeB expression in vivo, GAS avoids the dominant noncanonical IL-1β innate immune response during progression of invasive disease (Fig. 7A). This mechanism may tie back to our original clinical observation that pharmacological IL-1β inhibition results in a greater incidence of invasive GAS infection, as development of the pathoadaptive covR/S mutation may not be required in the absence of IL-1b signaling.
The findings of this paper illustrate a paradigm in which IL-1β and the inflammasome are not functionally redundant, with implications for the treatment of inflammatory disease. The success of IL-1β–neutralizing therapy demonstrates the critical contribution of this cytokine to autoinflammatory disease (29). Because these treatments preserve inflammasome function, pyroptosis and the other remaining inflammatory signaling and antimicrobial processes of the cell can still provide significant protection from pathogen infection (3). Furthermore, IL-1β neutralization can provide therapeutic benefit in instances when IL-1β is activated by alternative mechanisms (30). Conversely, therapeutic targeting of the inflammasome (for example, NLRP3 or caspase-1 inhibitors) could provide greater advantage when IL-1β is not the sole driver of disease pathology. Knowing when these pathways act in concert or diverge can provide better targets for the control of inflammation because targeting each component of inflammasome signaling can led to different therapeutic benefits and carry different infection risks.
MATERIALS AND METHODS
Bacteria and plasmids
The invasive GAS isolate M1T1 5448, its isogenic AP covRS−, ΔspeB, ΔcepA, Δmac, and Δralp3 variants, and plasmid complementation by speB (pSpeB) have been previously described (14, 17, 31–33). GAS strains were routinely propagated statically at 37°C in Todd Hewitt broth (Difco), washed twice in phosphate-buffered saline (PBS), and diluted to a multiplicity of infection (MOI) of 10 for in vitro experiments.
Animal experiments
GAS cultures were grown statically overnight at 37°C in Todd Hewitt broth and washed twice in PBS, and 1×108 colony-forming units (CFU) in 100 μL of PBS were injected subcutaneously into 7- to 10-week-old C57BL/6 (Jackson Laboratories), caspase-1/-11−/− (R. Flavell), or il1β−/− (D. Chaplin) mice, as previously described (34). Where indicated, anakinra [50 mg/kg of body weight; Amgen] or MCC950 [20 mg/kg (13)] were delivered intravenously daily starting 24 hours before infection. At 72 hours, mice were euthanized by CO2 asphyxiation, blood was collected by cardiac puncture, and lesions were excised and homogenized. Blood and homogenate were dilution plated onto Todd-Hewitt agar plates for enumeration of bacterial CFU or used for cytokine quantification by enzyme-linked immunosorbent assay (ELISA) (R&D Systems or BioLegend). Mice were housed under specific pathogen-free conditions, and all use and procedures were approved by the University of California, San Diego (UCSD) Institutional Animal Care and Use Committee.
Cell culture
Macrophages were generated from femur exudates of wild-type C57BL/6 (Jackson Laboratories) or caspase-1/11−/− (R. Flavell), il1R−/− (Jackson Laboratories), il1β−/− (D. Chaplin), il18R−/− (Jackson Laboratories), or nlrp3−/− (Millennium Pharmaceuticals) mice as previously described (35). One hour before infection, the medium was replaced with medium lacking fetal bovine serum and antibiotics. Inhibitor treatments were added 1 hour before infection and included anakinra (20 mg/ml; Amgen); 10 μM VX-765 (Invivogen); 50 μM MCC950 (13); 10 μg/mL complete protease inhibitor cocktail (Roche); recombinant IL-1β (100 ng/mL; R&D Systems); 5 μM caspase inhibitors zVAD-fmk, YVAD-fmk, DEVD-fmk, and IETD-fmk (R&D Systems); 50 μM E-64 (Sigma); or 10 μM benzyl-(cyanomethyl)carbamate (Sigma). GAS 5448, GBS COH1, GCS MGCS10565, or MRSA USA300 was grown overnight at 37°C and diluted in PBS to a MOI of 10 for in vitro experiments. Purified PAMPs include LPS (100 ng/mL, from E. coli O111:B4; Sigma), 5 mM ATP (Sigma), FliC (1 μg/mL, from S. typhimurium; Invivogen), poly(dA:dT) (1 μg/mL; Boehringer-Mannheim), and monosodium urate crystals (100 μg/mL; Invivogen). Cells were analyzed 2 hours after infection or treatment unless otherwise stated. Only for intracellular bacterial survival experiments, the medium was supplemented with gentamicin (100 μg/mL) 30 min before the terminal time point, and then, the cells were washed once in PBS and lysed with 0.05% Triton X-100 for dilution plating and CFU enumeration on Todd Hewitt agar plates.
HEK293 cells cultured in RPMI supplemented with 10% fetal bovine serum were transfected with pIL-1β (36), pCaspase-1 or pCaspase-7 (35), or pSpeB or catalytic mutant pSpeBC192A (14) using Lipofectamine (Thermo Fisher) following the manufacturer’s protocol. Cells or supernatants were harvested 24 hours after transfection for analysis, unless otherwise stated. Human neutrophils [polymorphonuclear neutrophils (PMNs)] isolated from healthy donors using Polymorphprep (Axis-Shield) in accordance with the UCSD Human Research Protections Program and HaCaT keratinocytes were cultured in RPMI as previously described (34).
Measurements of cytokines and cell death
Cell death was quantified by release of lactate dehydrogenase (CytoTox 96 kit; Promega) and cytokine release by ELISA (R&D Systems or BioLegend). Caspase-1 activation was determined by Fam-YVAD-FMK (ImmunoChemistry Technologies) staining of macrophages infected on glass coverslips, as previously described (35). Cell permeability was monitored in parallel by staining with propidium iodide (10 μg/ml; ImmunoChemistry Technologies). Caspase-1 activation and cell death were enumerated by counting the fraction of positive cells in at least four separate fields. Subcellular localization of SpeB and IL-1β was examined in macrophages 1 hour after infection with GAS at MOI of 10. Cells were washed in PBS, fixed with Cytoperm (BD Biosciences), incubated with anti-IL-1β (R&D Systems) or anti-SpeB (Toxin Technology, Inc.) antibodies and detected with Alexa Fluor-conjugated secondary antibodies (Invitrogen). Immunofluorescence images were collected using an Axio Observer D1 microscope (Zeiss). Images were processed by identical adjustments for brightness and contrast in ImageJ.
Protein methods
Western blots for IL-1β cleavage were performed on cells infected or transfected as outlined above. In blots of secreted proteins, samples were concentrated by mixing supernatants with an equal volume of saturated ammonium sulfate (Teknova). After SDS–polyacrylamide gel electrophoresis (PAGE) and transfer to polyvinylidene difluoride membranes, blots were probed with anti-IL-1β antibody (R&D Systems). Anti-SpyA (7) and anti-SLO (Abnova) antibodies were used to examine expression of canonical GAS PAMPs in cultured GAS. Blots were developed with infrared-conjugated secondary antibodies (LI-COR Biosciences) and visualized on an Odyssey infrared scanner (LI-COR Biosciences).
For assays with purified proteins, 300 ng of recombinant pro-IL-1β (Sino Biological Inc.) was incubated with 1.24 ng of SpeB at room temperature in assay buffer (PBS with 2 mM dithiothreitol). At the indicated time intervals, reactions were quenched by the addition of LDS-PAGE buffer and heated to 95°C for 5 min, and then run on SDS-PAGE; proteins visualized by silver stain (Thermo Fisher), or incubated with 10 μM E-64 (Sigma); and activity was measured with IL-1R reporter cells (Invivogen). For sequencing of the protein cleavage site, 3 μg of recombinant pro-IL-1β (Sino Biological Inc.) was incubated for 18 hours with 1.24 ng of SpeB at room temperature in assay buffer (PBS with 2 mM dithiothreitol). Proteins were run on SDS-PAGE, transferred to Immobilon-PSQ membrane (Millipore), and then stained with Coomassie blue. The indicated band was cut out of the membrane and sequenced by Edman degradation by Bio-Synthesis Inc.
Internally-quenched peptides of the sequences of IFFDTWDNE, TWDNEAYVH, EAYVHDAPV, and HDAPVRSLN, corresponding to amino acids 103 to 111, 107 to 115, 111 to 119, and 115 to 123 of the reference human pro-IL-1β sequence (UniProt: P01584), were labeled on the N terminus with Mca and the C terminus with Lys-Dnp (CPC Scientific). In triplicate, 10 μM peptides were incubated in assay buffer (PBS with 5 mM dithiothreitol and 0.01% Tween-20) with 5 nM human neutrophil elastase (VWR), human caspase-1 (Enzo), or SpeB from 5448 GAS. The reaction was continuously monitored using an EnSpire plate reader (PerkinElmer) with fluorophore excitation at 323 nm and emission at 398 nm, and the maximum kinetic velocity in triplicate samples was calculated, as previously described (37).
IL-1 signaling assay
Relative IL-1 signaling by macrophages was measured by removing 50 μl of supernatants from infected or treated cells onto transgenic IL-1R reporter cells (Invivogen). After 18 hours, reporter cell supernatants were analyzed for secreted alkaline phosphatase activity by addition of 30 μl of IL-1R reporter cell supernatant onto 70 μl of HEK-Blue Detection reagent (Invivogen) and reading the optical density at 620 nm via EnSpire plate reader (PerkinElmer).
A protease-labile mAmetrine-tdTomato FRET construct (21) was used as a biosensor reference for total proteolysis activity. HEK293T cells (2 × 104) stably expressing the FRET sensor were transiently transfected with 400 ng of pIL-1β (36) in black 96-well plates (Costar). Twenty-four hours after transfection, cells were gently washed twice in RPMI free of serum and phenol red. Neutrophil elastase (1 μg/mL; VWR), caspase-1 (Enzo), or SpeB was made in fourfold dilutions from this concentration and packaged into TransFast liposomes (Promega) using established methods (38). In parallel, LPS (100 ng/mL, from E. coli O111:B4; Sigma), 5 mM ATP (Sigma), FliC (1 μg/mL, from Salmonella typhimurium; Invivogen), poly(dA:dT) (1 μg/mL; Boehringer-Mannheim), Streptococcus pyogenes SLO (1 μg/mL; Fisher), or Bacillus anthracis lethal factor (100 ng/mL; List Biological Laboratories) was prepared with these liposomes as controls. Cells transfected with proteases or inflammasome PAMPs were monitored with an EnSpire plate reader (PerkinElmer) measuring change in FRET with emission at 526 and 581 nm after excitation at 406 nm. Cell supernatants were analyzed by the relative IL-1R signaling assay.
SpeB switching assay
Single CFU were assayed for total protease activity was measured by hydrolysis of azocasein (Sigma) or casein (Sigma) by methods previously described (31). Alternatively, switching was confirmed by monitoring hyperencapsulation by hyaluronic acid assay (Corgenix), as previously described (25). At least eight isolates from each biological replicate were analyzed, and phenotypic conversion is expressed as the percentage of isolates with a heritable loss of casein proteolysis. In each instance, wild-type GAS inoculum was found to contain only a SpeB+ cell population.
FAERS database mining
The FAERS database (http://www.fda.gov/Drugs/InformationOnDrugs/ucm135151) was reviewed as in (39) for reports of infections associated with Anakinra use from 2004 to 2014. Reports were manually screened, and cases with identifiers indicative of duplicated records were excluded from analysis, as outlined in the Supplementary Methods. Proportional reporting risk analysis was assigned by incidence of adverse outcome (infection) in reports containing anakinra relative to incidence of the same outcome in all reports where anakinra is not indicated.
Statistical analysis
The Prism software package (GraphPad) was used to calculate statistical significance by unpaired two-sided Student’s t test (*P < 0.05). All calculations are presented in table S1.
Supplementary Material
Fig. S1. Examination of inflammasome dependence of IL-1β activation.
Fig. S2. Potentiation of GAS infection by NLRP3 inhibition.
Fig. S3. SpeB colocalization with IL-1β.
Fig. S4. SpeB does not activate caspase-8 or caspase-8-like proteolysis.
Fig. S5. SpeB induces cell death and antagonizes canonical inflammasome activation.
Fig. S6. Kinetics of activation by caspase-1 or SpeB during infection.
Table S1. Source data statistics for all figures (Excel).
Table S2. Report IDs positive for indicated drug-adverse reaction.
Table S3. List of suspected duplicate drug adverse reports.
One Sentence Summary.
A microbial protease directly activates IL-1β, resulting in an inflammasome-independent innate immune response.
Acknowledgments
We thank members of the Nizet laboratory for helpful discussions. We also thank M. Walker for SpeB transfection plasmids, M. Karin for caspase and IL-1β transfection plasmids, and P. Ghosh for advice and assistance with protein purification.
Funding: This work was supported by a fellowship from the A.P. Giannini Foundation (C.N.L.), National Health and Medical Research Council fellowship APP1059354 (M.A.C.), and NIH grants AI052453, AI096837 and AI077780 (V.N.) and AI52430 (H.M.H.)
Footnotes
Author contributions:
C.N.L., A.J.O., H.M.H., and V.N. designed the experiments; C.N.L., J.T., D.L.L., J.O., and A.J.O. performed the experiments; C.N.L., J.T., D.L.L., A.J.O., and V.N. analyzed the data; A.A.B.R., M.A.C., and H.M.H. provided reagents necessary for this research; and C.N.L and V.N. drafted the paper with input from all authors.
Competing interests:
The authors declare that they have no competing interests.
References and Notes
- 1.Saavedra PH, Demon D, Van Gorp H, Lamkanfi M. Protective and detrimental roles of inflammasomes in disease. Semin Immunopathol. 2015;37:313–322. doi: 10.1007/s00281-015-0485-5. [DOI] [PubMed] [Google Scholar]
- 2.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. The inflammasome and autoinflammatory syndromes. Ann Rev Pathol. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 3.LaRock CN, Cookson BT. Burning down the house: cellular actions during pyroptosis. PLoS pathogens. 2013;9:e1003793. doi: 10.1371/journal.ppat.1003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev. 2014;27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, Yu GY, Lai LC, Temkin V, Sinzig U, Aung T, Nizet V, Weissman I, Karin M. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder J, Franchi L, Muñoz-Planillo R, Park J-H, Reimer T, Núñez G. Activation of the NLRP3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-κB activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol. 2009;183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin AE, Beasley FC, Keller N, Hollands A, Urbano R, Troemel ER, Hoffman HM, Nizet V. A group A Streptococcus ADP-ribosyltransferase toxin stimulates a protective interleukin 1β-dependent macrophage immune response. mBio. 2015;6:e00133–00115. doi: 10.1128/mBio.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem. 2009;284:862–871. doi: 10.1074/jbc.M804632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRock CN, Nizet V. Inflammasome/IL-1β responses to streptococcal pathogens. Front in Immunol. 2015;6:518. doi: 10.3389/fimmu.2015.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, Modafferi D, Poulakos J, Sun G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r‐metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo‐controlled trial. Arthritis Rheum. 2003;48:927–934. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/
- 12.Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, Hoffman HM. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175:2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 13.Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnett TC, Liebl D, Seymour LM, Gillen CM, Lim JY, LaRock CN, Davies MR, Schulz BL, Nizet V, Teasdale RD. The globally disseminated M1T1 clone of group A Streptococcus evades autophagy for intracellular replication. Cell Host Microbe. 2013;14:675–682. doi: 10.1016/j.chom.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang AY, González-Páez GE, Wolan DW. Identification and co-complex structure of a new S. pyogenes SpeB small molecule inhibitor. Biochemistry. 2015;54:4365–4373. doi: 10.1021/acs.biochem.5b00607. [DOI] [PubMed] [Google Scholar]
- 16.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aziz RK, Pabst MJ, Jeng A, Kansal R, Low D, Nizet V, Kotb M. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol Microb. 2004;51:123–134. doi: 10.1046/j.1365-2958.2003.03797.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Sanchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, Spiller D, White M, Daniels MJD, Mortellaro A, Penalver M, Paszek P, Steringer JP, Nickel W, Brough D, Pelegrin P. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 2016;23:1219–31. doi: 10.1038/cdd.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapur V, Majesky MW, Li L-L, Black RA, Musser JM. Cleavage of interleukin-1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson DC, Garbe J, Collin M. Cysteine proteinase SpeB from Streptococcus pyogenes- a potent modifier of immunologically important host and bacterial proteins. Biol Chem. 2011;392:1077–1088. doi: 10.1515/BC.2011.208. [DOI] [PubMed] [Google Scholar]
- 21.Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Meth. 2008;5:401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- 22.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Druck T, Cannizzaro LA, Huebner K, Black RA. Molecular cloning of the interleukin-1β converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 23.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJ, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin T, Lee TD, Shively JE, Maccross M, Mumford RA, Schmidt JA, Tocci MJ. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 24.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin-1β and inflammatory disease. J Biol Chem. 1990;265:6318–6322. [PubMed] [Google Scholar]
- 25.Cole JN, Pence MA, Köckritz-Blickwede M von, Hollands A, Gallo RL, Walker MJ, Nizet V. M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A Streptococcus. MBio. 2010;1:e00191–00110. doi: 10.1128/mBio.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiebig A, Loof TG, Babbar A, Itzek A, Koehorst JJ, Schaap PJ, Nitsche-Schmitz DP. Comparative genomics of Streptococcus pyogenes M1 isolates differing in virulence and propensity to cause systemic infection in mice. Int J Med Microbiol. 2015;305:532–543. doi: 10.1016/j.ijmm.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Angosto D, López-Castejón G, López-Muñoz A, Sepulcre MP, Arizcun M, Meseguer J, Mulero V. Evolution of inflammasome functions in vertebrates: Inflammasome and caspase‐1 trigger fish macrophage cell death but are dispensable for the processing of IL-1β. Innate immun. 2012;18:815–824. doi: 10.1177/1753425912441956. [DOI] [PubMed] [Google Scholar]
- 29.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Disc. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe J, Kuida K, Flavell RA, Dinarello CA. Response to local inflammation of IL-1β-converting enzyme-deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 31.Kwinn LA, Khosravi A, Aziz RK, Timmer AM, Doran KS, Kotb M, Nizet V. Genetic characterization and virulence role of the RALP3/LSA locus upstream of the streptolysin S operon in invasive M1T1 group A Streptococcus. J Bacteriol. 2007;189:1322–1329. doi: 10.1128/JB.01256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okumura CYM, Anderson EL, Döhrmann S, Tran DN, Olson J, von Pawel-Rammingen U, Nizet V. IgG Protease Mac/IdeS is not essential for phagocyte resistance or mouse virulence of M1T1 group A Streptococcus. mBio. 2013;4:e00499–13. doi: 10.1128/mBio.00499-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4:170–178. doi: 10.1016/j.chom.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaRock CN, Döhrmann S, Todd J, Corriden R, Olson J, Johannssen T, Lepenies B, Gallo R, Ghosh P, Nizet V. Group A streptococcal M1 protein sequesters cathelicidin to evade innate immune killing. Cell Host Microbe. 2015;18:1–7. doi: 10.1016/j.chom.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Donoghue AJ, Jin Y, Knudsen GM, Perera NC, Jenne DE, Murphy JE, Craik CS, Hermiston TW. Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PloS One. 2013;8:e75141. doi: 10.1371/journal.pone.0075141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin-1β via IPAF. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 39.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor α‐neutralizing agent. New Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 40.Chan ATY, Cleeve V, Daymond TJ. Necrotising fasciitis in a patient receiving infliximab for rheumatoid arthritis. Postgrad Med J. 2002;78:47–48. doi: 10.1136/pmj.78.915.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Sande MGH, van Slobbe-Bijlsma ER. Necrotizing fasciitis in a rheumatoid arthritis patient treated with tocilizumab. Rheumatology. 2012;51:577–578. doi: 10.1093/rheumatology/ker336. [DOI] [PubMed] [Google Scholar]
- 42.Jarrett P, Rademaker M, Duffill M. The clinical spectrum of necrotising fasciitis. A review of 15 cases. Aust N Z J Med. 1997;27:29–34. doi: 10.1111/j.1445-5994.1997.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan C, Sullivan P, Eadie P, Veale D. Necrotizing fasciitis in patients on methotrexate therapy. J Clin Rheumatol. 2013;19:289–291. doi: 10.1097/RHU.0b013e31829d4d6f. [DOI] [PubMed] [Google Scholar]
- 44.Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine. 2003;82:225–235. doi: 10.1097/01.md.0000085060.63483.bb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Examination of inflammasome dependence of IL-1β activation.
Fig. S2. Potentiation of GAS infection by NLRP3 inhibition.
Fig. S3. SpeB colocalization with IL-1β.
Fig. S4. SpeB does not activate caspase-8 or caspase-8-like proteolysis.
Fig. S5. SpeB induces cell death and antagonizes canonical inflammasome activation.
Fig. S6. Kinetics of activation by caspase-1 or SpeB during infection.
Table S1. Source data statistics for all figures (Excel).
Table S2. Report IDs positive for indicated drug-adverse reaction.
Table S3. List of suspected duplicate drug adverse reports.


