Abstract
Sentinel behaviour, a form of coordinated vigilance, occurs in a limited range of species, mostly in cooperative breeders. In some species sentinels confirm their presence vocally by giving a single sentinel call type, whereby the rate and subtle acoustic changes provide graded information on the variation of perceived predation risk. In contrast, meerkat (Suricata suricatta) sentinels produce six different sentinel call types. Here we show that manipulation of perception of danger has different effects on the likelihood of emitting these different call types, and that these call types affect foraging individuals differently. Increasing the perceived predation risk by playing back alarm calls decreased the production rate of the common short note calls and increased the production rate of the rare long calls. Playbacks of short note calls increased foraging behaviour and decreased vigilance in the rest of the group, whereas the opposite was observed when playing long calls. This suggests that the common call types act as all-clear signals, while the rare call types have a warning function. Therefore, meerkats increase the efficiency of their sentinel system by producing several discrete call types that represent changes in predation risk and lead to adjustments of the group’s vigilance behaviour.
Accurate risk assessment is important for every animal living in an environment with unpredictable levels of predation risk1,2. In group-living species, public information, information that is obtained by monitoring other group members’ interactions with their environment, plays an important role in the assessment of predation risk3,4. Many species use alarm calls to warn other group members about immediate threats, which typically elicit appropriate responses, interrupting their foraging and for example running for shelter5,6,7. Variation in alarm calls of a vast number of different species are known to contain information on the type of threat5,8, predator size9, distance10, location11,12, or the type and urgency of a flight response6. Due to the costs involved in these responses, information about changes in predation risk before an immediate response is required seems beneficial. It allows receivers experiencing different risk levels, e.g. due to the variation of how close an individual is to shelter, or their investment into pursuing a specific prey item, to adjust their vigilance and foraging behaviour accordingly13,14. A limited number of species, typically cooperative breeders, such as the dwarf mongoose15 (Helogale parvula), meerkats16, or the pied babblers17,18 (Turdoides bicolor) evolved a sentinel system, a form of coordinated vigilance behaviour, where one individual is on guard while the rest of the group is involved in other activities, mainly foraging13,19,20. In species, where dense vegetation or foraging strategies prevent the visual location of other group members, sentinels emit the Watchman’s song21, soft vocalisations of low amplitude, to announce their presence. All detailed studies of sentinel vocalisations found that sentinels give a single, potentially graded (with a continuous change along one or several acoustic parameters), type of sentinel call (reviewed in ref. 20), whereby the rate of these calls seems to provide information on the variation of the perceived predation risk14,22. Here we show that meerkat sentinels produce a variety of different call types, besides emitting alarm calls when predators approach16,23.
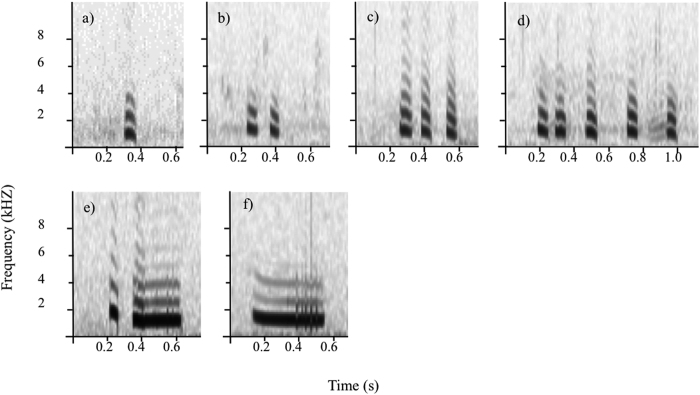
Meerkats are a small, highly cooperative mongoose species that occur in arid, semi-desert areas of southern Africa and live in groups from three to 50 individuals24,25. They are diurnal and spend most of their time foraging for invertebrates and small vertebrates24, often moving within a distance of 20–50 meters from the closest shelter26,27. During foraging, meerkats mainly dig for their prey in the sand, which prevents them from visually scanning their surrounding for predators, such as martial eagles (Polemaetus bellicosus), jackals (Canis mesomelas) and caracals (Felis caracal)6,28. These factors together, namely the open environment, the inability to scan it during foraging and the variety of predators, resulted not only in the evolution of functionally referential alarm calls, containing information about both the predator type and the urgency level6,29, but also in the evolution of sentinel behaviour to coordinate group vigilance and hence optimize the foraging-vigilance trade-off for the whole group23. Meerkats on guard produce at least six different call types of soft calls during the entire length of the guarding session (Fig. 1)23. Most of these calls, especially the short note calls are also given in other contexts, such as foraging, or sunning30. However, the functions of these different call types in the sentinel context and the ultimate reason for this diversity in sentinel call types to evolve are still unknown.
Figure 1.
Spectrograms of the six sentinel call types divided into: i) short note calls: (a) single note call, (b) double note call, (c) triple note call; ii) long calls: (d) multiple note call, (e) di-drrr call, and (f) wheek call.
We investigated the functions of the different meerkat sentinel call types in regards to public information about perceived predation risk. Based on previous work on meerkat sentinel vocalisations23, we grouped four of the six known call types into two different contextual categories. The most frequently produced “single-” and “double note” calls were grouped together into a ‘tonal short note calls’ category (hereafter short note calls) and the rare “di-drrr” and “wheek” calls into a ‘modulated long calls’ category (hereafter long calls) (Fig. 1), hypothesising that the former have a calming and the latter a warning function for the receivers of the signal. In order to understand the function of acoustic signals in animals, it is important to consider not only the contexts in which they are produced, but also the information receivers extract from these signals31,32. We tested whether changes in the perceived predation risk, induced by playing back meerkat alarm calls had an influence on call rate or call type emitted by the sentinel, similar to playback experiments performed with dwarf mongooses22. We expected sentinels to adjust their vocalisations according to the perceived predation risk in terms of decreasing the rate of short note calls while at the same time increasing the rate of long calls with an increase in perceived predation risk. We then investigated the receiver side by analysing how foraging test subjects responded to sentinel calls from the two different categories focusing on foraging and vigilance behaviour. We expected the two sentinel call categories to contain different information about the temporary perceived predation risk and hence to lead to a corresponding change in the vigilance behaviour of the foraging group, i.e. a decrease in vigilance after playbacks of short note calls and an increase in vigilance after the playbacks of long calls.
Results
Influence of changes in predation risk on sentinel vocalisations
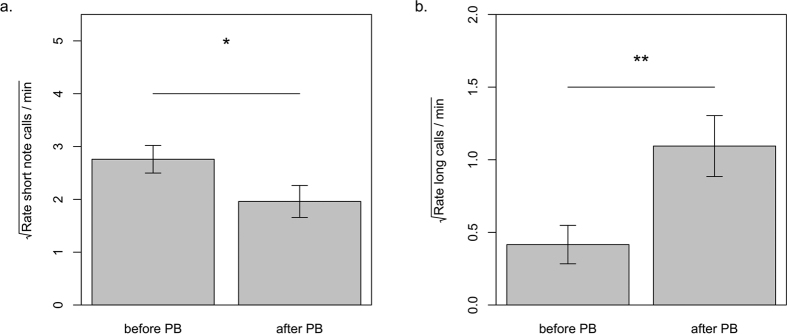
Playing back alarm calls to the sentinel in order to increase the perceived predation risk had different effects on the production rate of the different sentinel call types. Meerkat sentinels decreased the rate of short note calls (LRT; 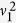 = 5.41, df = 1, p = 0.025; Fig. 2a) and at the same time increased the rate of long calls (LRT;
= 5.41, df = 1, p = 0.025; Fig. 2a) and at the same time increased the rate of long calls (LRT; 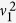 = 10.03, df = 1, p = 0.002, Fig. 2b) within 30 seconds after the playback of an alarm call compared to the time analysed before the playback. When the entire recording period of five minutes after the playback was taken into account, there was no effect of the alarm calls on the production rate of short note calls (LRT;
= 10.03, df = 1, p = 0.002, Fig. 2b) within 30 seconds after the playback of an alarm call compared to the time analysed before the playback. When the entire recording period of five minutes after the playback was taken into account, there was no effect of the alarm calls on the production rate of short note calls (LRT; 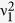 = 3.341, df = 1, p = 0.067) or long calls (LRT;
= 3.341, df = 1, p = 0.067) or long calls (LRT; 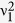 = 0.747, df = 1, p = 0.387).
= 0.747, df = 1, p = 0.387).
Figure 2. Influence of increased predation risk on production rates of the two categories of sentinel calls.
Rates of (a) short note calls and (b) long calls two minutes before and 30 seconds after the playback (PB) of an alarm call. Shown are the transformed values of the mean and SE used for the LMM with asterixes indicating significance levels (*p < 0.05; **p < 0.01).
Influence of different sentinel call categories on foraging group members
Testing the response of foraging subjects within 30 seconds after a playback showed that different playback conditions (i.e. short note calls, long calls, and the two control conditions: close calls and background noise), had significant overall impacts on all of the measured behaviours: foraging (LRT;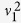 = 39.36, df = 2, p < 0.001), scanning (LRT;
= 39.36, df = 2, p < 0.001), scanning (LRT; 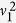 = 24.58, df = 2, p < 0.001) and bipedal scanning (LRT;
= 24.58, df = 2, p < 0.001) and bipedal scanning (LRT; 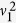 = 925.34, df = 2, p < 0.001). Behavioural responses did not differ significantly between the playbacks of close calls and background noise for any of the analysed behaviours (Table 1; Fig. 3), validating the use of close calls as control condition that not any soft call might have a calming or warning effect.
= 925.34, df = 2, p < 0.001). Behavioural responses did not differ significantly between the playbacks of close calls and background noise for any of the analysed behaviours (Table 1; Fig. 3), validating the use of close calls as control condition that not any soft call might have a calming or warning effect.
Table 1. LMM model outputs comparing the behavioural responses of foraging test subjects to the playbacks of the different call types (short note calls, close calls and long calls) to background noise (t-values).
| Behaviour | Playback comparison | t/z | df | P | |
|---|---|---|---|---|---|
| Foraging | Background | Close calls | 0.86 | 3362 | 0.39 |
| Background | Short notes | 1.14 | 3362 | 0.26 | |
| Background | Long calls | −5.19 | 3376 | <0.001*** | |
| Short notes | Close calls | −2.01 | — | 0.045* | |
| Short notes | Long calls | −6.35 | — | <0.001*** | |
| Close calls | Long calls | −4.34 | — | <0.001*** | |
| Quadrupedal scanning | Background | Close calls | 0.53 | 395 | 0.59 |
| Background | Short notes | −0.06 | 395 | 0.29 | |
| Background | Long calls | 4.05 | 397.2 | <0.001*** | |
| Short notes | Close calls | 1.55 | — | 0.123 | |
| Short notes | Long calls | 4.95 | — | <0.001*** | |
| Close calls | Long calls | 3.43 | — | <0.001*** | |
| Bipedal scanning | Background | Close calls | 0.47 | 394.7 | 0.47 |
| Background | Short notes | −1.11 | 394.7 | 0.27 | |
| Background | Long calls | 3.1 | 395.9 | <0.001*** | |
| Short notes | Close calls | 1.6 | — | 0.11 | |
| Short notes | Long calls | 4.86 | — | <0.001*** | |
| Close calls | Long calls | 3.29 | — | 0.001** | |
Multiple comparison post-hoc tests showing the remaining comparisons among call types (z-values).
Figure 3. Influence of different sentinel call and control categories on foraging and vigilance behaviour.
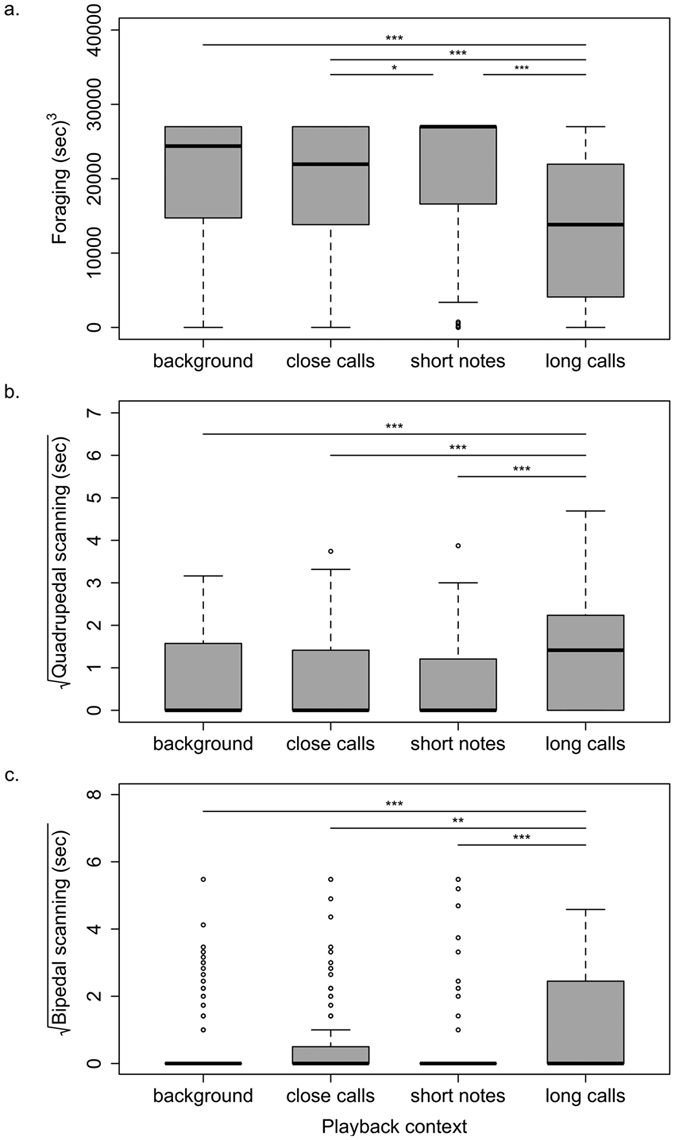
Time meerkats spent (a) foraging, (b) quadrupedal scanning and (c) on bipedal scanning during the different types of playbacks (calming calls, close calls and warning calls). Shown are the transformed values of the mean and SE used for the LMM with asterixes indicating significance levels (*p < 0.05; **p < 0.01; ***p < 0.001).
For the foraging behaviour, we found that meerkats spent more time foraging during the playback of short note calls compared to playbacks of close calls and long calls (Table 1; Fig. 3a). Considering close calls and long calls, meerkats spent more time foraging during the playback of close calls compared to long calls (Table 1; Fig. 3a). Focusing on vigilance behaviour, meerkats spent about the same amount of time scanning their surrounding quadrupedal during the playback of short note calls and close calls (Table 1; Fig. 3b). However, in the context of long calls being played back, meerkats showed a significant increase in quadrupedal scanning compared to both short note calls and close calls (Table 1; Fig. 3b). A similar pattern was found for the second type of vigilance we focused on, bipedal scanning, where meerkats spent about the same amount of time on bipedal scanning during the playback of short note and close calls (Table 1; Fig. 3c). But as soon as long calls were being played back, there was a significant increase in bipedal scanning compared to both short note calls and close calls (Table 1; Fig. 3c).
Discussion
Our study provides evidence that meerkat sentinels alter their vocalisations based on temporary changes in the perceived predation risk and that foraging group members use this information to adjust their own vigilance and foraging behaviour accordingly. In contrast to other species using only one, potentially graded, call type as Watchman’s song, meerkats seem to increase the efficiency of their sentinel system, by producing additional types of sentinel calls (i.e. long calls) that indicate an increase in perceived predation risk, but no need for an immediate flight response, which allows the rest of the group to continue foraging.
Production of different sentinel call categories depending on predation risk
Increasing the potential predation risk, by playing back alarm calls, affected the likelihood of the production of the two categories of sentinel call types in opposite directions. Sentinels decreased the rate of short note calls and at the same time increased the rate of long calls after hearing an alarm call. The decrease in the rate of short note calls provides evidence that these calls (“single-” and “double note” sentinel calls) act as all-clear signals, which have also been referred to as the Watchman’s song21, similar to what is known for sentinel calls of reed buntings33 (Emberiza schoeniclus), dwarf mongooses22 and pied babblers34. In these species, a higher call rate refers to low predation risk, while a decrease in call rate or the eventual absence of the calls, signals an increase in the temporal predation risk and leads to behavioural adjustment of the groups own vigilance behaviour13,14,22,35. This is in accordance with our findings that the playback of an alarm call, which was assumed to represent an increase in the perceived predation risk in the sentinel by simulating the presence of a predator, led to a decrease of this signal.
The increase in the rate of long calls after the playback of alarm calls provides evidence that “di-drrr” and “wheek” sentinel calls have a warning function and are mainly given after an increase in the perceived predation risk. Contrary to species using only one type of vocalisation as watchman’s song21,22,33,34, meerkat sentinels not only decrease in the rate of short note calls, but additionally emit long calls. Thereby, they specifically inform the group about an increase in the temporary predation risk, as well as the fact that there is still a sentinel on guard, which would give the appropriate alarm calls if a flight response of the rest of the group was needed.
The disadvantage of a sentinel system in which the decrease of the Watchman’s song, or even silence, in extreme cases35, signals an increase in predation risk is that this information could mistakenly be interpreted as the absence of a sentinel. Hence, the rest of the group has to respond in any case: either they have to react to the potential threat detected by the sentinel or they have to increase their vigilance as response to the finished guarding bout. However, foregoing foraging opportunities when there is no urgent threat has costs in terms of a reduced food intake, which would be even higher in harsh environments with food being rare or hard to extract. For meerkats, which live in a harsh environment, this problem seems to be solved by the presence of additional warning call types produced by sentinels. Therefore, this additional call category, which has not been described before in any other sentinel system, seems to further increase the efficiency of information transfer between the sentinel and its group in order to maximise food intake and hence optimize the trade-off between foraging and vigilance. At the same time this brings up the question why other species, such as pied babblers, a cooperative breeding bird living in similar environmental conditions do not show this differentiation in sentinel calls and what is the ultimate cause for the diversity in discrete sentinel calls in meerkats.
These effects of an increase in the perceived predation risk on the rate of short note and long calls, however, were only present within a short time period of 30 seconds after the playback, but not when taking the longer time period of five minutes into account. This can be explained by previous findings, which demonstrate that the time it takes for meerkats to relax after hearing alarm calls is on average no more than 60 seconds36. This reflects the need of a quick recovery after a predator incident in order to keep a positive balance towards acquiring food versus avoiding predation in an environment in which high fluctuations in predation risk are frequent.
Response of foraging group members to different sentinel call categories
Foraging test subjects responded very differently to the playbacks of the two different sentinel call categories. Playing back short note calls led to less vigilance behaviour and more time invested in foraging. This confirms the findings of a decrease of alertness in foraging group members when playing back sentinel calls, of which the short note calls are the most frequently emitted calls23. It supports our hypothesis that short note calls act as an all-clear signal and are therefore very similar to sentinel calls referred to as Watchman’s song in other species. Playing back long calls, on the other hand, led to a general increase in vigilance behaviour in foraging test subjects. This confirms that long calls function as pre-stage of alarm calls, leading to receivers being on average more vigilant, but also informing them that there is no need for an immediate flight response. This enables group members that have already invested energy by digging a hole to extract food to be able to continue foraging. These long calls hence further increase the efficiency of the sentinel system by having a call, which is given when the predation risk increases and therefore enables the group to adjust their own vigilance behaviour accordingly. At the same time, long calls imply that the sentinel is still on guard, which allows the group to continue foraging despite higher vigilance levels rather than all of them interrupting foraging, as they typically do when they hear an alarm call36. These findings provide further evidence that short note calls function as an all-clear signal, while long calls have an alerting function and that the receivers of the signal use this information to adjust their behaviour according to the temporary predation risk.
Conclusions
Our study provides evidence that meerkat sentinel calls convey public information about subtle changes in the current predation risk, which then leads to behavioural adjustment in the rest of the group. We suggest the use of the terms ‘calming calls’ and ‘warning calls’ for the two discrete and functionally different categories of sentinel calls. While meerkat sentinels optimize the trade-off between foraging and avoiding predation with multiple distinct call types, other species express variation in predation risk in changes of call rate only. This brings up the question under what circumstances discrete vocalisations are of advantage, and when graduation of a single call type by means of variation in call rate is sufficient for the same function. Further research needs to investigate how the remaining two sentinel calls (“triple-” and “multiple note” calls) fit into the context of calming and warning calls and, potentially, whether the call order of specific sentinel calls provide the group with valuable information about changes in the predation risk. This would shed light into how discrete and graded the meerkat sentinel calls are and how flexibly these vocalisations are used. Vocal flexibility and the importance of social learning in vocal communication in mammals is still a controversial topic37. However, our study is in accordance with the increasing evidence that animals are capable of voluntary control of the onset and offset of vocalisations, known as contextual learning37,38,39, in order to optimize the trade-off between foraging and antipredator behaviour.
Methods
Study site and species
The study was conducted between May and November 2014 at the Kuruman River Reserve in the southern Kalahari Desert, South Africa (for more information about habitat and climate at the study site see refs 28 and 40. As part of the Kalahari Meerkat Project’s long term data collection, all group members were uniquely dye marked to allow individual recognition, and one or two individuals of each group were fitted with radio-collars to facilitate localisation of the group (see ref. 41 for details of capture and collaring procedures). We conducted playback experiments in a total of nine groups with group size of eight to 24 individuals. All groups were habituated to close human observations and to the sound recording and playback equipment, allowing us to perform the recordings within a distance of 0.2–0.5 m from the calling meerkat.
Sound recordings
All recordings from sentinel vocalisations were done using a Sennheiser directional microphone (ME66/K6) connected to a Marantz PMD-670 solid-state recorder (Marantz Japan Inc.; sampling frequency 44.2 kHz, 16 bits accuracy). A Rainhardt microphone windshield (W200) was permanently attached to the microphone to ensure high quality ‘recordings in the meerkats’ natural environment. Whenever the sentinel was calling from a tree or any other position difficult to access, the microphone was fixed to a telescopic pole in order to maintain the recording distance of less than 0.5 meters and thereby maintaining a high signal-to-background ratio.
Playback experiments
To edit the sound files for the playback experiments, we used Cool Edit Pro (Syntrillium Software Corporation) to select single calls with a high signal-to-noise ratio. To play the selected calls back, we used a Marantz PMD-670 solid-state recorder connected to an iHome rechargeable mini speaker (iHM79SC). The amplitude was assessed according to how the calls occur under similar natural weather and wind conditions. Playback experiments were only conducted when no predator had been seen for at least five minutes and only if the group was foraging normally.
Experimental set-up and observations
Influence of alarm calls on sentinel vocalisations
To measure the influence of an increased level of predation risk on sentinel vocalisations, playback experiments of a wide range of alarm calls, i.e. low and high urgency alarm calls for aerial and terrestrial predators, were conducted. The 15 unique alarm calls chosen for the playbacks were recorded from different individuals from the same meerkat population during previous years, as it is known that meerkats do not differentiate between alarm calls of their own group compared to alarm calls from a foreign group42. Each playback consisted of one alarm call bout (5–30 alarm calls given shortly after each other) of three to ten seconds and each alarm call was used a maximum of three times and always in different groups. For each of our eight groups we played a total of 4 alarm call playbacks (aerial high, aerial low, terrestrial high and terrestrial low), resulting in a total sample size of 32 playbacks. To investigate the effect of alarm calls being played back to individuals on guard, we recorded sentinel vocalisations for two minutes prior to the playback and five minutes after the playback. The distance between the speaker and the sentinel on guard was at least seven meters while the distance between the sentinel and the microphone was 0.3–0.5 m. As alarm calls are given quite frequently under natural conditions (on average every 45 minutes)6, it was possible to conduct two alarm playbacks during the same foraging session over typically 3 hours, but with at least a 30 minutes break in between, without risking subjects habituating to the playback procedure.
Influence of different sentinel call categories on foraging group members
To investigate the information group members extract from the two different categories of sentinel vocalisations (short note and long calls) we compared the behavioural response of foraging group members to playbacks of these two call categories, focusing on two behaviours: foraging and vigilance. The playbacks were conducted on four adult individuals (>12 months) per group, resulting in a total sample size of 32 playbacks. One playback file was composed of five minutes of short note calls (“single note” and “double note” sentinel calls) and another five minutes of long calls (“di-drrr” and “wheek” sentinel calls) of the same individual. Before, between these two categories and afterwards five minutes of close calls (cc) from the same individual were played back. This resulted in playbacks of a length of 30 minutes each (e.g. cc-short note-cc-long calls-cc-background). Close calls (cc) are the most commonly emitted vocal signals used by the meerkats for group coordination while foraging43 and were used as control condition. At the beginning or end of each playback, another five minutes of background noise were added to control for the impact of any call being played back and hence to check the utility of close calls as control to demonstrate baseline behaviour. To avoid any order effects, the order of short note calls and long calls was alternated between the different playbacks. The rates of the close calls and short note sentinel calls were kept the same for the playbacks as calculated from the natural recordings with background noise between each call (close calls: 8.25 ± 2.28 calls/min; single note calls: 3.79 ± 0.43 calls/min; double note calls: 3.19 ± 0.37 calls/min). For the long calls context we always played a total of four calls, two “di-drrr” and two “wheek” calls in alternating order and with at least one minute of background noise in between, which also lies in the range of natural recordings (di-drr: 0.34 ± 0.12 calls/min; wheek calls: 0.39 ± 0.09 calls/min). Each of the playbacks consisted of calls from at least six different recordings from the same individual (n = 8) recorded during three weeks prior to the start of the playbacks. Each group was subjected to playback calls from at least three different individuals.
Playback experiments were only conducted when no group member was on sentinel guard, to prevent any interference with other sentinel calls, and only when the group was foraging (i.e. more than 50% of the group members were foraging). If any of the conditions, including the absence of predators, were violated after the playback had been started, the playback was paused and resumed only after the majority of the group was back to normal foraging behaviour for a minimum of five minutes or the sentinel finished its guarding session. The speaker was kept at a height of 0.3–0.4 m as this has been shown to be a frequent height chosen by natural sentinels44 and also represented a good compromise to play back sentinel calls as well as close calls from the same height. The distance to the foraging test subject was no less than one meter but no more than two meters throughout the whole playback. In the case when a second playback was conducted in the same session, the second playback file differed in order of the five-minute playbacks and the calling individual. To analyse the behaviour of the test subject, behavioural focals were performed simultaneously to the playback of the different call types using a handheld Palm TX Tungsten (Palm Inc, 2005) with the focal program written in Cybertracker (Cybertracker Conservation 2013 version 3.376).
Analysis of sound recordings and behavioural focals
Sound recordings of naturally occurring sentinel sessions with a minimum duration of one minute were analysed using Cool Edit Pro (Syntrillium Software Corporation). In line with other studies on call combinations30,45, for a call to be classified as a call combination (i.e. “double note”, “triple note” or “multiple note”) the silence interval within the call combination had to be shorter than the silence interval to the previous and the following call. Work on meerkat call combination showed that the silence interval between two calls is almost 20 times longer than the silence interval within call combinations30, enabling call categorization of each call, by visual and audio inspection, as one of the six already described sentinel calls23 (Fig. 1). To quantify the immediate and short-term effect of alarm calls being played back, we analysed the call rates of the two different sentinel categories during two minutes prior to the playback of an alarm call and 30 seconds as well as five minutes afterwards separately.
For the analysis of the behavioural focals conducted during the sentinel playbacks, we focused on the behaviours recorded during 30 seconds after the occurrence of a call, rather than analysing the whole five minutes of the focal. Furthermore, because call rates of the different sentinel playback contexts (i.e. short note sentinel calls, long sentinel calls and close calls) differed according to their natural frequencies, we randomly selected four calls of every playback context to be analysed by using the sample function in R. As foraging behaviour we pooled all the different food-related behaviours, including foraging (digging in a hole for prey), scrabbling (scratching at multiple small holes or surface while steadily moving), processing (processing food items in sand, or chewing off tail of scorpions, etc.) and eating. Regarding the alert-related behaviours, we focused on two types of vigilance behaviour: quadrupedal scanning of surroundings, which is common and usually of short duration and bipedal scanning of surroundings, which is less frequent and of longer duration.
Statistical analysis
All statistical analyses were done using R, release GUI 2.1 (R Development Core Team 2015). For all analyses linear mixed models46 were used in order to determine the relationship between call rate of sentinels or length of behaviours and the parameters of interest. To analyse changes in call rate of the sentinel after the playback of alarm calls, we used the rate of short note and long calls respectively as response variables and the relative timing of the playback (i.e. before and after the playback) as fixed effect. For the second part, when we analysed the behavioural responses of foraging group members to different calls, duration of each of the three focal behaviours (foraging, quadrupedal vigilance and bipedal vigilance) were used in three separate models and playback context (short note calls, long calls, close calls and background noise) was used as fixed effect. All the models contain individual identity nested in group identity as a random effect to control for differences in call rate between different individuals as well as between different groups. To determine whether alarm calls or the playback context had a significant effect on the response variable, likelihood ratio tests (LRT, v) were used to compare whether the model with the fixed effect included differs significantly from the same model with the fixed effect excluded47. To determine the fit of the linear mixed models we examined the model diagnostic plots and predictor variables were transformed where assumptions of the models were not met. Whenever the explanatory variable consisted of more than two categories multiple comparison test with manually set contrasts (multcomp function in R) were used to compare the different categories not specified by the intercept48.
Ethical note
All research was conducted under the permission of the ethical committee of Pretoria University and the Northern Cape Conservation Service, South Africa (Permit number: EC011-10). All methods were carried out adhering to the approved guidelines in this permit.
Additional Information
How to cite this article: Rauber, R. and Manser, M. B. Discrete call types referring to predation risk enhance the efficiency of the meerkat sentinel system. Sci. Rep. 7, 44436; doi: 10.1038/srep44436 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank the Kalahari Research Trust and Tim Clutton-Brock for the permission to work at the Kuruman River Reserve, as well as the Northern Cape Conservation Authority for research permission (FAUNA 1020/2016). We also thank Dave Gaynor for organising the field site and managers and volunteers of the Kalahari Meerkat Project (KMP) for maintaining habituation and basic data collection on the meerkats and helping out with the performance of the playback experiments. We thank Tim Clutton-Brock, Sabrina Engesser, Katie Collier, Jack Thorley and Patricia Lopes for comments on the manuscript and Jamie Samson for statistical advice. RR was self-funded and research expenses as well as MM were funded by the University of Zurich and the Claraz Foundaton. The long-term field site KMP was financed by University of Cambridge and Zurich. This paper has relied on records of individual identities and/or life histories maintained by the KMP, which has been supported by the European Research Council (Grant No 294494 to T.H. Clutton-Brock since 1/7/2012), the University of Zurich and the Mammal Research Institute of the University of Pretoria.
Footnotes
The authors declare no competing financial interests.
Author Contributions R.R. and M.M. were equally involved in planning the experiments and writing the manuscript, while R.R. collected and analysed the data.
References
- Lima S. L. & Dill L. M. Behavioral decisions made under the risk of predation - a review and prospectus. Canadian Journal of Zoology-Revue Canadienne De Zoologie 68, 619–640, doi: 10.1139/z90-092 (1990). [DOI] [Google Scholar]
- Stankowich T. & Blumstein D. T. Fear in animals: a meta-analysis and review of risk assessment. Proceedings of the Royal Society B-Biological Sciences 272, 2627–2634, doi: 10.1098/rspb.2005.3251 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E., Giraldeau L. A., Valone T. J. & Wagner R. H. Public information: From nosy neighbors to cultural evolution. Science 305, 487–491, doi: 10.1126/science.1098254 (2004). [DOI] [PubMed] [Google Scholar]
- Krause J. & Ruxton G. D. Living in groups. Living in groups, i (2002).
- Seyfarth R. M., Cheney D. L. & Marler P. Monkey responses to 3 different alarm calls - evidence of predator classification and semantic communication. Science 210, 801–803, doi: 10.1126/science.7433999 (1980). [DOI] [PubMed] [Google Scholar]
- Manser M. B. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proceedings of the Royal Society B-Biological Sciences 268, 2315–2324, doi: 10.1098/rspb.2001.1773 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbuhler K. Vol. 40 Advances in the Study of Behavior (eds Naguib M., Zuberbuhler K., Clayton N. S. & Janik V. M.) 277–322 (Elsevier Academic Press Inc, 2009). [Google Scholar]
- Zuberbuhler K., Noe R. & Seyfarth R. M. Diana monkey long-distance calls: Messages for conspecifics and predators. Animal Behaviour 53, 589–604, doi: 10.1006/anbe.1996.0334 (1997). [DOI] [Google Scholar]
- Templeton C. N., Greene E. & Davis K. Allometry of alarm calls: Black-capped chickadees encode information about predator size. Science 308, 1934–1937, doi: 10.1126/science.1108841 (2005). [DOI] [PubMed] [Google Scholar]
- Owings D. H. & Virginia R. A. Alarm Calls Of California Ground Squirrels (Spermophilus-Beecheyi). Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology 46, 58–70 (1978). [Google Scholar]
- Evans C. S., Evans L. & Marler P. On The Meaning Of Alarm Calls - Functional Reference In An Avian Vocal System. Animal Behaviour 46, 23–38, doi: 10.1006/anbe.1993.1158 (1993). [DOI] [Google Scholar]
- Casar C., Zuberbuhler K., Young R. J. & Byrne R. W. Titi monkey call sequences vary with predator location and type. Biology Letters 9, doi: 10.1098/rsbl.2013.0535 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S. L. & Bednekoff P. A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. American Naturalist 153, 649–659, doi: 10.1086/303202 (1999). [DOI] [PubMed] [Google Scholar]
- Bell M. B. V., Radford A. N., Rose R., Wade H. M. & Ridley A. R. The value of constant surveillance in a risky environment. Proceedings of the Royal Society B-Biological Sciences 276, 2997–3005, doi: 10.1098/rspb.2009.0276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasa O. A. E. Coordinated vigilance in dwarf mongoose family groups - the watchmans song hypothesis and the costs of guarding. Ethology 71, 340–344 (1986). [Google Scholar]
- Clutton-Brock T. H. et al. Selfish sentinels in cooperative mammals. Science 284, 1640–1644, doi: 10.1126/science.284.5420.1640 (1999). [DOI] [PubMed] [Google Scholar]
- Ridley A. R., Raihani N. J. & Bell M. B. V. Experimental evidence that sentinel behaviour is affected by risk. Biology Letters 6, 445–448, doi: 10.1098/rsbl.2010.0023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollen L. I., Bell M. B. V. & Radford A. N. Cooperative sentinel calling? Foragers gain increased biomass intake. Current Biology 18, 576–579, doi: 10.1016/j.cub.2008.02.078 (2008). [DOI] [PubMed] [Google Scholar]
- Bednekoff P. A. Mutualism among safe, selfish sentinels: A dynamic game. American Naturalist 150, 373–392, doi: 10.1086/286070 (1997). [DOI] [PubMed] [Google Scholar]
- Bednekoff P. A. In Advances in the Study of Behavior, Vol 47 Vol. 47 Advances in the Study of Behavior (eds Naguib M. et al.) 115–145 (Elsevier Academic Press Inc, 2015). [Google Scholar]
- Wickler W. Coordination of vigilance in bird groups - the watchmans song hypothesis. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology 69, 250–253 (1985). [Google Scholar]
- Kern J. M. & Radford A. N. Call of duty? Variation in use of the watchman’s song by sentinel dwarf mongooses, Helogale parvula. Animal Behaviour 85, 967–975, doi: 10.1016/j.anbehav.2013.02.020 (2013). [DOI] [Google Scholar]
- Manser M. B. Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proceedings of the Royal Society B-Biological Sciences 266, 1013–1019, doi: 10.1098/rspb.1999.0737 (1999). [DOI] [Google Scholar]
- Doolan S. P. & Macdonald D. W. Diet and foraging behaviour of group-living meerkats, Suricata suricatta, in the southern Kalahari. Journal of Zoology 239, 697–716 (1996). [Google Scholar]
- Clutton-Brock T. H. et al. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068, doi: 10.1038/nature05386 (2006). [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H. et al. Costs of cooperative behaviour in suricates (Suricata suricatta). Proceedings of the Royal Society B-Biological Sciences 265, 185–190 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser M. B. & Bell M. B. Spatial representation of shelter locations in meerkats, Suricata suricatta. Animal Behaviour 68, 151–157, doi: 10.1016/j.anbehav.2003.10.017 (2004). [DOI] [Google Scholar]
- Clutton-Brock T. H. et al. Predation, group size and mortality in a cooperative mongoose, Suricata suricatta. Journal of Animal Ecology 68, 672–683, doi: 10.1046/j.1365-2656.1999.00317.x (1999). [DOI] [Google Scholar]
- Furrer R. D. & Manser M. B. The Evolution of Urgency-Based and Functionally Referential Alarm Calls in Ground-Dwelling Species. American Naturalist 173, 400–410, doi: 10.1086/596541 (2009). [DOI] [PubMed] [Google Scholar]
- Collier K., Townsend S. W. & Manser M. B. Call concatenation in wild meerkats. Animal Behvaiour online, doi: 10.1016/j.anbehav.2016.12.014 (2017). [Google Scholar]
- Marler P. et al. Animal signals: motivational, referential, or both? Nonverbal vocal communication: comparative and developmental approaches., 66–86 (1992). [Google Scholar]
- Macedonia J. M. & Evans C. S. Variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93, 177–197 (1993). [Google Scholar]
- Wingelmaier K., Winkler H. & Nemeth E. Reed bunting (Emberiza schoeniclus) males sing an ‘all-clear’ signal to their incubating females. Behaviour 144, 195–206, doi: 10.1163/156853907779947319 (2007). [DOI] [Google Scholar]
- Hollen L. I. et al. Calling by Concluding Sentinels: Coordinating Cooperation or Revealing Risk? Plos One 6, doi: 10.1371/journal.pone.0025010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Bird calls: a cornucopia for communication. Nature’s music: the science of birdsong 132–177, doi: 10.1016/b978-012473070-0/50008-6 (2004). [DOI] [Google Scholar]
- Manser M. B., Bell M. B. & Fletcher L. B. The information that receivers extract from alarm calls in suricates. Proceedings of the Royal Society B-Biological Sciences 268, 2485–2491, doi: 10.1098/rspb.2001.1772 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik V. M. & Slater P. J. B. The different roles of social learning in vocal communication. Animal Behaviour 60, 1–11, doi: 10.1006/anbe.2000.1410 (2000). [DOI] [PubMed] [Google Scholar]
- Radford A. N. & Ridley A. R. Individuals in foraging groups may use vocal cues when assessing their need for anti-predator vigilance. Biology Letters 3, 249–252, doi: 10.1098/rsbl.2007.0110 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. W., Rasmussen M., Clutton-Brock T. & Manser M. B. Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behavioral Ecology 23, 1360–1364, doi: 10.1093/beheco/ars129 (2012). [DOI] [Google Scholar]
- Russell A. F. et al. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. Journal of Animal Ecology 71, 700–709, doi: 10.1046/j.1365-2656.2002.00636.x (2002). [DOI] [Google Scholar]
- Jordan N. R., Cherry M. I. & Manser M. B. Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Animal Behaviour 73, 613–622, doi: 10.1016/j.anbehav.2006.06.010 (2007). [DOI] [Google Scholar]
- Schibler F. & Manser M. B. The irrelevance of individual discrimination in meerkat alarm calls. Animal Behaviour 74, 1259–1268, doi: 10.1016/j.anbehav.2007.02.026 (2007). [DOI] [Google Scholar]
- Engesser S. Function of ‘close’ calls in a group foraging carnivore, Suricata suricatta. MSc thesis, University of Zurich, Zurich, Switzerland (2011).
- Tatalovic M. First quantitative description of sentinel posts in wild meerkats. Natura Croatica 21, 493–496 (2012). [Google Scholar]
- Crockford C. & Boesch C. Call combinations in wild chimpanzees. Behaviour 142, 397–421, doi: 10.1163/1568539054012047 (2005). [DOI] [Google Scholar]
- Bates D M. M., Bolker B. & Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7 (2014).
- Crawley M. J. The R Book. Jon Wiley & Sons Ltd., Chichester (2007). [Google Scholar]
- Hothorn T., , Westfall P. & Heiberger R. M. multcomp: Simultaneous inference in general parametric models (2008). [DOI] [PubMed]