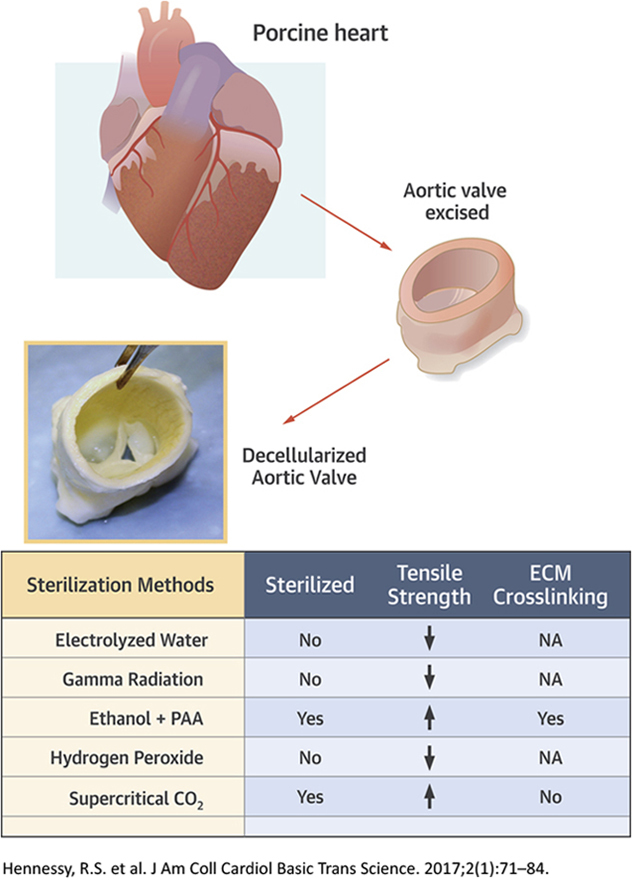
Visual Abstract
Key Words: decellularized, decontamination, heart valve, tensile properties, tissue engineering
Abbreviations and Acronyms: B&B, Brown and Brenn gram stain; DSC, differential scanning calorimetry; ECM, extracellular matrix; EOW, electrolyzed water; ETPA, 96% ethanol with 2% peracetic acid; GAG, glycosaminoglycans; H&E, hematoxylin and eosin; LHP, 6% liquid hydrogen peroxide; PAA, peracetic acid; PAS, Periodic acid-Schiff; PBS, phosphate-buffered saline; scCO2, supercritical carbon dioxide; SDS, sodium dodecyl sulfate; SEM, scanning electron microscopy; UTS, ultimate tensile strength
Highlights
-
•
Sterilization of a decellularized aortic valve was investigated.
-
•
Various sterilization techniques including EOW, gamma radiation, ETPA, LHD, and scCO2 were applied.
-
•
Brown and Brenn staining, Periodic acid-Schiff staining, and aerobic broth culturing were used to characterize sterility.
-
•
Differential scanning calorimetry was used to determine tissue matrix cross-linking.
-
•
Scanning electron microscopy was used to characterize tissue matrix damage, at the structural level.
-
•
EOW sterilization, which is done with electrolyzed water, could not sterilize efficiently.
-
•
Gamma sterilization damaged the tissue matrix.
-
•
Ethanol and peracetic acid–treated samples were cross-linked.
-
•
Hydrogen peroxide sterilization damaged the tissue matrix.
-
•
Supercritical carbon dioxide sterilization method was found efficient to provide 100% sterility of the sample. It neither damages nor cross-links the tissue.
Summary
Sterilization of grafts is essential. Supercritical carbon dioxide, electrolyzed water, gamma radiation, ethanol-peracetic acid, and hydrogen peroxide techniques were compared for impact on sterility and mechanical integrity of porcine decellularized aortic valves. Ethanol-peracetic acid– and supercritical carbon dioxide–treated valves were found to be sterile using histology, microbe culture, and electron microscopy assays. The cusp tensile properties of supercritical carbon dioxide–treated valves were higher compared with valves treated with other techniques. Superior sterility and integrity was found in the decellularized valves treated with supercritical carbon dioxide sterilization. This sterilization technique may hold promise for other decellularized soft tissues.
Tissue grafting is a technique to replace or repair damaged tissue or organs in our bodies 1, 2. One method of tissue grafting is xenografting in which one obtains viable or similar tissue constructs from another animal species. Decellularizing xenografts can be useful for tissue engineering and involves the removal of any cells and several proteins, including DNA, which decrease antigenicity, inflammation, and calcification. During collection, processing, and engineering of tissues intended for grafting, the obtained tissue grafts can become contaminated with bacteria, fungi, and other organisms 3, 4. Therefore, before implantation, these grafts need to be sterilized. There are many sterilization techniques that are being used to sterilize soft tissues, including cardiac tissues; however, each technique has its own beneficial or detrimental effects.
The effects of sterilization on various tissue grafts and engineered tissues have been extensively studied for the last 2 decades 5, 6, 7, 8, 9, 10. However, information on the effect of sterilization on decellularized soft tissue grafts such as heart valves has been infrequent. Further, most sterilization techniques did not show ample effectiveness in sterilizing the tissues without any damage or change in their structure. Somers et al. (11) showed that gamma irradiation altered the ultrastructure of decellularized valves with in vitro testing (12). The alteration included cross-linking, molecular fragmentation, and degradation of protein materials through peptide chain scission, leading to significant modification of mechanical properties. This unfavorable structural change contributes to inferior cell adhesion (13). In our preliminary studies, we examined the effect of gamma irradiation on porcine decellularized valves in a sheep model, and the studies showed that doses of gamma irradiation, enough to sterilize our valves, damaged the valves, leading to maladaptive wound remodeling (14). This occurs via extra damage to the extracellular matrix and disruption of collagen by gamma rays. Sterilization of decellularized tissue engineered porcine liver by ethanol treatment showed significant loss in collagen content, cross-linking of peptide materials, inability to kill the bacterial spores, and ultimately, reduction of cell attachment and proliferation on the decellularized liver tissue (15). When treated with peracetic acid (PAA) by other researchers, the decellularized tissue was found to be sterilized; however, there was loss of glycosaminoglycans (GAG) from the tissue, and only 56% of the original GAG remained due to the oxidative effect of PAA (15). Sterilization with electrolyzed water (EOW) showed reduction of the aerobic colony count and staphylococci on the surfaces, and the aerobic colony count remained below pre-cleaning level after 48 h (16). However, the methicillin-susceptible Staphylococcus aureus, and methicillin-resistant S. aureus counts exceeded the original levels at 24 h after sterilization. The drawbacks in the sterilization techniques discussed in the preceding text encouraged us to apply a novel technique—supercritical carbon dioxide (scCO2) treatment—to sterilize the decellularized porcine aortic valves in coordination with the company, NovaSterilis (Lansing, New York). Sterilization using scCO2 requires additive sterilant to effectively inactivate bacterial endospores. The NovaSterilis process uses peracetic acid to achieve sterility assurance level 6 sterilization required for medical devices and can be applied to both wet and dry samples 17, 18, 19. Sterilization using scCO2 involves low temperatures (31°C) and high pressure (1,100 psi). The low temperature makes the technique ideal for the materials that are temperature sensitive or reactive with other forms of sterilization. The method was, for example, applied to bone and tendon allograft tissue (20), and amniotic tissue (21).
The heart valve has delicate tissues with different thicknesses and structure (cusps, root, aortic wall), which makes it an ideal organ for analyzing mechanical integrity and the impact after sterilization 22, 23. Because the majority of this tissue is heterogeneous and contains very fragile cusps, as well as a strong root, the sterilization process can really affect the integrity of cusps easily, that is, the functionality of the valve 24, 25, 26. To verify the superiority of the scCO2 technique, decellularized porcine aortic valves were sterilized with various sterilization techniques, including the scCO2 technique. The sterility and mechanical properties of these treated valve samples were compared by characterizing different parts of the treated samples.
Materials and Methods
Decellularization of porcine aortic valve
Freshly harvested (within 2 h) whole porcine hearts were randomly selected and obtained from a local abattoir (Hormel Food Corporation, Austin, Minnesota). The aortic valves were dissected, leaving the aortic root, as well as the anterior leaflet of the mitral valve. Most myocardium was trimmed, leaving enough to maintain all aortic valve cusps. After harvest, aortic valves were decellularized with 1% sodium dodecyl sulfate (SDS) for 4 days followed by 2 days of 2% DNase I (Roche Diagnostics GmbH, Mannheim, Germany) washing with TRIS buffer. The valves were then washed with 1% PAA solution and then with phosphate-buffered saline (PBS) for 21 days to remove residual SDS. With these decellularization parameters, we were successful in decellularization of valves. Other researchers have taken fewer or more days to complete their decellularization depending on their method parameters.
Sterilization techniques
Decellularized valves (n = 12 for each technique) were randomly selected and then sterilized with 5 different approaches: 1) electrolyzed water (EOW); 2) 3,000-Gy gamma radiation; 3) 96% ethanol with 2% PAA (ETPA); 4) 6% liquid hydrogen peroxide (LHP) solution; and 5) scCO2. Nonsterile decellularized valves were used as a control.
Electrolyzed water
EOW containing sodium hypochlorite was obtained from a disinfectant machine (Hydris HS000111, Ecolab, St. Paul, Minnesota). Decellularized valves were placed into the EOW for 2 h in a sterile culture hood with resuspension every 15 min. Valves were then washed 8 times with sterile PBS at 15-min intervals as previously described (15).
3,000-Gy gamma radiation
Decellularized valves (in PBS) were gamma irradiated in an irradiator (MK125, J L Shepherd and Associates, San Fernando, California) with a setting of 7.44 Gy/min for 403.23 min at room temperature, yielding a dose of 3,000 Gy. This optimum dose of 3,000 Gy was determined ideal for our tissue construct, considering sterility and trying to maintain the structural integrity (14).
96% ethanol with 2% PAA
Decellularized valves were placed into a 96% ethanol/2% PAA solution for 4 h at room temperature with continuous agitation. Valves were then washed by placing into sterile PBS for 4 days with resuspension every 24 h, similar to Dohmen et al. (27).
6% liquid hydrogen peroxide
Decellularized valves were placed into LHP for 30 min with continuous agitation at room temperature (28). Valves were then placed into sterile PBS for 4 days with a change of PBS every 24 h.
Supercritical carbon dioxide
Decellularized valves were sterilized using the scCO2 technique (NovaSterilis). Decellularized valves were individually packaged in 2 Tyvek (DuPont, Wilmington, Delaware) sterilization pouches with Chevron seal and sterilized in batches of 7 or less. Twenty-five milliliters of water was misted inside of the commercial Nova2200 supercritical vessel (20-l capacity) to increase humidity during sterilization, along with 16 ml of the Novakill Gen2 reagent added onto a cellulose pad placed at the bottom of the vessel inside a stainless steel holder in a 1-inch tall stainless steel basket positioned directly over a stirrer. The Novakill Gen2 reagent contains peracetic acid (certified 13.5% to 18.5%) and hydrogen peroxide (certified 4.5% to 6%). Sterilization was completed in the vessel with a 2-h humidification cycle (chamber heated to 35°C with agitation by stirrer) followed by a 2-h supercritical cycle (target temperature 35°C and pressure 1,436 psi). We cannot provide more information due to the manufacturer’s proprietary rights.
Staining and imaging of sterile valves for their characterizations
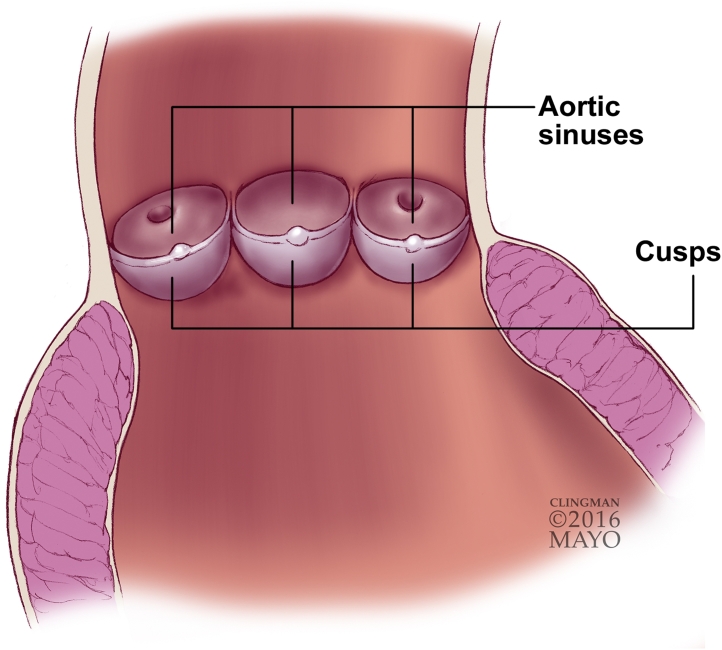
After sterilization of porcine aortic valves through different techniques, all 3 cusps and 3 sinus regions (Figure 1) were stored in PBS at 4°C for 3 days. The 3 sinus sections were placed in 10% buffered formalin overnight. They were then washed 2× in PBS followed by 2 rinses in distilled water to prevent phosphate precipitation. The samples were then embedded in paraffin and cut into 5-μm sections. Hematoxylin and eosin (H&E) staining, Brown and Brenn gram stain (B&B), and fungal Periodic acid-Schiff (PAS) staining were performed on the sections. The slides were imaged with light microscopy at 20×, 40×, and 100× magnification. In H&E staining, any collagen and the nucleus are seen as pale pink and blue, respectively. In B&B staining, any gram-positive bacteria are seen as blue. In PAS staining, any fungus wall is seen as magenta. Sinus samples of each type were characterized through scanning electron microscopy (SEM) analysis. All these stained images and SEM images were inspected by a professional pathologist, and their input is reflected in this paper. Fresh native aortic valve sinus samples were stained with H&E and imaged with light microscopy for structural comparison. Fresh sinus samples were also imaged with SEM for structural comparison and to find any presence of microbes.
Figure 1.
Schematic Diagram of a Sectioned Aortic Valve Containing Cusps and Sinuses
Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Sterility tests
The sinus sections were placed into separate aerobic, anaerobic, and fungal broths, and incubated at 37°C for 1 week (29). Sabouraud dextrose broth (Teknova, Hollister, California) was used for fungal identification. Anaerobic culture (BBL Fluid Thioglycollate Medium, BD, Franklin Lakes, New Jersey) and aerobic culture (Tryptic Soy Broth, Moltox, Boone, North Carolina) were performed on other sinus sections. The broths/media were observed daily for turbidity changes. The turbidity test was performed by visual inspection. The transparency of the original medium was considered as the threshold. At 7 days, broths were sent for analysis to a professional investigating company, Marshfield Labs (Marshfield, Wisconsin), where anaerobic, aerobic, and fungal identification and characterization of organisms was performed.
Tensile tests
Cusps of sterile aortic valves from each sterilization technique (n = 12) were removed and cut with a razor blade punch into a dumbbell shape of 8-mm height. The width at the center of the dumbbell-shaped sample was 2 mm. The circumferentially oriented collagen fibers were aligned along the long axis of the dumbbell-shaped samples. Digital calipers were used to measure the thickness at the center of the samples. Samples were then clamped into custom-made clasps attached to a tensile tester (Bose ElectroForce 3200, Bose Corporation, Eden Prairie, Minnesota) utilizing sandpaper and cyanoacrylate adhesive. A uniaxial tensile test was performed at crosshead speed of 0.1667 mm/s per ASTM standards. The ultimate strength and stiffness were calculated from the stress and strain diagram using load versus displacement data in WinTest Bose software (version 7.0).
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was performed on the treated cusps of aortic valves to quantify cross-linking of the extracellular matrix (ECM) components on the basis of calorimetric heat data against temperature (30). DSC experiments and analyses of their results were performed by a professional investigating company (Legend Technical Services, St. Paul, Minnesota). A tissue sample weighing in the range of 12 to 19 mg was cut and sealed hermetically in a stainless steel O-ring sealable DSC pan. This tissue sealed pan and an empty reference pan were heated from 0°C to 140°C at a heating rate 10°C/min in a Thermal Analysis Instrument (DSC 800, Perkin Elmer, Waltham, Massachusetts). The heat flow was recorded as a function of time, that is, temperature. The heat flow versus temperature endotherm was analyzed with Pyris software. The temperature at the maximum heat flow was considered as the peak temperature. A portion of each sample was also dried, with the percent solids calculated. The sample weight, based on the dry weight, was used for the enthalpy calculations.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, California). For all sterilization groups, the tensile property data including the stiffness and ultimate tensile strength met the criteria for normality using the D’Agostino & Pearson normality test. Data expressed as mean ± SD were compared by 1-way analysis of variance followed by the Bonferroni multiple comparison test for statistical significance. Two outliers were identified (Q = 5.0%) due to the specimen slipping in the grips during mechanical testing, and were therefore removed from the statistical analysis. Values of p < 0.05 were considered statistically significant.
Results
Decellularized aortic valves were sterilized by various sterilization techniques: scCO2, EOW, gamma radiation, ethanol-peracetic acid, and LHP. The sterility and mechanical integrity of the treated valves were investigated by characterizing sinuses and cusps of each sterilized valve as shown in Figure 1.
Tests of sterility (n = 12)
For the sterility test, we performed 3 experiments: microbe culture, staining, and SEM imaging to find any presence of bacteria or fungi in any samples. We performed multiple experiments to be sure that all experiments on a sample produced negative results and the sample could be declared as sterile.
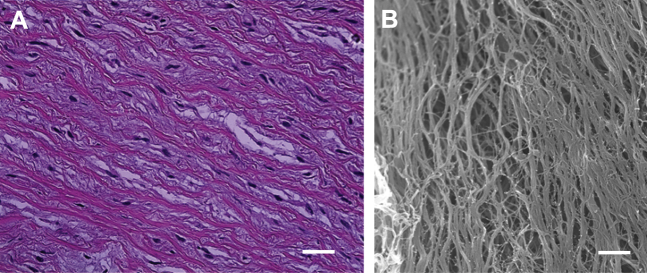
To show the difference between native valve tissue and sterile valve tissue, H&E staining and SEM imaging of the fresh native valve sinus were obtained (Figure 2). The H&E results (Figure 2A) display blue/purple nuclei and thus demonstrate that cells were present in the valve sinus. The pale pink collagen fibrils have distinct orientation. These oriented collagen fibrils are also confirmed in the SEM image. No presence of microbes was found in the fresh native aortic sinus.
Figure 2.
H&E-Stained Image and SEM Image of Sinus of a Native Aortic Valve
(A) In hematoxylin and eosin (H&E) staining, nuclei are seen as blue/purple. Collagen fibrils are seen as pale pink. Scale: 5 μm. (B) Collagen fibrils were aligned and dense as shown in the scanning electron microscopy (SEM) image. Scale = 8 μm.
Nonsterilized decellularized valves
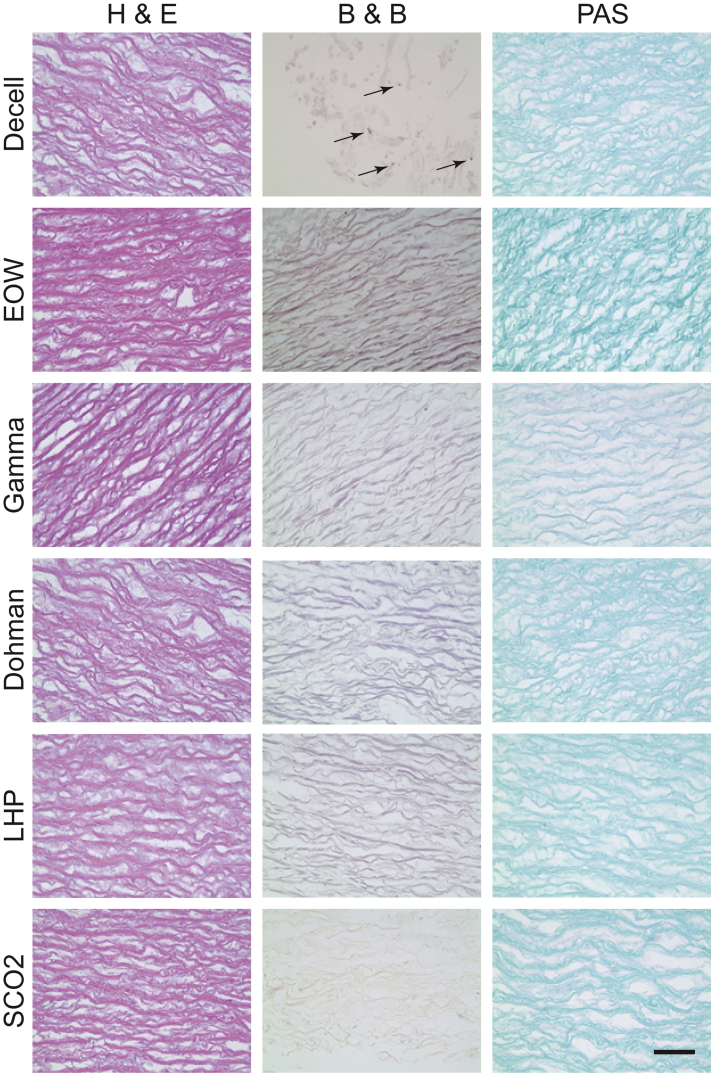
The decellularization process itself did not result in a sterile valve. H&E staining showed no evidence of nuclei (blue), indicating that there was no evidence of porcine cells in the decellularized valve (Figure 3). B&B stain showed the presence of gram-positive cocci (purplish blue, shown by the arrows). No fungi were visible with PAS staining (magenta). Aerobic, anaerobic, and fungal broths were turbid at 3 days. Analysis showed no evidence of anaerobic or fungal growth present at baseline; however, there were 4 species of bacteria present in the aerobic cell culture: Citrobacter freundii, Escherichia coli, Enterobacter cloacae, and Stenotrophomonas maltophilia. To observe any presence of microbes in the decellularized samples, they were imaged with SEM, and microbes were visible in the SEM images (Figure 4A).
Figure 3.
Decellularized Aortic Valve Samples Stained With H&E, B&B, and PAS for Each Sterilization Technique
In hematoxylin and eosin (H&E) staining, no blue/purple-colored nuclei are seen. Collagen fibrils are seen as pale pink. In Brown and Brenn (B&B) staining, gram-positive bacteria are seen as blue. In Periodic acid-Schiff (PAS) staining, the fungus wall is seen as magenta. Arrows show the gram-positive+ bacteria. Scale = 10 μm. Decell = decellularized; Dohman = Dohman technique (Dohman et al. [27]); EOW = electrolyzed water; LHP = 6% liquid hydrogen peroxide; scCO2 = supercritical carbon dioxide.
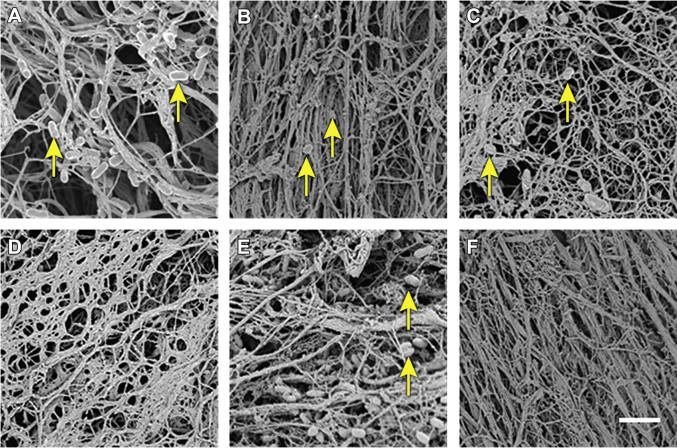
Figure 4.
SEM Images of Sinuses After Sterilization by Various Techniques
(A) Nonsterile decellularized sinuses showed the presence of microbes (arrows). (B) Sterilization with the EOW technique could not remove the microbes (arrows). (C) Microbes (arrows) were evident after sterilization with gamma radiation. (D) No microbes were found after sterilization with the EPTA technique. (E) Presence of microbes (arrows) proved that the LHP technique could not sterilize sinus tissue completely. (F) Sinus tissues were completely sterile because no microbes were present after sterilization with the scCO2 technique. Scale = 12 μm. Abbreviations as in Figures 2 and 3.
Electrolyzed water
EOW did not effectively sterilize decellularized valve tissue. H&E staining showed no evidence of porcine cells due to absence of nuclei (blue) (Figure 3). B&B and PAS staining showed no apparent presence of cocci (purplish blue) and fungal infiltration (magenta), respectively. However, aerobic broths were turbid at 2.5 days with infiltration of C. freundii, E. cloacae, and S. maltophilia. There were no anaerobic or fungal cultures that were positive. Microbes were evident under SEM (Figure 4B).
Gamma
H&E staining showed no evidence of nuclei (blue), and thus, there were no porcine cells in the treated tissue (Figure 3). B&B stain showed no bacteria (purplish blue), and there were no visible spores or fungal particles (magenta) with PAS staining. Aerobic, anaerobic, and fungal broths showed no turbidity at 14 days. Through SEM imaging, microbes were observed in the tissue samples (Figure 4C).
96% ethanol with 2% PAA
Valves were analyzed after treatment with ETPA for their sterilization. The sterilized samples were stained with H&E staining, and the stained sample did not show any evidence of nuclei (blue) of porcine cells (Figure 3). The samples were stained with B&B, and no bacteria (purplish blue) were found. PAS staining of the treated samples showed no presence of fungus (magenta). Aerobic, anaerobic, and fungal broths showed no turbidity at 14 days. There were no microbes in the treated samples when observed in the SEM (Figure 4D).
6% liquid hydrogen peroxide
Treated valves were then washed in sterile PBS for analysis. H&E staining was performed on the samples and showed no presence of porcine cells (blue) (Figure 3). B&B and PAS staining was performed and showed no presence of bacteria (purplish blue) or fungi (magenta) in the samples. Aerobic broths were all turbid and grew E. coli, whereas the anaerobic and fungal broths showed no turbidity at 14 days. SEM imaging confirmed the presence of microbes in the samples (Figure 4E).
Supercritical carbon dioxide
H&E staining of the treated samples showed no evidence of porcine cells (blue) (Figure 3). Both B&B (purplish blue) and PAS (magenta) staining were negative for microorganisms such as bacteria and fungi. Aerobic, anaerobic, and fungal broths were analyzed, and they showed no turbidity at 14 days. Microbes were not observed with SEM imaging of the samples (Figure 4F).
A microbial summary for evaluated sterilization techniques is provided in Table 1. Comparing the SEM images of the native valve sinus and sterile decellularized valve sinus (Figures 2B and 4), it is observed that during decellularization and sterile processing, there were some alterations in orientation and density of collagen fibrils.
Table 1.
Microbial Summary for Evaluated Sterilization Techniques
| Technique | Culture (Aerobic) | Staining | SEM for Bacteria |
|---|---|---|---|
| Decellularized valve | + | + | + |
| EOW | + | − | + |
| Gamma | − | − | + |
| ETPA | − | − | − |
| LHP | + | − | + |
| scCO2 | − | − | − |
EOW = electrolyzed water; ETPA = 96% ethanol with 2% peracetic acid; LHP = 6% liquid hydrogen peroxide; scCO2 = supercritical carbon dioxide; SEM = scanning electron microscopy.
Tensile testing
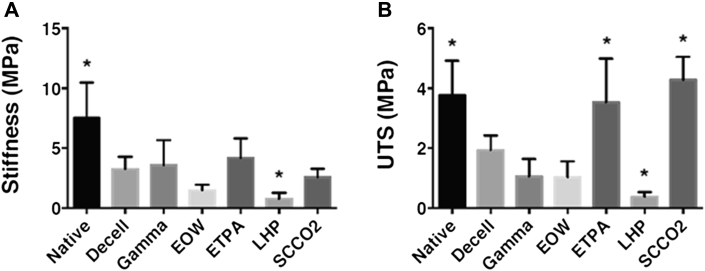
Besides sterility testing, tensile testing on the sterile valve tissues was performed to verify their mechanical integrity. Cusps are weaker than sinuses and were chosen for tensile testing. In the case of valve tissue, mechanical integrity is an important parameter because the valve tissue faces a dynamic environment including transvalvular pressure, shear stress, and sudden impact. From the obtained results, it was observed that stiffness of the nonsterile decellularized leaflets (3.24 ± 0.29 MPa) was less than one-half of the stiffness for the native leaflets (7.50 ± 0.85 MPa) (Figure 5A). After sterilization through various techniques, the tensile stiffness of sterile leaflets was either lowered or marginally increased. Leaflets sterilized by gamma radiation (3.57 ± 0.60 MPa) and ETPA (4.18 ± 0.47 MPa) techniques showed an increase in stiffness compared with the stiffness of the nonsterile decellularized leaflets (3.24 ± 0.29 MPa), likely due to cross-linking of the peptide materials existing in the leaflets. However, leaflets sterilized by the EOW (1.67 ± 0.44 MPa) and LHP (0.76 ± 0.14 MPa) techniques showed a decrease in stiffness compared with the stiffness of the decellularized leaflets (3.24 ± 0.29 MPa), possibly due to damage of the construct, proteins, and other essential materials. On the other hand, there was no statistically significant difference found between the tensile stiffness of leaflets sterilized by the scCO2 technique (2.92 ± 0.38 MPa) compared with nonsterile decellularized leaflets (3.24 ± 0.29 MPa).
Figure 5.
Mechanical Integrity of Sterile Valve Cusps
(A) Stiffness of the sterile valve leaflets was compared with that of non-sterile decellularized valve leaflet. Stiffness of native valve leaflet (∗p < 0.0001) and LHP treated leaflet (∗p = 0.002) were significantly different from decellularized leaflet by 1-way ANOVA. (B) Ultimate tensile strength (UTS) of the sterile valve leaflets was compared with that of non-sterile decellularized valve leaflet. UTS of native valve leaflet (∗p < 0.0001), ETPA treated (∗p < 0.0001), LHP treated (∗p = 0.0001), and scCO2 treated leaflets (∗p < 0.0001) were significantly different from decellularized leaflet by 1-way ANOVA. ETPA = 96% ethanol with 2% peracetic acid; other abbreviations as in Figure 3.
The ultimate tensile strength (UTS) of the decellularized leaflets (1.93 ± 0.14 MPa) was less than one-half that of the native leaflets (4.21 ± 0.55 MPa) (Figure 5B). After sterilization of the decellularized leaflets with gamma radiation (1.25 ± 0.25 MPa) or EOW (1.02 ± 0.15 MPa) techniques, the UTS values were even lower compared with the decellularized process (1.93 ± 0.14 MPa) alone. Sterilization by the LHP technique (0.37 ± 0.04 MPa) had an 80% lower UTS compared with decellularized leaflets (1.93 ± 0.14 MPa). However, sterilization with the ETPA technique (3.53 ± 0.41 MPa) showed a 45% increase in UTS compared with decellularized leaflets (1.93 ± 0.14 MPa), which was comparable to the UTS of the native leaflet. Interestingly, sterilization through the scCO2 technique (4.28 ± 0.22 MPa) had the largest increase in UTS with approximately 55% higher values compared with the decellularized leaflet (1.93 ± 0.14 MPa), and was found to be statistically significant (p < 0.0001).
DSC analysis
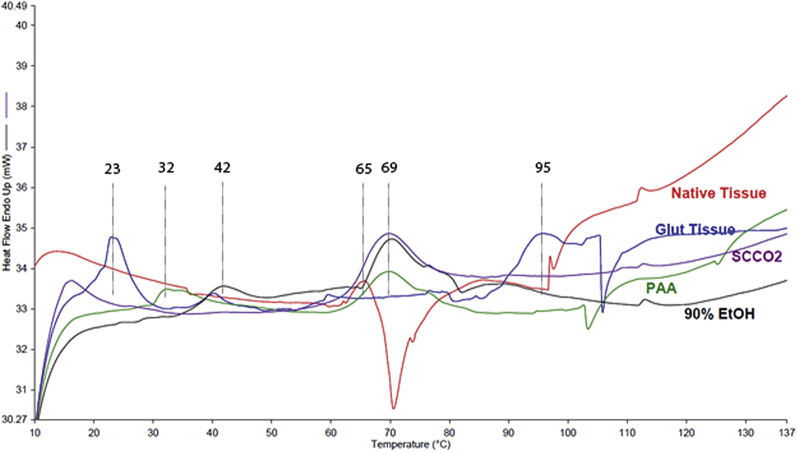
Calorimetric heat data against temperature provided information on cross-linking of the ECM during each sterilization process. This cross-linking was found to be directly related to a change in mechanical properties for each sterilization technique. DSC testing was performed on PAA, 90% ethanol, scCO2, native (negative control), and glutaraldehyde-treated (positive control) valves. ETPA is a combination of 90% ethanol and PAA (both chemicals have the capability to fix the tissue); therefore, to remove any confounding results, both 90% ethanol and PAA samples were tested independently. The DSC curves for the samples sterilized by various techniques compared to native and glutaraldehyde-treated samples are shown in Figure 6.
Figure 6.
DSC Curves of Treated and Nontreated Valve Samples
Differential scanning calorimetry (DSC) curves of a leaflet of a native aortic valve (red) and glutaraldehyde-treated (Glut) (blue) valve samples. DSC curves of a leaflet of 90% ethanol–treated (EtOH) (black), PAA-treated (green), and scCO2-treated (purple) valve samples. The treatments were performed on decellularized valves. Abbreviations as in Figure 3.
DSC is used to evaluate denaturation properties of the sterilized tissue, especially its main constituent, collagen (31). The native leaflet of an aortic valve contained a weak endothermic transition at 65°C. This is a typical denaturation temperature (shrink temperature) of non–cross-linked collagen materials. The typical range is from 60°C to 70°C. In the glutaraldehyde-treated leaflet sample, a very weak endothermic transition was noted at 59°C (shrink temperature). Two larger endothermic transitions were also noted, one at 23°C and the other at 95°C. The transition at 23°C is indicative of substantial denaturation of the collagen (32). The PAA-treated sample contained a main endothermic transition (shrink temperature) at 69°C. A smaller endothermic transition was noted at 32°C, indicating some denaturation. The slightly elevated shrink temperature indicates some cross-linking of the material. In the 90% ethanol–treated sample, a main endothermic transition (shrink temperature) was noted at 70°C. A smaller endothermic transition was noted at 42°C, indicating some denaturation. This sample had a thermal profile similar to the PAA-treated sample. The scCO2-sterilized leaflet sample exhibited 1 main endothermic transition (shrink temperature) at 69°C, which is close to the endothermic transition that occurred in the native leaflet at 65°C. Except for the aforementioned endothermic transitions in the DSC curves of different samples, no other significant transitions were noted. Because both the nonsterile native leaflet and the leaflet sterilized with the scCO2 technique showed endothermic transition in almost the same temperature zone, it seemed that significant cross-linking did not occur in the scCO2-sterilized leaflet compared with the native leaflet. Nonexistence of an endothermic transition at 23°C and 95°C in the scCO2-treated leaflet further confirms that the scCO2 technique did not cross-link the valve significantly.
Discussion
In our first in vivo experiments with decellularized heart valves placed into an ovine model, we observed that terminal sterilization techniques can affect the valve integrity and durability (14). EOW has been shown to have some efficacy in removing bacteria and fungus; however, the majority of the antibiotic-resistant bacteria such as streptococcal and staphylococcal species are resistant to its effect (16). The method of ethanol and PAA not only fixes the tissue, thereby preventing remodeling of the tissue, but also can affect the amount of collagen and protein retained, thus affecting cellularization (15). This method for sterilization also has issues in removing bacterial spores that have been known to survive extreme dryness, subzero temperatures, and extreme environments. Peracetic acid has been shown to affect the amount of GAG retention in decellularized constructs (15). Gamma irradiation has been showed to change the microenvironment of decellularized constructs in in vitro settings 11, 12. This ensues via protein breakdown, denaturation, and other methods that can affect the mechanical properties of these tissue constructs. This change that occurs can also change this once cell-friendly environment to an environment that inhibits cellularization. Not only does gamma irradiation affect the cellular adhesion, but it also can promote maladaptive changes by promoting inflammatory cells and other factors that help disrupt this damaged, exposed matrix. This can affect the whole construct and remodel the tissue to a defective state. Thus, it was important to test sterilization techniques to find a technique that not only sterilizes effectively, but also preserves the mechanical integrity of the tissue after sterilization.
The first part of evaluating a sterilization technique is testing whether the technique actually is efficient in sterilization of the tissue. Most published reports that described in vivo experiments of decellularized tissues do not provide detail about sterilization techniques or validation of tissue sterility 33, 34. Our preliminary experiments showed that gamma-radiated samples exposed to 3,000 Gy resulted in negative cultures; however, their SEM images showed the presence of bacteria. scCO2 technique showed no culture growth, as well as no bacteria present in SEM images. The LHP technique showed no anaerobic or fungal growth, yet aerobic cultures were turbid and had the presence of E. coli. Thus, multiple testing measures are paramount for sterility success, and our more stringent confirmation methodology included H&E staining; gram staining; fungal staining; growth of microbes in aerobic, anaerobic, and fungal broths; and SEM imaging. All of these methods have helped to determine the levels of sterility for the different tissue sterilization techniques.
During sterilization, valves were processed in different solutions and environments according to their protocols, leading to their sterilization and changes in mechanical properties. Some techniques such as ETPA and scCO2 were effective to sterilize the valves completely. Other techniques were partially effective. In the gamma sterilization technique, bacteria were observed in SEM images; however, there were no living microbes detected in the other 2 characterization (staining and culture) modalities. Sterilization with the LHP technique was not effective because microbes were observed in the aerobic culture. Thus, among all techniques studied here, the ETPA and scCO2 techniques showed effectiveness in sterilization of decellularized valve tissue.
During sterilization, tissue damage can occur due to the sterilant’s harsh environment. Testing of tensile properties of tissue after their sterilization could be useful to measure the possible damage. Orientation of collagen fibrils was also disrupted during the decellularization process, as well as during the sterilization process. Another important sterilization drawback is tissue fixation and the ability of the sterilant to cross-link tissues. Cross-linking and fixation can narrow and tighten the tissue via the Mannich reaction, leading to possible strength enhancement with decreasing cell functionality (35). Thus, cross-linking has the possibility for the impedance of the decellularized construct to remodel and incorporate cells.
Leaflets of the sterilized valves were used for tensile properties because they are the most vulnerable part of a heart valve. Both the stiffness and UTS of native leaflets were found to be decreased during decellularization due to use of chemical detergents that degrade the ECM in the leaflets (36). A similar observation was made by Liao et al. (37) in their mechanical property testing of the decellularized heart valve leaflet. They found effective flexural modulus decreased from 156 kPa for the native aortic valve leaflet to 24 kPa, 19 kPa, and 16 kPa for SDS-, Triton X-100–, and trypsin-treated leaflets, respectively. Tissue engineered heart valve leaflets also showed a decrease in circumferential stiffness from 7.4 MPa to 6.3 MPa after decellularization with SDS and Triton X-100 successively (38). In the presence of EOW, the ECM of each decellularized leaflet was degraded further, leading to decreased tensile properties.
However, sterilization by the ETPA technique increased both stiffness and UTS due to fixation of collagen in the leaflets (proved by DSC tests). Similarly, the EOW and LHP techniques of sterilization degraded the leaflets, leading to their decrease in stiffness and UTS. Interestingly, sterilized leaflets by the scCO2 technique showed an increment in UTS, maintaining a stiffness similar to that of native leaflets. The DSC analyses of the native leaflet and scCO2-sterilized leaflet indicated no fixation of any protein materials in the scCO2 sterilization process, and thus, their stiffness did not change. It may be possible that reactions during scCO2 sterilization assisted in minute reversible cross-linking not picked up by DSC of protein materials leading to an increase of UTS (38).
The DSC determines the temperature and heat flow associated with material transitions as a function of time and temperature. It also provides quantitative and qualitative data on endothermic transition (heat absorption) and exothermic (heat evolution) processes of materials during physical transitions that are caused by phase changes, such as “shrinkage temperature” or collagen denaturation temperature. Table 2 lists the onset temperature(s), peak temperature(s), and enthalpy(ies) of denaturation for each sample.
Table 2.
Data From DSC Experiments of Various Treated and Nontreated Samples
| Treated Tissues | Onset Temp(s) (°C) | Peak Temp(s) (°C) | Enthalpy(ies) of Denaturation (ΔH) (J/g) |
|---|---|---|---|
| Native tissue | 61.49 | 65.75 | 1.4223 |
| Glutaraldehyde tissue | 21.03, 90.31 | 22.93, 94.72 | 3.1570, 2.2774 |
| PAA | 29.84, 62.97 | 32.31, 69.83 | 1.1922, 4.1005 |
| 90% ethanol | 36.89, 65.39 | 41.57, 70.29 | 1.1217, 3.4800 |
| scCO2 | 63.07 | 69.58 | 5.4707 |
PAA = peracetic acid; scCO2 = supercritical carbon dioxide.
Kinetic energy of the molecules of collagen increases with rise in temperature, leading to melting or denaturing of collagen. Melting or denaturing of degraded or damaged samples with less molecular stability generally occurs at low temperature and takes less energy. Melting or denaturing of collagen in native leaflets occurred at 65°C, and it consumes 1.42 J/g energy. Glutaraldehyde-treated fixed tissue showed 1 denature peak temperature at 23°C, which is lower than the denature temperature (65°C) for native leaflet. Commonly, glutaraldehyde-fixed tissue has more bonds between the molecules, and its denature temperature should increase, which is observed at 95°C. It seems that some damage of the collagen due to the corrosive nature of glutaraldehyde occurred, causing its first denature peak temperature at 23°C. However, besides glutaraldehyde, the PAA and 90% ethanol-treated fixed samples showed denaturation at a temperature (33°C and 42°C, respectively) lower than the denature temperature (65°C) of native tissue. The reason could be development of new molecular bonding from the treatment and that the bonding strength is less than the bonding strength that exists in the native tissue. In addition to this low bond strength, the treatments also created molecular bonding with a strength higher than the strength that exists in the native tissue. Thus, another denaturation peak (70°C and 71°C, respectively) was observed in each fixed sample. The higher denaturation peak (95°C) in the glutaraldehyde-treated sample confirms more fixation in this sample compared with the PAA or 90% ethanol-treated samples. Only the scCO2-treated sample showed 1 peak temperature, and it was within the native collagen denature temperature range (60°C to 70°C), which confirms that there was almost no fixation of the tissue with scCO2 treatment.
For denaturing of the treated tissues, there were increments in the enthalpy requirement with respect to that for native tissue, and the extra bonding among the molecules necessitated more energy for their disruption. Interestingly, a large increase in the enthalpy requirement for the scCO2-treated sample was observed. Although the rationale behind this enthalpy increment is unknown, similar observations were noticed when gamma-irradiated collagen samples were characterized by DSC assay 39, 40.
DSC analysis of the ECM protein in the treated leaflets revealed partial to full fixation of collagen. Sterilization by the gamma irradiation technique increased the stiffness and decreased the strength of decellularized leaflets, which indicates that the ECM became hardened after radiation exposure. Gamma irradiation causes scission of molecular bonding in the peptides, leading to a decrease in strength (40). However, the scission in peptide chains may lead to formation of new inter- and intramolecular bonds. Gamma irradiation brings an oxidative environment to the tissue sample, causing modification of amino acid side chains, hydroxylation, and an increase in carbonylation (41). It is possible that interaction of peptide chain with bound water and mineral alters and influences the stiffness of the sample (42).
On the basis of our objective methodology of testing sterilization techniques, we found that the scCO2 technique had the best balance between sterility and preserving tissue strength. Because the main sterilant of this technique is carbon dioxide, use of a safe effective gas not only preserves strength without affecting the existing collagen/elastin matrix, it also removes both fungi and bacteria effectively and nontoxically. This was performed by the manipulation of pressure and temperature on the tissue, which was gentle in nature, and thus, the properties of leaflets were preserved.
The regulatory aspects for sterilization of heart valve replacements can be complicated and are described in the Food and Drug Administration heart valve guidance document, as well as in the Cardiovascular Implants ISO 5850 document (43). Sterilization manufacturers are required to perform a validation study demonstrating that their process provides a sterility assurance level of at least 10−6. The validation studies should include positioning a biological indicator in the worst-case valve location. Because the valve assembly is very intricate, these biological indicators might be placed behind the commissures where there are many suture sites, knots, and small layers of tissue. Another key location where biological indicators might be situated would be between the valve, cloth, and stent interfaces. In addition to placing indicators on the valve, it is important to identify the worst-case location for the valve sterilization within the sterilization chamber itself. This can be done by conducting a series of experiments such as temperature mapping of the sterilization chamber, which would indicate regions of low/high temperature variations observed throughout the sterilization process. Once these indicators are placed on the valve and the valve is in the worst-case location in the chamber, the validation specimens will be exposed to the process range including the minimum and maximum temperatures and times of the sterilization process. The resulting spore counts from the biological indicators that were placed on the valve will be analyzed. The organism chosen for the analysis must represent the worst-case organism for the sterilization process. Further details for valve and sterilization manufacturers refer to ISO 11134, ISO 11135, ISO 11137, ISO 14160, and ISO 14937 44, 45, 46, 47, 48.
Conclusions
In summary, we have tested different sterilization techniques to sterilize decellularized heart valves. Gamma irradiation with the required sterilization dose damaged the valve cusps. EOW and hydrogen peroxide were not effective enough for a terminal sterilization technique for our valve samples due to microbial remnants. Ethanol was effective in sterilization; however, the cellular remodeling potential was inhibited with fixation and cross-linking. The scCO2 sterilization technique proved superior to other techniques evaluated. It maintained good mechanical properties and structural integrity during the sterilization process. This scCO2 sterilization technique could be useful to other soft decellularized tissue constructs.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Decellularized tissues are often used for grafting. Before grafting, these tissues need to be sterilized. There are several methods that have been applied for sterilization of decellularized tissues; however, all the techniques have some drawbacks, including fixation and/or damage of the tissues. A supercritical carbon dioxide–based sterilization method has been found to be superior to other methods in accomplishing the sterilization of the decellularized heart valves and preserving their integrity during sterilization. This method holds promise for sterilization of decellularized soft tissues.
TRANSLATIONAL OUTLOOK: After sterilization of decellularized heart valve with supercritical carbon dioxide–based method, mild presence of peracetic acid, which is one of the ingredients in the sterilization method, was observed. Presence of acid helps keeping the sterility of the tissue intact for a long time. In our in vivo implantation study in animals, there were no adverse results observed due to the presence of acid in the heart valve tissue. Further investigations are being pursued on the use of the supercritical carbon dioxide–based method to obtain full sterile decellularized heart valves without any presence of peracetic acid.
Acknowledgments
The authors would like to thank Jenny Pattengill and Federico Franchi for their technical assistance with this project. The authors would also like to acknowledge NovaSterilis, Inc. for their collaboration regarding sterilization with supercritical CO2.
Footnotes
This work is supported by the HH Sheikh Hamed bin Zayed Al Nahyan Program in Biological Valve Engineering and the National Institutes of Health (#T32HL007111). The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Hennessy and Jana contributed equally to this work.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Galea G. The organization of tissue banking in Scotland. Scott Med J. 2012;57:225–231. doi: 10.1258/smj.2012.012124. [DOI] [PubMed] [Google Scholar]
- 2.van Kats J.P., van Tricht C., van Dijk A. Microbiological examination of donated human cardiac tissue in heart valve banking. Eur J Cardiothorac Surg. 2010;37:163–169. doi: 10.1016/j.ejcts.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Uriarte J.J., Nonaka P.N., Campillo N. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. J Mech Behav Biomed Mater. 2014;40:168–177. doi: 10.1016/j.jmbbm.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Rosario D.J., Reilly G.C., Ali Salah E., Glover M., Bullock A.J., Macneil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med. 2008;3:145–156. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 5.Akkus O., Rimnac C.M. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927–934. doi: 10.1016/S0736-0266(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 6.Athanasiou K.A., Niederauer G.G., Agrawal C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 7.Fideler B.M., Vangsness C.T., Lu B., Orlando C., Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- 8.Holy C.E., Cheng C., Davies J.E., Shoichet M.S. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials. 2000;22:25–31. doi: 10.1016/s0142-9612(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 9.Munting E., Wilmart J.-F., Wijne A., Hennebert P., Delloye C. Effect of sterilization on osteoinduction: comparison of five methods in demineralized rat bone. Acta Orthop. 1988;59:34–38. doi: 10.3109/17453678809149340. [DOI] [PubMed] [Google Scholar]
- 10.Noah E.M., Chen J., Jiao X., Heschel I., Pallua N. Impact of sterilization on the porous design and cell behavior in collagen sponges prepared for tissue engineering. Biomaterials. 2002;23:2855–2861. doi: 10.1016/s0142-9612(01)00412-4. [DOI] [PubMed] [Google Scholar]
- 11.Somers P., Cuvelier C.A., Somer F.D. Gamma radiation alters the ultrastructure in tissue-engineered heart valve scaffolds. Tissue Eng Part A. 2009;15:3597–3604. doi: 10.1089/ten.TEA.2008.0690. [DOI] [PubMed] [Google Scholar]
- 12.Gouk S.S., Lim T.M., Teoh S.H., Sun W.Q. Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation, and remodeling. J Biomed Mater Res B Appl Biomater. 2008;84:205–217. doi: 10.1002/jbm.b.30862. [DOI] [PubMed] [Google Scholar]
- 13.Matuska A.M., McFetridge P.S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold: implications for stem cell adhesion. J Biomed Mater Res B Appl Biomater. 2015;103:397–406. doi: 10.1002/jbm.b.33213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helder M., Hennessy R., Spoon D. Low-dose gamma irradiation of decellularized heart valves results in tissue injury in vitro and in vivo. Ann Thorac Surg. 2016;101:667–674. doi: 10.1016/j.athoracsur.2015.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein K.H., Park K.M., Teotia P.K. Sterilization using electrolyzed water highly retains the biological properties in tissue-engineered porcine liver scaffold. Int J Artif Organs. 2013;36:781–792. doi: 10.5301/ijao.5000246. [DOI] [PubMed] [Google Scholar]
- 16.Stewart M., Bogusz A., Hunter J. Evaluating use of neutral electrolyzed water for cleaning near-patient surfaces. Infect Control Hosp Epidemiol. 2014;35:1505–1510. doi: 10.1086/678595. [DOI] [PubMed] [Google Scholar]
- 17.Setlow B., Korza G., Blatt K.M., Fey J.P., Setlow P. Mechanism of Bacillus subtilis spore inactivation by and resistance to supercritical CO plus peracetic acid. J Appl Microbiol. 2016;120:57–69. doi: 10.1111/jam.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Q.Q., Leamy P., Brittingham J., Pomerleau J., Kabaria N., Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater. 2009;91:572–578. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- 19.White A., Burns D., Christensen T.W. Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol. 2006;123:504–515. doi: 10.1016/j.jbiotec.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Nichols A., Burns D.C., Christopher R. Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical carbon dioxide. J Orthop. 2009;6 e(1–9) [Google Scholar]
- 21.Wehmeyer J.L., Natesan S., Christy R.J. Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Engineering Part C Methods. 2015;21:649–659. doi: 10.1089/ten.TEC.2014.0304. [DOI] [PubMed] [Google Scholar]
- 22.Jana S., Lerman A. Bioprinting a cardiac valve. Biotechnol Advs. 2015;33:1503–1521. doi: 10.1016/j.biotechadv.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Jana S., Lerman A., Simari R.D. In vitro model of a fibrosa layer of a heart valve. ACS Appl Mater Interfaces. 2015;7:20012–20020. doi: 10.1021/acsami.5b04805. [DOI] [PubMed] [Google Scholar]
- 24.Jana S., Simari R.D., Spoon D.B., Lerman A. Drug delivery in aortic valve tissue engineering. J Control Release. 2014;196:307–323. doi: 10.1016/j.jconrel.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Jana S., Tefft B.J., Spoon D.B., Simari R.D. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014;10:2877–2893. doi: 10.1016/j.actbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Jana S., Tranquillo R.T., Lerman A. Cells for tissue engineering of cardiac valves. J Tissue Eng Regen Med. 2016;10:804–824. doi: 10.1002/term.2010. [DOI] [PubMed] [Google Scholar]
- 27.Dohmen P.M., da Costa F., Yoshi S. Histological evaluation of tissue-engineered heart valves implanted in the juvenile sheep model: is there a need for in-vitro seeding? J Heart Valve Dis. 2006;15:823–829. [PubMed] [Google Scholar]
- 28.Ganavadiya R., Chandra Shekar B.R., Saxena V., Tomar P., Gupta R., Khandelwal G. Disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments: a randomized trial. J Basic Clin Pharm. 2014;5:98–104. doi: 10.4103/0976-0105.141946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parveen S., Kaur S., David S.A., Kenney J.L., McCormick W.M., Gupta R.K. Evaluation of growth based rapid microbiological methods for sterility testing of vaccines and other biological products. Vaccine. 2011;29:8012–8023. doi: 10.1016/j.vaccine.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Chiu M.H., Prenner E.J. Differential scanning calorimetry: an invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J Pharm Bioallied Sci. 2011;3:39–59. doi: 10.4103/0975-7406.76463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozec L., Odlyha M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys J. 2011;101:228–236. doi: 10.1016/j.bpj.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samouillan V., Delaunay F., Dandurand J. The use of thermal techniques for the characterization and selection of natural biomaterials. J Funct Biomater. 2011;2:230–248. doi: 10.3390/jfb2030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt D., Stock U.A., Hoerstrup S.P. Tissue engineering of heart valves using decellularized xenogeneic or polymeric starter matrices. Philos Trans R Soc Lond B Biol Sci. 2007;362:1505–1512. doi: 10.1098/rstb.2007.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juthier F., Vincentelli A., Gaudric J. Decellularized heart valve as a scaffold for in vivo recellularization: deleterious effects of granulocyte colony-stimulating factor. J Thorac Cardiovasc Surg. 2006;131:843–852. doi: 10.1016/j.jtcvs.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Doughty M.J., Bergmanson J.P., Blocker Y. Shrinkage and distortion of the rabbit corneal endothelial cell mosaic caused by a high osmolality glutaraldehyde-formaldehyde fixative compared to glutaraldehyde. Tissue Cell. 1997;29:533–547. doi: 10.1016/s0040-8166(97)80054-7. [DOI] [PubMed] [Google Scholar]
- 36.Schenke-Layland K., Vasilevski O., Opitz F. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Liao J., Joyce E.M., Sacks M.S. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syedain Z.H., Bradee A.R., Kren S., Taylor D.A., Tranquillo R.T. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A. 2013;19:759–769. doi: 10.1089/ten.tea.2012.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun W.Q., Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4:817–826. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Burton B., Gaspar A., Josey D., Tupy J., Grynpas M.D., Willett T.L. Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone. 2014;61:71–81. doi: 10.1016/j.bone.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 42.Nyman J.S., Ni Q., Nicolella D.P., Wang X. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008;42:193–199. doi: 10.1016/j.bone.2007.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ANSI/ISO . ISO; Geneva, Switzerland: 2005. ISO 5840:2005—Cardiovascular Implants—Cardiac Valve Prostheses. [Google Scholar]
- 44.ISO . ISO; Geneva, Switzerland: 1994. ISO 11134:1994—Sterilization of Health Care Products—Requirements for Validation and Routine Control—Industrial Moist Heat Sterilization. [Google Scholar]
- 45.ISO . ISO; Geneva, Switzerland: 1994. ISO 11135:1994/Cor 1:1994—Medical Devices—Validation and Routine Control of Ethylene Oxide Sterilization. [Google Scholar]
- 46.ISO . ISO; Geneva, Switzerland: 1995. ISO 11135:1995—Sterilization of Health Care Products—Requirements for Validation and Routine Control—Radiation Sterilization. [Google Scholar]
- 47.ISO . ISO; Geneva, Switzerland: 1996. ISO 14160:1998—Sterilization of Single Use Medical Devices Incorporating Materials and Animal Origin—Validation and Routine Control of Sterilization by Liquid Sterilants. [Google Scholar]
- 48.ISO . ISO; Geneva, Switzerland: 2000. ISO 14937:2000—Sterilization of Health Care Products—General Requirements for Characterization of a Sterilizing Agent and the Development, Validation, and Routine Control of a Sterilization Process for Medical Devices. [Google Scholar]