ABSTRACT
Lysyl oxidase (LOX) catalyzes the oxidative deamination of lysine residues in collagen and elastin, key components of connective tissue. LOX is synthesized as an inactive 50 kD pre-proenzyme, and secreted to the extracellular matrix where it is cleaved into an active 32 kD LOX, and an 18kD free propeptide (LOX-PP), purportedly an inhibitor of fibroblast growth factor-2 (FGF-2) signaling. Given that adipocytes are distributed inside the connective tissue, it is likely that LOX-PP has an important regulatory role in adipogenesis, which has not been studied. Using NIH 3T3-L1 cells, we observed that FGF-2 inhibited adipogenesis, and LOX-PP promoted adipogenesis of 3T3-L1 cells in the presence of FGF-2; the expression of peroxisome proliferator-activated receptor (PPAR) γ and CCAAT-enhancer binding protein (C/EBP) α, two markers of adipogenesis, were enhanced in the presence of LOX-PP. We further observed that LOX-PP down-regulated AKT and ERK1/2, two proliferative signaling proteins down-stream of FGF-2 signaling. Similarly, inhibition of FGF-2 receptor signaling by canofin, a competitive inhibitor of FGF-2 receptor, promoted adipogenesis albeit less effective compared to LOX-PP. To further explore whether LOX-PP promoted adipogenesis through inhibition of FGF-2 signaling, site directed mutagenesis of LOX-PP, resulting in an Arg158 to Gln158 mutation which abolishes the inhibitory activity of LOX-PP to FGF-2 receptor, attenuated the adipogenic promoting properties of LOX-PP. In summary, for the first time, our data show that LOX-PP enhances adipogenesis at least partially through inhibition of FGF-2 receptor signaling. Our data suggest that LOX-PP may serve as a bona fide therapeutic target for regulating adipogenesis and adipose tissue development.
KEYWORDS: adipogenesis, FGF-2, lysyl oxidase, proliferation, propeptide, rs1800449
Introduction
Obesity is a serious health problem that results from both hypertrophy (increase in size) and hyperplasia (increase in number) of adipocytes leading to more serious diseases such as type II diabetes, coronary heart disease, and hypertension.1 By 2050 it is speculated that 58% of Americans may be obese.2 Considering the current obesity epidemic, it is critical to define mechanisms governing adipogenesis. Adipocytes are imbedded inside connective tissues. As a result, a local niche environment of connective tissue likely to have critical regulatory roles in adipogenic differentiation.3 Lysyl Oxidase (LOX) is a copper dependent enzyme that is secreted as a N-glycosylated, inactive pre-protein and then cleaved into a functional 32 kD enzyme (LOX) and an 18 kD propeptide (LOX-PP).4 LOX catalyzes the cross-linking of lysine residues in collagen and elastin, strengthening the extracellular matrix.5 In NIH 3T3-L1 fibroblasts, LOX inhibited the tumorigenic phenotype of ras-transformed cells and was thus named the ras-recision gene.6 Recently, the tumor inhibiting properties of LOX was mapped to the 162 amino acid LOX-PP domain where it inhibited ras-dependent transformation in NIH 3T3-L1 cells.7 Later, it was found that LOX-PP inhibits ERK1/2 signaling and subsequent proliferation of osteoblasts by blocking FGF-2 autocrine signaling.8 Also, LOX-PP inhibits FGF-2 induced ERK1/2 and AKT signaling pathways in DU 145 prostate cancer cells.9 These data suggest that LOX-PP inhibits FGF-2 signaling.
As a critical growth factor, FGF-2 promotes cell survival and proliferation.10 The effect of FGF-2 on adipogenesis has been controversial, though most data point towards FGF-2 as having an inhibitory effect on adipogenesis.11-13 Because LOX-PP inhibits FGF-2 signaling, we hypothesized that LOX-PP promotes adipogenic differentiation via inhibition of FGF-2 signaling. Here we report that LOX-PP up-regulates adipogenesis, at least partially through inhibition of FGF-2 signaling in differentiating pre-adipocytes; a point mutation of LOX-PP (Arg 152 to Glu 152), which is required to inhibit FGF-2 receptor, abolished the enhancing effect of LOX-PP on adipogenesis.
Methods
Cell culture
The NIH 3T3-L1 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotics at 37°C. Two days after reaching confluency, adipogenic differentiation was induced using a standard cocktail (1 µg/ml insulin, 0.1 µg/ml dexamethazone, 27.8 µg/ml isobutylmethylaxanthine and 10 µM troglitazone) as previously described.14
Cloning LOX-PP and site directed mutagenesis of Arg 152 to Glu 152
Total RNA was isolated from mature 3T3-L1 cells using the Trizol method and reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA). The DNA sequence encoding LOX-PP was amplified using forward primer: CCCAAGCTTTCAATACGGTGAAATTGTGCAGCCTGAGGCATA, and reverse primer: CCCAAGCTTGCCCACCATGCGATCTATGTG. Then, the amplicon was cloned into pCMV vector following digestion with BglII and Hind III (New England Biolabs, Ipswich, MA) resulting in a vector construct named PP-WT.
Site directed mutagenesis was performed using the PrimeSTAR® HS DNA Polymerase kit (Mountain View, CA) with forward primer: CGCAGCTCAGTAATCTGCAGCCACCCAGCCACATA, and reverse primer: TATGTGGCTGGGTGGCTGCAGATTACTGAGCTGCG, which resulted in the substitution of Arg 152 to Gln 152, and the resulting vector construct named PP-PM.
Transfection of 3T3-L1 cells with PP-WT, PP-PM and the empty pCMV vector (V) was conducted using the Turbofect transfection reagent according to the manual instruction (Fermentas, Glen Burnie, MD).
Preparation of recombinant LOX-PP
LOX-PP cDNA was amplified with a forward primer: CCCAAGCTTGCCCCGCAGACCCCGC, and a reverse primer: GGAATTCGCCCACCATGCGATCTATGTGGC.The amplicon was cloned into pFLAG bacterial expression vector. The pFlag-LOX-PP vector was transfected into E. coli and 500 µM IPTG was used to induce expression. To purify LOX-PP, cells were lysed and the supernatant was collected. The recombinant LOX-PP containing Flag was purified using anti-pFLAG resin (Sigma Aldrich, St. Louis, MO). The purity of prepared LOX-PP was confirmed by Western blot analysis using anti-FLAG antibody, where a single 18 kD band was observed.
Immunoblotting analysis
Antibodies against β-tubulin and PPARγ were purchased from Cell Signaling (Danvers, MA). IRDye 800CW goat anti-rabbit secondary antibody and IRDye 680 goat anti-mouse secondary antibody were purchased from Li-COR Biosciences (Lincoln, NE).
Proteins were separated by electrophoresis and transferred onto nitrocellulose membrane which was blotted with anti-PPARγ and anti-β-tubulin antibodies at 1:1000 dilution in 1:1 PBST/Odyssey blocking buffer. The image was visualized on Odyssey infrared imaging system. Band intensity was normalized according to β-tubulin content.15
Oil-Red O staining
3T3-L1 cells were seeded at 100% confluency, and purified PP was administered at 0, 1, 2, 4, or 8 µg/ml in the presence of 5 ng/ml FGF-2 and standard adipogenic cocktail as previously described.16 After 14 days of differentiation, cells were stained with Oil-Red O following the standard procedure.16 Briefly, cells were fixed in 4% paraformaldehyde for 10 min at room temperature, rinsed with 60% ethanol and stained with 0.2% (w/v) Oil-Red O in 60% ethanol (Sigma Aldrich) as described previously.17 Then, cells were destained with 60% ethanol for 5 min and used for microscopic observation. In addition, stained Oil-Red O was solubilized by 100% ethanol and used for spectrometry analysis of absorbance at 520 nm.
Cell proliferation
Cell proliferation was measured using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method according to the manual instruction of the kit (Invitrogen, Grand Island, NY). The absorbance was measured at 570 nm on the Synergy H1 hybrid Reader (BioTek, Winooski, VT).
Statistical analysis
Data were analyzed as a complete randomized design using GLM (General Linear Model of Statistical Analysis System, SAS, 2000). The differences in the mean values were compared by the Tukey's multiple comparison, and mean ± standard errors of mean (SEM) were reported. Statistical significance was considered as P < 0.05.
Results
LOX-PP abolishes the inhibitory effects of FGF-2 on adipogenesis
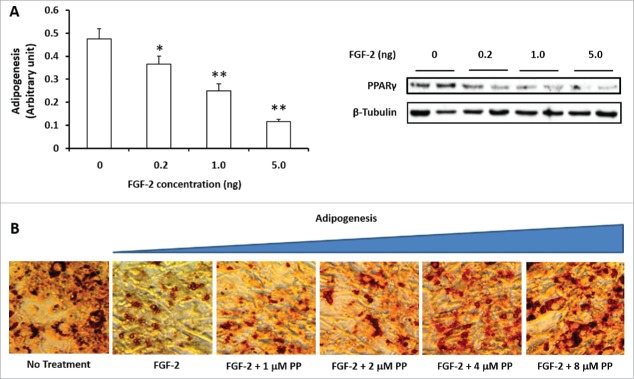
FGF-2 (0–5 ng/ml) was added to 3T3-L1 cells together with the standard adipogenic cocktail to induce adipogenesis. FGF-2 at 1 and 5 ng/ml clearly reduced the expression of PPARγ, and the 5 ng/ml dose was used for the subsequent experiments (Fig. 1A).
Figure 1.
Lysyl oxidase propeptide (LOX-PP) abolished the inhibitory effect of FGF-2 on adipogenesis in 3T3-L1 cells. (A). FGF-2 dose dependently inhibited adipogenesis of 3T3-L1 cells. FGF-2 was added to confluent 3T3-L1 cells at concentrations between 1 to 5 ng/ml in the presence of a adipogenic cocktail; (B). LOX-PP dose dependently abolished the inhibitory effects of FGF-2 (5 ng/ml) on adipogenesis. (Means ± SEM; *P < 0.05; **P < 0.01)
To test the role of LOX-PP in adipogenesis, we cloned and purified LOX-PP. Recombinant LOX-PP was used to treat 3T3-L1 cells, and the presence of lipid droplets in differentiated 3T3-L1 cells were analyzed by Oil-Red O staining. The 3T3-L1 cells were treated with different doses of purified LOX-PP in the presence of 5 ng/ml FGF-2. Purified LOX-PP dose dependently increased the lipid content of differentiated 3T3-L1 cells, with 4 to 8 μM PP completely abolished the inhibitory effect of FGF-2 on adipogenesis (Fig. 1B).
LOX-PP blocks FGF-2 signaling which promotes adipogenesis
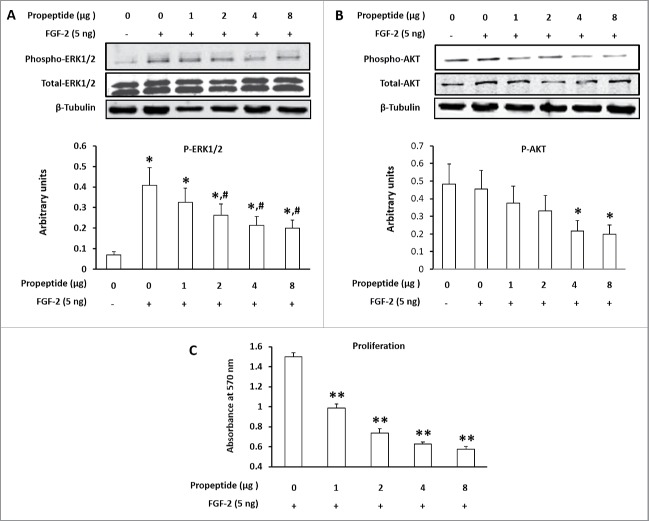
Purified LOX-PP (0–8 µg/ml) together with 5 ng/ml FGF-2 was added to confluent 3T3-L1 cells. ERK1/2 and AKT are crucial signaling mediators downstream of FGF-2 signaling. Indeed, FGF-2 induced phosphorylation of ERK1/2 and AKT, and the addition of purified LOX-PP dose dependently down-regulated AKT and ERK1/2 phosphorylation (Fig. 2A). Consistently, the proliferation of 3T3-L1 cells was reduced by LOX-PP (Fig. 2B).
Figure 2.
Lysyl oxidase propeptide (LOX-PP) inhibited phosphorylation of ERK1/2 and AKT, two key mediators of FGF-2 receptor signaling. (A) LOX-PP dose dependently inhibited ERK1/2 phosphorylation; (B) LOX-PP dose dependently inhibited AKT phosphorylation; (C) LOX-PP inhibited the prolifreative response of 3T3-L1 cells induced by FGF-2 treatment. (Means ± SEM; *vs. cells without FGF-2 and LOX-PP treatments, P < 0.05; #vs. cells with 5 ng/ml FGF-2 without LOX-PP, P < 0.05).
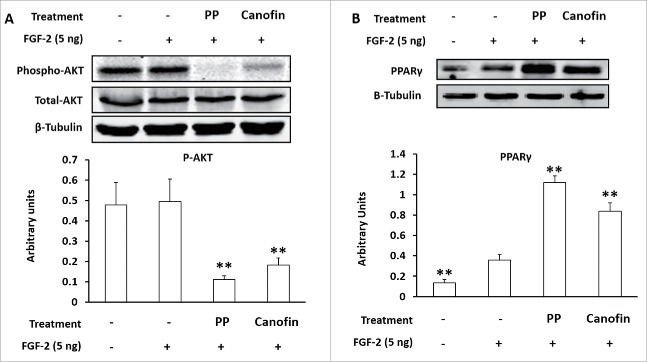
To further test the role of FGF-2 receptor in mediating the effect of LOX-PP on cell proliferation, we used a FGF-2 antagonist, canofin. Canofin is a peptide, which competitively inhibits FGF-2 receptor signaling in neurons.18 Confluent 3T3-L1 cells were treated with 8 µg LOX-PP or 2.3 µM canofin in the presence of 5 ng/ ml FGF-2.19 Both LOX-PP and canofin blocked FGF-2 signaling as determined by reduction in AKT phosphorylation, with LOX-PP more potent than canofin (Fig. 3A). Consistently, both LOX-PP and canofin enhanced PPARγ expression and adipogenesis (Fig. 3B)
Figure 3.
Canofin and Lysyl oxidase propeptide (LOX-PP) similarly inhibited AKT phosphorylation and promoted PPARγ expression. (A) LOX-PP and canofin, a peptide competitive inhibitor of FGF-2 receptor, inhibited AKT signaling; (B) LOX-PP and canofin abolished the inhibitory effects of FGF-2 on PPARγ expression. (Means ± SEM; **P < 0.01)
Nulling the inhibition of LOX-PP on FGF-2 signaling abolishes its promontory effect on adipogenesis
A naturally occurring polymorphism (Arg 152 to Glu 152) in LOX-PP promotes breast cancer development, and this polymorphism was later found to abolish its inhibitory effect of LOX-PP on FGF-2 receptor and its downstream signaling.19 To further evaluate whether LOX-PP promotes adipogenesis through inhibition of FGF-2 signaling, we cloned LOX-PP into a mammalian expression vector, and induced a point mutation (Arg 152 to Glu 152) to abolish the inhibitory function of LOX-PP on FGF-2 receptor. 3T3-L1 cells were transfected with PP-WT, PP-PM, and empty pCMV Vector (V), and cells were allowed to differentiate for 3 days. Although transfection of the pCMV vector alone dramatically reduced PPARγ and C/EBPα expression, transfection of LOX-PP restored their signals, while transfected with PP-PM did not (Fig. 4), demonstrating the necessity of FGF-2 signaling in promotion of adipogenesis by LOX-PP.
Figure 4.
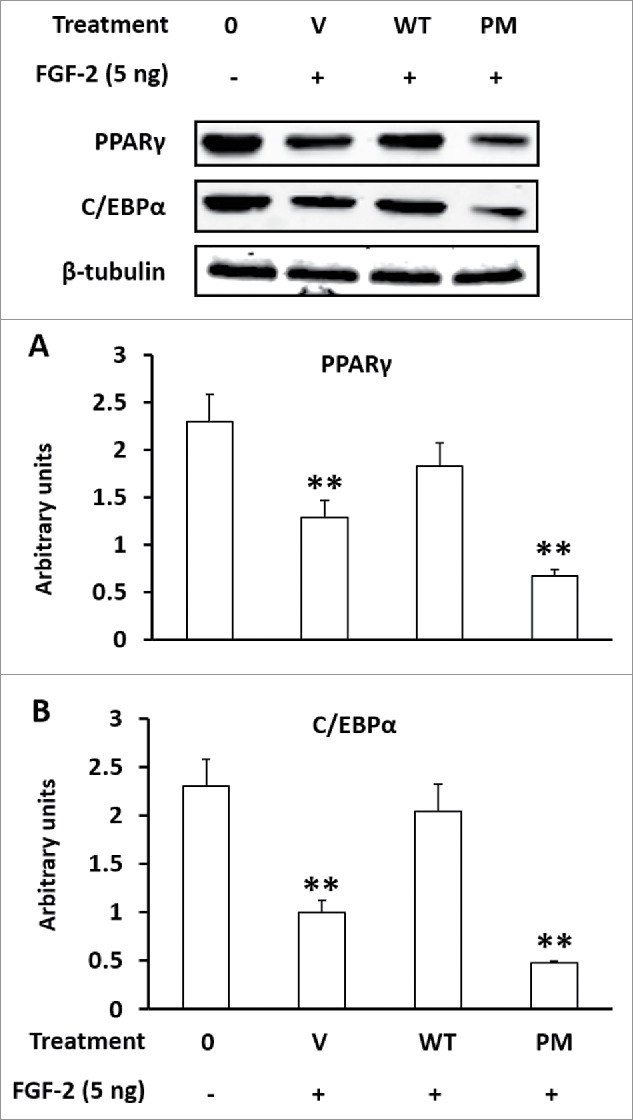
Point mutation of lysyl oxidase propeptide (LOX-PP) abolished its promotion effects on adipogenesis. (A) Over-expression of wild-type (WT) or mutated (Arg 152 to Glu) LOX-PP on PPARγ expression; (B) Over-expression of wild-type (WT) or mutated (PM, Arg 152 to Glu) LOX-PP on PPARγ expression. 3T3-L1 cells were transfected with control pCMV vector (V), WT vector, or PM vector with or without 5 ng/ml FGF-2. (Means ± SEM; **P < 0.01)
Discussion
FGF2 promotes cell proliferation and inhibits adipogenesis
FGF2 is one of the major molecular triggers for cell proliferation, and appears to mainly target cells with proliferation capacity including stem cells, fibroblasts and adipogenic progenitor cells.20,21 FGF2 is critical for stimulating embryonic development when undifferentiated stem cells and progenitor cells are abundant.20,21 During the postnatal stage, however, FGF2 are critical for wound healing and angiogenesis, where extensive proliferation of fibroblasts and progenitor cells are needed.22,23 FGF2 stimulates cell proliferation through enhancing both ERK1/2 and AKT signaling pathways, which are critical for cell proliferation and protein synthesis respectively.24
Despite extensive studies about the role of FGF2 in cell proliferation, its role in cell differentiation has been poorly explored. We speculated that FGF2 inhibits adipogenesis. Indeed, we observed that FGF2 dose dependently inhibits adipogenesis, which is consistent with an earlier report where FGF2 inhibited adipogenic differentiation and phenotypic maintenance in TA1 cells.25
LOX-PP inhibits FGF2 signaling and promotes adipogenesis
To date, studies with LOX-PP have been focused on its tumor suppressing properties and anti-proliferative effects. Initially, the anti-proliferative effects were contributed to LOX, not the LOX-PP, because LOX oxidizes FGF-2 receptor to inhibit downstream signaling pathways stimulating cell proliferation.26 Then, the tumor inhibiting properties of LOX was mapped to the LOX-PP domain which inhibited ras-dependent transformation in NIH 3T3-L1 cells.7 LOX-PP reduces tumor growth in both pancreatic and lung cancers through inhibition of ERK1/2 and AKT, down-stream effectors of FGF-2 signaling,27 and was further shown to inhibit smooth muscle cell proliferation by reducing ERK1/2 signaling.28 In cancer cell lines, accumulating evidence show that the anti-proliferative effects of LOX-PP is via blocking FGF-2 signaling at the level of the receptor.8,9 Although it is known that LOX-PP inhibits FGF-2 receptor signaling, the physiological effects of this inhibition, beyond proliferation remains unexplored.
Here we show that LOX-PP inhibits ERK1/2 and AKT activation by inhibiting FGF-2 receptor signaling in developing adipocytes. Our data are further supported with a competitive inhibitor of FGF2 receptor, canofin. We have shown that LOX-PP is a potent inhibitor of FGF-2 receptor similar with canofin. Our results indicate that LOX-PP inhibits ERK1/2 and at the same time promotes adipogenesis, likely by blocking FGF-2 receptor signaling. This is consistent with the report that ERK1/2 inhibits adipogenesis by directly phosphorylating PPARγ (Rosen et al., 2000). ERK1/2 must be attenuated to prevent PPARγ phosphorylation (Hu et al., 1996). Thus, LOX-PP could promote adipogenesis through inhibition of ERK1/2.
LOX-PP mutation abolishing its inhibitory effect on FGF2 signaling promotes adipogenesis
To further establish the role of LOX-PP as an inhibitor of FGF2 signaling, we conducted a mutation in LOX-PP. In studies with cancer patients, a single nucleotide polymorphism (SNP) (rs1800449) of LOX-PP was identified, which results in an Arg158Gln substitution at the allele (G483A). This mutation occurs at a high frequency in estrogen receptor-α-negative breast cancer cells19 as well as in patients with gastric cancer in South Koreans.29 Consistently, the LOX-PP G483A SNP abolishes its inhibitory role of FGF2 receptor and the anti-proliferative properties of LOX-PP in breast cancer cells.19 However, this polymorphism has not been studied in developing adipocytes. Here, we report that the LOX-PP SNP, which removes its ability to inhibit FGF2 signaling, attenuates the pro-adipogenic effect of LOX-PP, clearly showing that LOX-PP promotes adipogenesis via inhibition of FGF2 signaling. Interesting, it has been reported that FGF-2 up-regulates LOX expression.30 Thus, LOX-PP production forms a feedback inhibition on FGF-2 signaling.
Implication of LOX-PP in adipogenic differentiation and obesity
We have determined that LOX-PP, not LOX, promotes adipogenesis of 3T3-L1 pre-adipocytes and thus regulates adipocyte differentiation, which is likely linked to obesity. Huang et al. (2009) reports that expression of LOX is induced by Bone Morphogenic Protein 2 and 4 (BMP2 and BMP4) during the commitment phase of adipogenesis and knockdown of LOX expression negatively effects adipogenic commitment.31 Our study further demonstrates that the effect of LOX on adipogenesis induced by BMP2 and 4 is through LOX-PP; as a part of LOX pre-propeptide, its expression contingent on LOX expression. Thus, LOX-PP is likely a prime candidate for a physiological link between adipogenic commitment, and adipocyte hyperplasia during obesity.
It has recently been reported that high glucose triggers LOX expression in rat retinal endothelial cells similar to the up-regulation of LOX in diabetic retinas.32 Thus, given that LOX-PP promotes adipogenesis, it is very likely that the increased expression of LOX, accompanied by LOX-PP, due to high glucose conditions seen in diabetic patients, is partially responsible for the high adiposity in multiple tissues. Adipocytes are embedded inside connective tissue. Similar to its role in adipogenesis, FGF-2 signaling also inhibit collagen synthesis and fibrosis.33,34 Thus LOX-PP may also promote collagenous protein synthesis, in addition to its effect on adipogenesis. These data implicate LOX-PP as a bona fide therapeutic target for regulating adipose and associated connective tissue development induced due to inflammation or other pathophysiological changes.
Conclusion
In this study, we observe that FGF-2 inhibited adipogenesis, and LOX-PP promoted adipogenesis in 3T3-L1 cells. Site directed mutagenesis of LOX-PP, which abolishes the inhibitory activity of LOX-PP, attenuated the adipogenic promoting properties of LOX-PP. In summary, for the first time, our data show that LOX-PP enhances adipogenesis through inhibition of FGF-2 receptor signaling. Our data suggest that LOX-PP may serve as a bona fide therapeutic target for regulating adipogenesis and adipose tissue development.
Abbreviations
- AKT
protein kinase B
- C/EBPα
CCAAT-enhancer binding protein
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- FGF-2
fibroblast growth factor-2
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- PP
lysyl oxidase propeptide
- PM
point mutation
- PPARγ
peroxisome proliferator-activated receptor
- V
vector only
- WT
wild-type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work was funded by NIH R01HD067449 and R21AG049976.
References
- [1].Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mole Cell Biol 2006; 7:885-96; http://dx.doi.org/ 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- [2].Yanovski SZ, Yanovski JA. Obesity prevalence in the United States–up, down, or sideways?. N Engl J Med 2011; 364:987-9; PMID:21410367; http://dx.doi.org/ 10.1056/NEJMp1009229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Du M, Carlin KM. Meat science and muscle biology symposium: extracellular matrix in skeletal muscle development and meat quality. J Anim Sci 2012; 90:922-3; PMID:22345108; http://dx.doi.org/ 10.2527/jas.2011-4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kagan HM, Li WD. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 2003; 88:660-72; PMID:12577300; http://dx.doi.org/ 10.1002/jcb.10413 [DOI] [PubMed] [Google Scholar]
- [5].Smith-Mungo LI, Kagan HM. Lysyl oxidase: Properties, regulation and multiple functions in biology. Matrix Biol 1998; 16:387-98; PMID:9524359; http://dx.doi.org/ 10.1016/S0945-053X(98)90012-9 [DOI] [PubMed] [Google Scholar]
- [6].Dimaculangan DD, Chawla A, Boak A, Kagan HM, Lazar MA. Retinoic acid prevents downregulation of ras recision gene/lysyl oxidase early in adipocyte differentiation. Differentiation 1994; 58:47-52; PMID:7867896; http://dx.doi.org/ 10.1046/j.1432-0436.1994.5810047.x [DOI] [PubMed] [Google Scholar]
- [7].Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of Ras-transformed cells. J Biol Chem 2004; 279:40593-600; PMID:15277520; http://dx.doi.org/ 10.1074/jbc.M406639200 [DOI] [PubMed] [Google Scholar]
- [8].Vora SR, Palamakumbura AH, Mitsi M, Guo Y, Pischon N, Nugent MA, Trackman PC. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J Biol Chem 2010; 285:7384-93; PMID:20048148; http://dx.doi.org/ 10.1074/jbc.M109.033597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Palamakumbura AH, Vora SR, Nugent MA, Kirsch KH, Sonenshein GE, Trackman PC. Lysyl oxidase propeptide inhibits prostate cancer cell growth by mechanisms that target FGF-2-cell binding and signaling. Oncogene 2009; 28:3390-400; PMID:19597471; http://dx.doi.org/ 10.1038/onc.2009.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol 2001; 2:REVIEWS3005; PMID:11276432; http://dx.doi.org/ 10.1186/gb-2001-2-3-reviews3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010; 47:360-70; PMID:20510392; http://dx.doi.org/ 10.1016/j.bone.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hayashi K, Kurihara I, Uchida Y. Studies of ocular murine cytomegalovirus infection. Investigative Ophthalmol Vis Sci 1985; 26:486-93. [PubMed] [Google Scholar]
- [13].Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 2002; 277:46226-32; PMID:12270934; http://dx.doi.org/ 10.1074/jbc.M207776200 [DOI] [PubMed] [Google Scholar]
- [14].Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr 1994; 14:99-129; PMID:7946535; http://dx.doi.org/ 10.1146/annurev.nu.14.070194.000531 [DOI] [PubMed] [Google Scholar]
- [15].Zhao JX, Yue WF, Zhu MJ, Du M. AMP-activated protein kinase regulates beta-catenin transcription via histone deacetylase 5. J Biol Chem 2011; 286:16426-34; PMID:21454484; http://dx.doi.org/ 10.1074/jbc.M110.199372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yan X, Huang Y, Zhao JX, Rogers CJ, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates microRNA let-7 g expression, a possible mechanism for enhanced adipogenesis during ovine fetal skeletal muscle development. Int J Obes (Lond) 2013; 37:568-75; PMID: 22614057; http://dx.doi.org/10.1038/ijo.2012.6920361929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao J, Yue W, Zhu MJ, Sreejayan N, Du M. AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of beta-catenin at Ser 552. Biochem Biophys Res Commun 2010; 395:146-51; PMID:20361929; http://dx.doi.org/ 10.1016/j.bbrc.2010.03.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Manfe V, Kochoyan A, Bock E, Berezin V. Peptides derived from specific interaction sites of the fibroblast growth factor 2-FGF receptor complexes induce receptor activation and signaling. J Neurochem 2010; 114:74-86; PMID:20374425 [DOI] [PubMed] [Google Scholar]
- [19].Min CY, Yu ZY, Kirsch KH, Zhao YS, Vora SR, Trackman PC, Spicer DB, Rosenberg L, Palmer JR, Sonenshein GE. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res 2009; 69:6685-93; PMID:19654310; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sanalkumar R, Vidyanand S, Lalitha Indulekha C, James J. Neuronal vs. glial fate of embryonic stem cell-derived neural progenitors (ES-NPs) is determined by FGF2/EGF during proliferation. J Mole Neurosci 2010; 42:17-27; http://dx.doi.org/ 10.1007/s12031-010-9335-z [DOI] [PubMed] [Google Scholar]
- [21].Diecke S, Quiroga-Negreira A, Redmer T, Besser D. FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs 2008; 188:52-61; PMID:18334814; http://dx.doi.org/ 10.1159/000121282 [DOI] [PubMed] [Google Scholar]
- [22].Grazul-Bilska AT, Luthra G, Reynolds LP, Bilski JJ, Johnson ML, Adbullah SA, Redmer DA, Abdullah KM. Effects of basic fibroblast growth factor (FGF-2) on proliferation of human skin fibroblasts in type II diabetes mellitus. Exp Clin Endocrinol Diabetes 2002; 110:176-81; PMID:12058341; http://dx.doi.org/ 10.1055/s-2002-32149 [DOI] [PubMed] [Google Scholar]
- [23].Reidy MA, Lindner V. Basic FGF and growth of arterial cells. Ann N Y Acad Sci 1991; 638:290-9; PMID:1785807; http://dx.doi.org/ 10.1111/j.1749-6632.1991.tb49039.x [DOI] [PubMed] [Google Scholar]
- [24].Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995; 9:726-35; PMID:7601337 [PubMed] [Google Scholar]
- [25].Navre M, Ringold GM. Differential effects of fibroblast growth factor and tumor promoters on the initiation and maintenance of adipocyte differentiation. J Cell Biol 1989; 109:1857-63; PMID:2507555; http://dx.doi.org/ 10.1083/jcb.109.4.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li W, Nugent MA, Zhao Y, Chau AN, Li SJ, Chou IN, Liu G, Kagan HM. Lysyl oxidase oxidizes basic fibroblast growth factor and inactivates its mitogenic potential. J Cell Biochem 2003; 88:152-64; PMID:12461785; http://dx.doi.org/ 10.1002/jcb.10304 [DOI] [PubMed] [Google Scholar]
- [27].Wu M, Min CY, Wang XB, Yu ZY, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res 2007; 67:6278-85; PMID:17616686; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0776 [DOI] [PubMed] [Google Scholar]
- [28].Hurtado PA, Vora S, Sume SS, Yang D, Hilaire CS, Guo Y, Palamakumbura AH, Schreiber BM, Ravid K, Trackman PC. Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation. Biochem Bioph Res Communs 2008; 366:156-61; http://dx.doi.org/ 10.1016/j.bbrc.2007.11.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yoon JH, Park JK, Kang YH, Park YK, Nam SW, Lee JY, Park WS. Lysyl oxidase G473A polymorphism is closely associated with susceptibility to gastric cancer in a South Korean population. APMIS 2011; 119:762-8; PMID:21995629; http://dx.doi.org/ 10.1111/j.1600-0463.2011.02802.x [DOI] [PubMed] [Google Scholar]
- [30].Trackman PC, Graham RJ, Bittner HK, Carnes DL, Gilles JA, Graves DT. Inflammation-associated lysyl oxidase protein expression in vivo, and modulation by FGF-2 plus IGF-1. Histochem Cell Biol 1998; 110:9-14; PMID:9681684; http://dx.doi.org/ 10.1007/s004180050259 [DOI] [PubMed] [Google Scholar]
- [31].Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 2009; 106:12670-5; PMID:19620713; http://dx.doi.org/ 10.1073/pnas.0906266106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chronopoulos A, Tang A, Beglova E, Trackman PC, Roy S. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes 2010; 59:3159-66; PMID:20823103; http://dx.doi.org/ 10.2337/db10-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chaudhary LR, Avioli LV. Extracellular-signal regulated kinase signaling pathway mediates downregulation of type I procollagen gene expression by FGF-2, PDGF-BB, and okadaic acid in osteoblastic cells. J Cell Biochem 2000; 76:354-9; PMID:10649432; http://dx.doi.org/ 10.1002/(SICI)1097-4644(20000301)76:3%3c354::AID-JCB2%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- [34].Svystonyuk DA, Ngu JM, Mewhort HE, Lipon BD, Teng G, Guzzardi DG, Malik G, Belke DD, Fedak PW. Fibroblast growth factor-2 regulates human cardiac myofibroblast-mediated extracellular matrix remodeling. J Transl Med 2015; 13:147; PMID:25948488; http://dx.doi.org/ 10.1186/s12967-015-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]