ABSTRACT
The neural crest is a transient cell population that gives rise to various cell types of multiple tissues and organs in the vertebrate embryo. Neural crest cells arise from the neural plate border, a region localized at the lateral borders of the prospective neural plate. Temporally and spatially coordinated interaction with the adjacent tissues, the non-neural ectoderm, the neural plate and the prospective dorsolateral mesoderm, is required for neural plate border specification. Signaling molecules, namely BMP, Wnt and FGF ligands and corresponding antagonists are derived from these tissues and interact to induce the expression of neural plate border specific genes. The present mini-review focuses on the current understanding of how the NPB territory is formed and accentuates the need for coordinated interaction of BMP and Wnt signaling pathways and precise tissue communication that are required for the definition of the prospective NC in the competent ectoderm.
KEYWORDS: BMP, GDF6, neural crest, Neural plate border, neurocristophathies, Recessive Robinow Syndrome, Ror2, Wnt
Introduction
The neural crest (NC) is a vertebrate-specific population of multipotent progenitor cells that differentiate into a variety of derivatives including a subset of the cranial ganglia, the majority of cranial cartilage and bone, part of the cardiac outflow tract, melanocytes, the sensory dorsal root ganglia, the enteric nervous system and the medulla of the adrenal gland (reviewed in refs. 1 and 2). The NC is induced in early embryonic development in two stripes laterally adjacent to the open neural plate. As the neural folds form, elevate and fuse to form the neural tube during neurulation, the two populations of NC cells are brought together at the dorsal neural tube. Shortly after, NC cells undergo an epithelial to mesenchymal transition, leave the dorsal neural tube and migrate on defined routes through the embryonic body to their destinations where they differentiate. Development of the NC was studied extensively over the past decades in a variety of vertebrate model organisms including the basal extant vertebrate lamprey, in fish, frog and chicken embryos and in the mouse as a mammalian model system (for review see ref. 3).
In the last years, a number of studies in frog and chicken embryos have further expanded our knowledge on the earliest events in NC development: the specification the neural plate border (NPB). The NPB is first specified at late blastula to early gastrula stages when global signal gradients act to pattern the embryo. During gastrulation, a NPB specific gene expression pattern is further established and maintained. In addition, morphogenetic movements reposition and shape the mesoderm, the neural plate as well as the NPB.
Tissue interactions in NPB formation
In the early frog gastrula, the prospective NC cells arise from an ectodermal region located adjacent to the dorsolateral marginal zone (DLMZ) and between the future neural plate and the non-neural ectoderm that will give rise to the epidermis.4 Comparable fate maps have been drawn for mouse and chicken embryos.5,6 At early neurula stages, the neural plate has been shaped and the prospective NC cells are located at the lateral borders of the neural plate in the NPB territory.
Experimental evidence in frog and chick embryos demonstrated that NPB formation depends on interaction with juxtaposed tissues, which are sources of signaling molecules that specify the NPB. The capability of a tissue or protein to induce a certain cell fate is typically judged by the expression of one or more genes that are also endogenously expressed in these cells. The most common markers indicating NPB specification are the transcription factors msx1 and pax3 as well as hairy2 and dlx5. Subsequent adoption of NC fate is often analyzed by the expression of snail2/slug, foxD3 or sox9, which in both cases represent only a small subset of the transcriptional signature of the respective tissues.
Ectodermal tissue interactions
Experimental studies illustrated that Xenopus neural plate tissue is able to respond to signals from the lateral ectoderm and adopt NC fate as indicated by the induction of snail2/slug expression at the border region.7 Likewise, studies using tissue explants from chick embryos suggested that the epidermal ectoderm presents the signaling source for the induction of dorsal cell differentiation indicated by expression of pax3, msx1/2 and snail2/slug at the border region of the neural plate.8 In vitro assays of cell differentiation using neural plate explants from chick embryos have further shown that patterning of the neuroectoderm is initiated by a contact-dependent inductive signal from the epidermal ectoderm and this interaction leads to NPB specification as indicated by the expression of the early NPB markers pax3 and msx1 and the NC marker HNK-1.7-9 Although these grafting experiments show that NC fate can be induced in the neuroectoderm, the requirement of the neural plate has been challenged by studies in chick and recently, in human embryonic stem cells (hESCs). Epiblast explants from stage 3 chick embryos develop into NC in the absence of definitive neural or mesodermal tissue.8,10 Just recently, analysis of NC induction in hESCs demonstrated that the differentiation of hESCs into NC cells as indicated by expression of NPB genes including pax3 and msx1 takes place independent of the presence of neural precursors or neuroectoderm.11
The role of the dorsolateral marginal zone
In contrast to the abovementioned experimental evidence that the NPB is induced by interactions between the prospective neural plate and the epidermis, tissue recombination experiments in gastrula stage Xenopus embryos suggested that the dorsolateral mesoderm can be considered as the key mesodermal tissue able to induce NC fate. Co-culture of naïve ectoderm with dorso-lateral marginal zone (DLMZ) mesoderm induced the expression of NC marker genes including snail2/slug, foxD3 and sox9.4,12-14 Consistently, Xenopus embryos in which the dorsolateral mesoderm has been experimentally removed fail to develop NC as indicated by the lack of snail2/slug expression at neurula stage and the failure to form NC derivatives such as melanocytes.12,13 The cross-species conservation of the mechanisms underlying these inductive capacities was demonstrated by explant assays performed with inducing tissue from posterior nonaxial mesoderm from chick and responding ectoderm from Xenopus. Chick posterior nonaxial mesoderm was able to induce pax3 and msx1 expression and thus NPB fate in naïve Xenopus ectoderm.15
The apparent contradiction concerning the requirement of dorsolateral mesoderm versus non-neural ectoderm has at least partially been resolved by a study by Steventon and colleagues, who showed that the DLMZ induces NPB in the adjacent ectoderm in tissue explants marked by expression of hairy2, dlx5 and msx1 whereas the subsequent induction of NC markers such as snail2/slug requires the additional presence of epidermis.4 Since most of the abovementioned ectodermal tissue recombination experiments have been performed in late gastrula to neurula stage embryos, these likely reflect later events in NPB and NC induction when the initial NPB specification has already taken place and subsequent NC induction requires interaction with the epidermis.
When is the NPB specified?
Considering the more recent studies, the timing of events in NPB specification and NC induction appears less divergent than previously assumed. In Xenopus the NPB is specified in early gastrula stages.16 At this time, the DLMZ lies adjacent to the prospective NPB territory and tissue rearrangements namely internalization and convergent extension of the mesoderm occur subsequently in parallel to NPB formation. The NPB is fully determined at the end of gastrulation. In vitro cultures of explants and recombination assays confirmed that the neural folds are specified autonomously to express snail2/slug and the ectoderm loses the competence to respond to NPB inducing signals by this time.7
In chick embryos, epiblast cells apparently are already specified to form NC in late blastula stages.17 In chick as well as in Xenopus and zebrafish, NPB markers are expressed at mid- to late gastrula stages.10,18,19 A sharp and defined NPB is observed in chick embryos during and after neurulation,10,20 and it appears that the competence to form NC is retained longer, well into neurula stages, in chick ectoderm as compared to the frog.7,21,22 This discrepancy is likely due to the timing of gastrulation and neurulation in amphibian and chick embryos. In contrast to amphibia, gastrulation and neurulation occur simultaneously in an anterior-posterior wave in chick embryos, resulting in completed neural tube closure in the anterior region when in the same embryo the posterior neural plate is still open. Consistently, the NPB is more defined in the anterior neural folds as compared to the more posterior regions.20
Growth factor signaling in NPB specification
What are the signals emanating from the dorsolateral mesoderm, the neural plate and non-neural ectoderm that interact to specify first the NPB and subsequently the NC? Multiple signaling pathways have been shown to influence NPB formation and subsequent NC induction. In the frame of this mini-review, we will focus on BMP and Wnt signaling involved in NPB formation, which does not imply that other pathways are less important.
BMP signaling
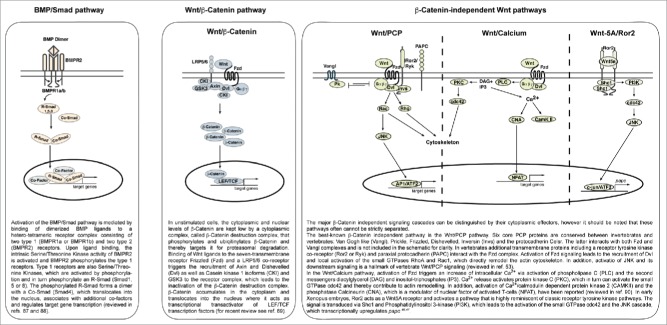
Global dorso-ventral patterning of the ectoderm is predominantly mediated by the activity of BMP signaling, which is high in the ventral ectoderm that gives rise to the epidermis and absent in the dorsal prospective neuroectoderm.23-25 In vertebrates, dorsal inhibition of BMP activity is mediated by secreted BMP antagonists derived from the organizer and the dorsal mesoderm (reviewed in ref 26). The NPB forms at the lateral borders of the neural plate in an area with low to intermediate BMP activity. Consistent with a dorso-ventral gradient of BMP signaling, studies in early Xenopus laevis and zebrafish embryos confirmed that increasing amounts of BMP shift the specification of naïve ectodermal precursors from neural to NC to epidermis.27,28 BMP signaling is transduced to the activation of Smad1, 5 and 8 transcription factors and thereby regulates the expression of Smad-responsive genes (Fig. 1). Among those genes expressed in the NPB during gastrulation, BMP regulates the expression of msx1, msx2, dlx5 and dlx6; interestingly, the related dlx3 is expressed in a more lateral region and its upregulation requires higher BMP activity as compared to dlx5/6.27,29 The transcription factor AP2-alpha (gene: tfap2a) is considered as the central player in NPB specification.30 In blastula and early gastrula stage Xenopus embryos, tfap2a is expressed in the entire non-neural ectoderm and the prospective NPB territory and a recent study showed that this expression depends on BMP activity.31,32 The source of BMP ligands is likely the non-neural ectoderm or the prospective NPB ectoderm itself, which expresses BMP ligands in zebrafish, frog, chick and mouse embryos.28,33-35 Consistently, the potential of epidermis to induce NPB or NC is supposedly attributable to the expression of BMP ligands. The endogenous or experimental juxtaposition of epidermis and prospective or definitive neuroectoderm, which is essentially devoid of BMP activity, results in moderate activation of BMP signaling in the contact area and induces NPB and NC.
Figure 1.
Schematic and simplified illustration of BMP/Smad, Wnt/β-Catenin and β-Catenin independent Wnt pathways.
These studies demonstrate a requirement of BMP signaling for NPB induction, whereas other researchers showed that inhibition of BMP signaling during gastrulation is required for NC induction in zebrafish and Xenopus.4,36 In chicken embryos, inhibition of BMP signaling in the area opaca induces the expression of pax7, dlx5 and msx1.37 However, few studies attempted a quantification of BMP activity in the prospective NPB; data obtained in Xenopus tissue explants shows that endogenous BMP activity is low but detectable in the prospective NPB at early gastrula stages, decreases even further by mid-gastrula and increases again in early neurula stages.4 Accordingly, low BMP activity in late blastula and early gastrula stages is present endogenously in the early NPB, which is supposedly sufficient to allow NPB specification at this early stage.
Wnt signaling
In addition to attenuated BMP activity in the prospective NPB ectoderm, Wnt signaling plays a key role in NPB and NC induction. Wnt ligands activate different intracellular signaling pathways that are traditionally subdivided into the β-Catenin-dependent or Wnt/β-Catenin pathway and a more divergent group of β-Catenin-independent pathways (for details see Fig. 1). In Xenopus, Wnt/β-Catenin signaling, putatively activated by Wnt8, directly regulates the expression of the earliest NPB markers tfap2a and gbx2.30,38 AP2-alpha is considered as the key transcription factor responsible for further establishing and reinforcing NPB specification, since it is required for the upregulation of multiple other early NPB specifiers including hairy2, msx1 and pax3 at early gastrula stages.30 Similarly and at approximately the same time in development, Gbx2 depletion resulted in failure to upregulate pax3 and msx1.38 So far, direct transcriptional regulation of gbx2 by AP2-alpha or vice versa has not been reported. Therefore, the upregulation of tfap2a and gbx2 by the combined activity of BMP, Wnt/β-Catenin signaling and, although indirectly, FGF signaling30,38 might be considered the earliest event in NPB specification.
After the initial upregulation of tfap2a and gbx2 expression, NPB fate is maintained by positive feedback loops between these factors and their target genes, in particular pax3 and msx1.30,39 BMP and Wnt/β-Catenin signaling functionally interact with Pax3, Zic1 and Ap2a in later phases to induce bona fide NC fate.19,30,40,41 Likewise, Wnt/β-Catenin signaling is essential for NPB specification in epiblast explants from chick blastulae, where Wnt activity precedes and upregulates bmp4,17 as well as for expression of NPB genes in hESCs.11
Steventon and colleagues have analyzed Wnt/β-Catenin activity in Xenopus embryos using the TOPFlash reporter gene assay. They observed low Wnt/β-Catenin activity at early gastrula stage 10 in the prospective NC, which rises slightly already till late gastrula stage 12 and continuously increases till mid-neurula stage 17.4 Since ectopic activation of Wnt/β-Catenin signaling during gastrulation downregulated the NPB genes hairy2 and dlx5, it has been suggested that an initial inhibition of Wnt/β-Catenin signaling is required for NPB specification.16 This model contradicts the essential role of Wnt/β-Catenin signaling in NPB specification discussed above and also studies in chick embryos, which showed that activation of Wnt signaling precedes BMP activation in late gastrula stage embryos.17 Notably, Steventon and colleagues induced ectopic expression of β-Catenin only at the onset of gastrulation.16 However, both direct Wnt/β-Catenin target genes, tfap2a and gbx2, are already expressed at that stage and in the case of tfap2a this early expression also depends on BMP signaling.30,32,38 Moreover, tfap2a induction in naïve ectodermal explants required only low levels of Wnt/β-Catenin activity,30 suggesting that low endogenous activity is sufficient to induce tfap2a in the NPB. During gastrulation, NPB genes apparently exhibit differential sensitivity toward Wnt/β-Catenin signaling. Increasing Wnt/β-Catenin activity enhances expression of tfap2a and gbx2,30,38 but inhibits expression of hairy2 and dlx5,16 which are apparently more sensitive to elevated Wnt/β-Catenin signaling. Dissecting the contributions of early signaling inputs to NPB specification is complicated by the different time points from late blastula to mid-neurula stages chosen for expression analyses in different studies, which impede discrimination between early induction, maintenance and the influence of positive or negative feedback regulation.
In our own recent work, we have demonstrated that the receptor tyrosine kinase Ror2 is essential for correct NPB specification during gastrulation in Xenopus embryos. Ror2 elicits multiple functions in Wnt signaling (see also Fig. 1): (1) Ror2 interacts with Frizzled (Fzd) receptors and the intracellular protein Dishevelled (Dvl) to activate Wnt/PCP signaling,42,43 (2) Ror2 is able to antagonize Wnt/β-Catenin signaling in cells and embryonic tissues,44,45 and (3) Ror2 activates a PI3K-dependent signaling cascade that transcriptionally regulates expression of paraxial protocadherin (PAPC).46,47 PAPC and the closely related PCNS are the two paralogs of Protocadherin 8 (PDCH8) in Xenopus laevis. Notably, PAPC itself acts as a modulator of Wnt/PCP signaling in the dorsal mesoderm.48,49
Antisense-morpholino-induced loss of Ror2 function in Xenopus embryos leads to defective NPB formation and downregulation of most NPB genes such as tfap2a, gbx2, zic1, and msx1 and msx2.50 Moreover, the BMP ligand gdf6 was downregulated in Ror2 morphant embryos already at late blastula and early gastrula stages, which is consistent with an early positive role of BMP signaling in NPB specification and importantly, functionally links Wnt and BMP signaling.
Ror2 function in NPB specification depends on Wnt/PCP signaling. Expression of NPB markers in Ror2-depleted embryos was restored by a PCP-activating mutant of DVL, which lacks the DIX domain,51 and by both Xenopus PCDH8 paralogs, PAPC and PCNS, which is consistent with a previous study on the role of PCP signaling in post-gastrula NC induction.52 At early neurula stages, the elevation of the neural folds marks the onset of neurulation. At the same time, the gdf6-positive NPB has concomitantly thickened, suggesting that also the NBP undergoes morphogenetic changes. In Ror2 depleted embryos, both, elevation of the neural folds and thickening of the NPB was impaired or delayed.50 Moreover, cell polarity in the lateral neural plate was disrupted, indicating that Ror2-dependent PCP signaling controls not only NPB specification but also cell polarity in the NPB and neural plate as well as NPB morphogenesis. Thus, Ror2 appears to coordinate and integrate tissue specification and morphogenesis. Notably, Wnt/PCP signaling also depends on cell-cell contacts (reviewed in ref. 53). Considering that contact-dependent signals between neural and non-neural ectoderm have been proposed in NC induction, an additional role of Ror2-mediated Wnt/PCP signaling could be envisioned, which however awaits experimental confirmation.
Sources of Wnt and FGF ligands
In addition to BMP and Wnt signaling, FGF signaling is the third major pathway required for NPB specification and NC induction. FGFs play multiple roles in patterning the dorsal ectoderm. FGFs have been shown to sensitize the dorsal ectoderm to respond to BMP inhibition, transcriptionally regulate BMPs and Wnts, and to contribute directly to NPB specification.19,20,54-56
In Xenopus, the DLMZ expresses a number of paracrine growth factors at the onset of gastrulation including wnt8, fgf4, fgf8, gdf6 and the BMP antagonist chordin (Fig. 2) and further expresses hairy2, fgfr1, fgfr4a, bmpr1a, pcns and ror2.4,14,15,50 The early expression of hairy2, a Notch target, suggested a contribution of Notch signaling already in this early stage of NPB specification. However, the requirement of Notch signaling in NC development has been shown only in late gastrula and early neurula stages whereas hairy2 expression in the NPB is dependent on BMP, FGF and Wnt but independent of Notch.57 Notably, the expression levels of bmpr1a, pcns and gdf6 are higher in the adjacent ectoderm at the onset of gastrulation, which additionally expresses bmpr1b, fgfr1, fgfr2, fgfr4 and weakly fgfr3,50,58,59 suggesting that Wnt, FGF and BMP ligands secreted by the DLMZ are received in the adjacent ectoderm.
Figure 2.
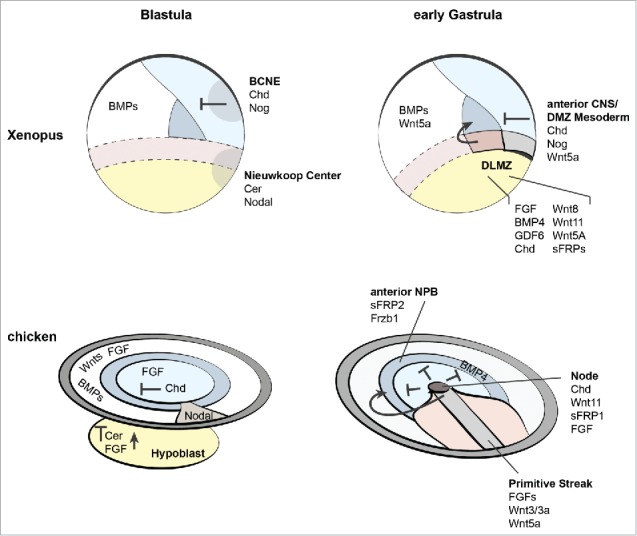
Expression of diffusible growth factors and antagonists in blastula and early gastrula stage in Xenopus and chick embryos. In blastula stages, BMP ligands are expressed in the ectoderm and their expression is antagonized by secreted BMP antagonists. In Xenopus these are derived from the Blastula Chordin and Noggin Expressing center (BCNE91). In early gastrula stages, BMP ligands are expressed in the ectoderm and BMP antagonists are continuously expressed in the presumptive anterior neural plate ectoderm and in the dorsal marginal zone (DMZ). BMP4, GDF6, FGF and Wnt8 as well as BMP and Wnt antagonists are derived from the DLMZ. In chick blastulae, FGF is expressed in the entire epiblast, BMPs, Wnts only in the lateral epiblast60 and Chordin is expressed in a subset of pre-node epiblast cells and the multi-specific Wnt/BMP and Nodal antagonist Cerberus is secreted by the hypoblast (for review see ref. 61 and references therein).61 In gastrula stages, BMP4 is expressed in the entire NPB area, the anterior NPB additionally expresses the Wnt antagonists sFRP2 and Frzb1. Wnts and FGFs are expressed in the primitive streak and the node, the latter further expresses Chordin.62,63
In the chick blastula, BMPs, FGF and Wnts are expressed in the lateral epiblast, whereas the medial epiblast expresses only FGFs.60 A medial, posterior population of pre-node cells expresses the BMP antagonist Chordin and the hypoblast represents an additional source of FGFs and the BMP and Wnt antagonist Cerberus, which contribute to early patterning of the ectoderm (for review see ref. 61 and references therein). In early gastrula chick embryos, FGFs and Chordin are secreted by the node while several Wnts and FGFs are expressed in the primitive streak (Fig. 2).62,63 An early study showed that FGFs are required for NPB specification in early epiblast explants20 and the authors suggest the paraxial mesoderm as endogenous source of FGFs.
A model involving inducing signals from the DLMZ or the node/primitive streak appears contradictory to studies arguing that mesoderm is dispensable for NPB specification in zebrafish, Xenopus and chick embryos. In chick embryos, this conclusion is based on experiments using epiblast explants prepared from blastula stage embryos, which are further cultured in a serumfree growth factor supplemented medium in vitro without forming any mesoderm as judged by the lack of mesodermal marker gene expression such as chordin, brachyury (t) and tbx6L.64 Other studies in the chick explanted neural plate tissue at neurula stages8 or used transplants or implantation of growth factor soaked beads,20 neither of which precludes early, endogenous signals and tissue interactions.
In zebrafish and Xenopus embryos, the proposed dispensability of mesoderm in NPB specification is based on experimental inhibition of Nodal signaling, the major mesoderm inducing signal, in intact embryos.36,65 Although major mesodermal markers such as brachyury (t) are absent in such embryos, it cannot be excluded that factors crucial for NPB specification are still expressed. Indeed, it has been shown in zebrafish that Nodal is dispensable for expression of Wnt8 in the DLMZ. Wnt8 is required for NPB specification, thus experimental inhibition of Nodal signaling blocks mesoderm induction but neither Wnt8 expression nor NPB induction.65 It might be assumed that the same is true for Nodal-inhibited Xenopus embryos. Moreover, an interesting study has identified an additional role of Snail2 in pre-gastrula mesoderm induction and patterning. Knock-down of Snail2 impaired both, mesoderm induction and NPB formation. Defective NPB specification in Snail2-deficient embryos was attributed to the downregulation of bmp4 and wnt8 and concomitant upregulation of BMP and Wnt antagonists in the DLMZ,66 which further emphasized the essential role of BMP and Wnt in NPB specification. By contrast, excision of the DLMZ results in embryos that fail to develop NC cells,13 which clearly demonstrates the endogenous requirement of the DLMZ in NPB specification and NC induction. Hence, the presence of DLMZ and mesoderm in general is required as source of growth factors that induce NPB fate although full mesodermal specification might be dispensable as long as expression of NPB inducing factors, namely Wnt and FGF, is maintained.
From NPB to NC
Shortly after gastrulation, in early neurula stages, the NPB/prospective NC territory is characterized by a marked increase of BMP/Smad signaling in Xenopus and chick embryos.24,59 Consistently, the NPB expresses the BMP ligands bmp4 and gdf6 as well as the receptors bmpr1a and bmpr1b.8,27,50,67 Expression of gdf6 at the NPB depends on Ror2 signaling during gastrulation and seems to be important for the spatially restricted activation of BMP signaling at the NPB, which is required for specification and maintenance of NC cell fate also in zebrafish embryos.68,69
Physical, genetic and functional interactions between BMP, Wnt and FGF combined with the transcription factors expressed in the NPB and additional signaling pathways form a gene regulatory network (for review see ref. 70). Although definite mesoderm induction appears to be dispensable for NPB specification, the mesoderm is important in positioning the NPB. The axial and prechordal plate mesoderm repress NC induction, and thus contribute to restriction of NC induction to the lateral and posterior NPB. The notochord secretes BMP antagonists and SHH71 and thereby represses NC induction in the neural plate. In addition, the prechordal mesoderm was identified as the tissue that inhibits NC fate in the anterior region via expression of the Wnt/β-Catenin antagonist DKK1.72
The gene regulatory mechanisms of NPB formation and NC induction are largely conserved among vertebrates. In human embryos, the earliest embryos have been analyzed at the end of neural tube closure, when the NC has already been induced and NC migration has been initiated. In these embryos, Pax3, Sox9 and Sox10 have been detected in premigratory NC, whereas migrating NC cells additionally expressed Ap2a and Pax7.73 Importantly, a recent study revealed that NC induction in hESCs is preceded by the expression of NPB markers Pax3, Pax7, Msx1 and Ap2a, shortly after also the NC marker snail2 is detected. This study further demonstrated that NC induction in hESCs requires Wnt/β-Catenin, BMP and FGF signaling, which indicates that the basic mechanisms of early NPB and NC induction are also conserved in humans.11
It remains to be shown whether the recently discovered role of β-Catenin independent Wnt signaling is likewise conserved. A role of Ror2 in NC development is indicated by the phenotypes of Ror2 knockout mice and humans affected with recessive Robinow syndrome (RRS). One characteristic of RRS are severe craniofacial deformations and occasional cardiac outflow tract malformations,74 which suggest a defect in the development of the cranial NC, whereas derivatives of trunk NC such as melanocytes or the enteric nervous system are unaffected. Similar phenotypes have been described in a Ror2 knockout mouse model,75 however, to date it remains unresolved whether these phenotypes originate from defective NPB formation or are caused by Ror2 function in later phases of NC development. However, similar facial appearance and defects in craniofacial development are observed in a number of neurocristopathies that have been associated with mutations in the NPB genes pax3, ap2a, msx1, msx2 and dlx5 (Table 1).
Table 1.
Examples of human neurocristopathies associated with mutations in NPB genes.
| Human congenital syndrome | Gene | Phenotype | NC derivatives |
|---|---|---|---|
| Craniofacial-deafness-hand syndrome (CDHS) | PAX376 | flat facial profile, hypertelorism, hypoplastic nose with slitlike nares, and a sensorineural hearing loss, micrognathia, hypertelorism, high arched palate | craniofacial cartilage and bone |
| Waardenburg syndrome, type 1 and type 3 | PAX377,78 | pigmentary abnormalities of the hair, skin, and eyes; congenital sensorineural hearing loss; lateral displacement of the ocular inner canthi and upper limb abnormalities | melanocytes |
| Branchiooculofacial syndrome | TFAP2A79 | branchial cleft sinus defects, ocular anomalies such as microphthalmia and lacrimal duct obstruction, a dysmorphic facial appearance including cleft or pseudocleft lip/palate, and autosomal dominant inheritance | craniofacial cartilage and bone |
| Craniosynostosis 2 | MSX280 | primary abnormality of skull growth involving premature fusion of the cranial sutures | craniofacial cartilage and bone |
| Parietal foramina 1 | MSX281 | symmetric, oval defects in the parietal bone situated on each side of the sagittal suture and separated from each other by a narrow bridge of bone | craniofacial cartilage and bone |
| Orofacial cleft 5; Tooth agenesis, selective, 1, with or without orofacial cleft | MSX1, WNT10A82,83 | isolated cleft lip with or without cleft palate Absence of primary or permanent teeth and cleft lip/palate | craniofacial cartilage and bone |
| Split-hand/foot malformation 1 | DLX584-86 | limb malformation with syndactyly, median clefts of the hands and feet, and aplasia and/or hypoplasia of the phalanges, metacarpals, and metatarsals; occasionally mental retardation, ectodermal and craniofacial findings, orofacial clefting and neurosensory hearing loss as well as congenital heart defects | craniofacial cartilage and bone, cardiac neural crest |
Note. The table lists syndromes with craniofacial malformations without claiming to be exhaustive. Description of disease phenotype from OMIM (Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD). World Wide Web URL: https://omim.org/)
Summarizing the current knowledge on tissues and signals involved in NPB specification, the following model emerges: the NPB is specified in late blastula/early gastrula stage embryos in a territory characterized by attenuated BMP activity combined with FGF, Wnt/β-Catenin and – in Xenopus laevis – Ror2-mediated β-Catenin independent Wnt signaling. The interaction of these factors leads to moderate activation of BMP/Smad and Wnt/β-Catenin signaling in the NPB ectoderm. Maintenance of NPB identity during gastrulation requires continuously attenuated BMP and also Wnt/β-Catenin signaling in parallel with active β-Catenin independent Wnt/PCP signaling, which controls morphogenetic movements of the dorsal mesoderm and the neuroectoderm and in Xenopus also upregulates the expression of GDF6 in the NPB. At the end of gastrulation, both, BMP and Wnt/β-Catenin signaling are locally upregulated in the NPB and interact with FGF, Notch, Retinoic acid and the transcription factors expressed in the NPB to induce NC fate.
Acknowledgments
The authors thank Dr. Marc Gentzel for critical reading of the manuscript and constructive discussions.
Funding
The authors' original work was funded by the German Research foundation (DFG) under grant numbers SCHA965/7–2 and 965/9–1.
References
- [1].Mayor R, Theveneau E. The neural crest. Development 2013; 140:2247-51; PMID:23674598; http://dx.doi.org/ 10.1242/dev.091751 [DOI] [PubMed] [Google Scholar]
- [2].Zhang D, Ighaniyan S, Stathopoulos L, Rollo B, Landman K, Hutson J, Newgreen D. The neural crest: A versatile organ system. Birth Defect Res C 2014; 102:275-98; PMID:25227568; http://dx.doi.org/ 10.1002/bdrc.21081 [DOI] [PubMed] [Google Scholar]
- [3].Simoes-Costa M, Bronner ME. Insights into neural crest development and evolution from genomic analysis. Genome Res 2013; 23:1069-80; PMID:23817048; http://dx.doi.org/ 10.1101/gr.157586.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 2009; 136:771-79; PMID:19176585; http://dx.doi.org/ 10.1242/dev.029017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ezin AM, Fraser SE, Bronner-Fraser M. Fate map and morphogenesis of presumptive neural crest and dorsal neural tube. Dev Biol 2009; 330:221-36; PMID:19332051; http://dx.doi.org/ 10.1016/j.ydbio.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tam PP. Regionalisation of the mouse embryonic ectoderm: Allocation of prospective ectodermal tissues during gastrulation. Development 1989; 107:55-67; PMID:2627894 [DOI] [PubMed] [Google Scholar]
- [7].Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: Mechanisms of Xslug induction. Dev Biol 1996; 177:580-89; PMID:8806833; http://dx.doi.org/ 10.1006/dbio.1996.0187 [DOI] [PubMed] [Google Scholar]
- [8].Liem KF, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 1995; 82:969-79; PMID:7553857; http://dx.doi.org/ 10.1016/0092-8674(95)90276-7 [DOI] [PubMed] [Google Scholar]
- [9].Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development 1995; 121:525-38; PMID:7768190 [DOI] [PubMed] [Google Scholar]
- [10].Basch ML, Bronner-Fraser M, García-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 2006; 441:218-22; PMID:16688176; http://dx.doi.org/ 10.1038/nature04684 [DOI] [PubMed] [Google Scholar]
- [11].Leung AW, Murdoch B, Salem AF, Prasad MS, Gomez GA, García-Castro MI. Wnt/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 2016; 143:398-410; PMID:26839343; http://dx.doi.org/ 10.1242/dev.130849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bonstein L, Elias S, Frank D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev Biol 1998; 193:156-68; PMID:9473321; http://dx.doi.org/ 10.1006/dbio.1997.8795 [DOI] [PubMed] [Google Scholar]
- [13].Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol 1998; 198:319-29; PMID:9659936; http://dx.doi.org/ 10.1016/S0012-1606(98)80008-0 [DOI] [PubMed] [Google Scholar]
- [14].Monsoro-Burq A-H, Fletcher RB, Harland RM. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 2003; 130:3111-24; PMID:12783784; http://dx.doi.org/ 10.1242/dev.00531 [DOI] [PubMed] [Google Scholar]
- [15].Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the lateral neural plate is dependent on a Wnt-mediated signal from posterior nonaxial mesoderm. Dev Biol 1999; 212:366-80; PMID:10433827; http://dx.doi.org/ 10.1006/dbio.1999.9319 [DOI] [PubMed] [Google Scholar]
- [16].Steventon B, Mayor R. Early neural crest induction requires an initial inhibition of Wnt signals. Dev Biol 2012; 365:196-207; PMID:22394485; http://dx.doi.org/ 10.1016/j.ydbio.2012.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 2009; 136:73-83; PMID:19060333; http://dx.doi.org/ 10.1242/dev.025890 [DOI] [PubMed] [Google Scholar]
- [18].Seo HC, Saetre BO, Håvik B, Ellingsen S, Fjose A. The zebrafish Pax3 and Pax7 homologues are highly conserved, encode multiple isoforms and show dynamic segment-like expression in the developing brain. Mech Dev 1998; 70:49-63; PMID:9510024; http://dx.doi.org/ 10.1016/S0925-4773(97)00175-5 [DOI] [PubMed] [Google Scholar]
- [19].Monsoro-Burq A-H, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell 2005; 8:167-78; PMID:15691759; http://dx.doi.org/ 10.1016/j.devcel.2004.12.017 [DOI] [PubMed] [Google Scholar]
- [20].Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: Involvement of FGF and BMP activity. Mech Dev 1999; 82:51-66; PMID:10354471; http://dx.doi.org/ 10.1016/S0925-4773(99)00013-1 [DOI] [PubMed] [Google Scholar]
- [21].Ruffins S, Bronner-Fraser M. A critical period for conversion of ectodermal cells to a neural crest fate. Dev Biol 2000; 218:13-20; PMID:10644407; http://dx.doi.org/ 10.1006/dbio.1999.9555 [DOI] [PubMed] [Google Scholar]
- [22].Basch ML, Selleck MA, Bronner-Fraser M. Timing and competence of neural crest formation. Dev Neurosci 2000; 22:217-27; PMID:10894985; http://dx.doi.org/ 10.1159/000017444 [DOI] [PubMed] [Google Scholar]
- [23].Faure S, Lee MA, Keller T, Ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development 2000; 127:2917-31; PMID:10851136 [DOI] [PubMed] [Google Scholar]
- [24].Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol 2002; 244:44-65; PMID:11900458; http://dx.doi.org/ 10.1006/dbio.2002.0579 [DOI] [PubMed] [Google Scholar]
- [25].Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development 2002; 129:37-52; PMID:11782399. [DOI] [PubMed] [Google Scholar]
- [26].De Robertis EM. Evo-devo: Variations on ancestral themes. Cell 2008; 132:185-95; PMID:18243095; http://dx.doi.org/ 10.1016/j.cell.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tríbulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 2003; 130:6441-52; PMID:14627721; http://dx.doi.org/ 10.1242/dev.00878 [DOI] [PubMed] [Google Scholar]
- [28].Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 1998; 199:93-110; PMID:9676195; http://dx.doi.org/ 10.1006/dbio.1998.8927 [DOI] [PubMed] [Google Scholar]
- [29].Luo T, Matsuo-Takasaki M, Lim JH, Sargent TD. Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int J Dev Biol 2001; 45:681-84; PMID:11461005 [PubMed] [Google Scholar]
- [30].de Crozé N, Maczkowiak F, Monsoro-Burq AH. Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proc Natl Acad Sci USA 2011; 108:155-60; PMID:21169220; http://dx.doi.org/ 10.1073/pnas.1010740107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo T, Matsuo-Takasaki M, Thomas ML, Weeks DL, Sargent TD. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Dev Biol 2002; 245:136-44; PMID:11969261; http://dx.doi.org/ 10.1006/dbio.2002.0621 [DOI] [PubMed] [Google Scholar]
- [32].Nordin K, LaBonne C. Sox5 Is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm. Dev Cell 2014; 31:374-82; PMID:25453832; http://dx.doi.org/ 10.1016/j.devcel.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hawley SH, Wünnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev 1995; 9:2923-35; PMID:7498789; http://dx.doi.org/ 10.1101/gad.9.23.2923 [DOI] [PubMed] [Google Scholar]
- [34].Clement JH, Fettes P, Knöchel S, Lef J, Knöchel W. Bone morphogenetic protein 2 in the early development of Xenopus laevis. Mech Dev 1995; 52:357-70; PMID:8541221; http://dx.doi.org/ 10.1016/0925-4773(95)00413-U [DOI] [PubMed] [Google Scholar]
- [35].Patthey C, Gunhaga L, Edlund T. Early development of the central and peripheral nervous systems is coordinated by Wnt and BMP signals. PLoS One 2008; 3:e1625; PMID:18286182; http://dx.doi.org/ 10.1371/journal.pone.0001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu MY, Ramel M-C, Howell M, Hill CS. SNW1 is a critical regulator of spatial BMP activity, neural plate border formation, and neural crest specification in vertebrate embryos. PLoS Biol 2011; 9:e1000593; PMID:21358802; http://dx.doi.org/ 10.1371/journal.pbio.1000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Linker C, De Almeida I, Papanayotou C, Stower M, Sabado V, Ghorani E, Streit A, Mayor R, Stern CD. Cell communication with the neural plate is required for induction of neural markers by BMP inhibition: Evidence for homeogenetic induction and implications for Xenopus animal cap and chick explant assays. Dev Biol 2009; 327:478-86; PMID:19162002; http://dx.doi.org/ 10.1016/j.ydbio.2008.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development 2009; 136:3267-78; PMID:19736322; http://dx.doi.org/ 10.1242/dev.036954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene network. Proc Natl Acad Sci USA 2008; 105:20083-88; PMID:19104059; http://dx.doi.org/ 10.1073/pnas.0806009105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Luo T, Lee Y-H, Saint-Jeannet J-P, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc Natl Acad Sci USA 2003; 100:532-37; PMID:12511599; http://dx.doi.org/ 10.1073/pnas.0237226100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sato T, Sasai N, Sasai Y. Neural crest determination by co-activation of Pax3 and Zic1 genes in Xenopus ectoderm. Development 2005; 132:2355-63; PMID:15843410; http://dx.doi.org/ 10.1242/dev.01823 [DOI] [PubMed] [Google Scholar]
- [42].Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell 2008; 15:23-36; PMID:18606138; http://dx.doi.org/ 10.1016/j.devcel.2008.05.007 [DOI] [PubMed] [Google Scholar]
- [43].van Amerongen R. Alternative Wnt Pathways and Receptors. Cold Spring Harb Perspect Biol 2012; 4:a007914-a007914; PMID:22935904; http://dx.doi.org/ 10.1101/cshperspect.a007914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem 2009; 284:30167-76; PMID:19720827; http://dx.doi.org/ 10.1074/jbc.M109.041715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Witte F, Bernatik O, Kirchner K, Masek J, Mahl A, Krejci P, Mundlos S, Schambony A, Bryja V, Stricker S. Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J 2010; 24:2417-26; PMID:20215527; http://dx.doi.org/ 10.1096/fj.09-150615 [DOI] [PubMed] [Google Scholar]
- [46].Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 2007; 12:779-92; PMID:17488628; http://dx.doi.org/ 10.1016/j.devcel.2007.02.016 [DOI] [PubMed] [Google Scholar]
- [47].Feike AC, Rachor K, Gentzel M, Schambony A. Wnt5a/Ror2-induced upregulation of xPAPC requires xShcA. Biochem Biophys Res Commun 2010; 400:500-6; PMID:20732301; http://dx.doi.org/ 10.1016/j.bbrc.2010.08.074 [DOI] [PubMed] [Google Scholar]
- [48].Medina A, Swain RK, Kuerner K-M, Steinbeisser H. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. EMBO J 2004; 23:3249-58; PMID:15272309; http://dx.doi.org/ 10.1038/sj.emboj.7600329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. EMBO J 2004; 23:3259-69; PMID:15297873; http://dx.doi.org/ 10.1038/sj.emboj.7600332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schille C, Bayerlová M, Bleckmann A, Schambony A. Ror2 signaling is required for local upregulation of GFD6 and activation of BMP signaling at the neural plate border. Development 2016; 143:3182-94; PMID:27578181; http://dx.doi.org/ 10.1242/dev.135426 [DOI] [PubMed] [Google Scholar]
- [51].Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 1998; 12:2610-22; PMID:9716412; http://dx.doi.org/ 10.1101/gad.12.16.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ossipova O, Sokol SY. Neural crest specification by noncanonical Wnt signaling and PAR-1. Development 2011; 138:5441-50; PMID:22110058; http://dx.doi.org/ 10.1242/dev.067280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: Cellular orientation by facing the wind (Wnt). Annu Rev Cell Dev Biol 2015; 31:623-46; PMID:26566118; http://dx.doi.org/ 10.1146/annurev-cellbio-100814-125315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Londin ER, Niemiec J, Sirotkin HI. Chordin, FGF signaling, and mesodermal factors cooperate in zebrafish neural induction. Dev Biol 2005; 279:1-19; PMID:15708554; http://dx.doi.org/ 10.1016/j.ydbio.2004.11.016 [DOI] [PubMed] [Google Scholar]
- [55].Hong C-S, Park B-Y, Saint-Jeannet J-P. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 2008; 135:3903-10; PMID:18997112; http://dx.doi.org/ 10.1242/dev.026229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stuhlmiller TJ, García-Castro MI. FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development 2012; 139:289-300; PMID:22129830; http://dx.doi.org/ 10.1242/dev.070276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nichane M, de Crozé N, Ren X, Souopgui J, Monsoro-Burq AH, Bellefroid EJ. Hairy2-Id3 interactions play an essential role in Xenopus neural crest progenitor specification. Dev Biol 2008; 322:355-67; PMID:18721802; http://dx.doi.org/ 10.1016/j.ydbio.2008.08.003 [DOI] [PubMed] [Google Scholar]
- [58].Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Dev Dyn 2009; 238:1467-79; PMID:19322767; http://dx.doi.org/ 10.1002/dvdy.21913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schille C, Heller J, Schambony A. Differential requirement of bone morphogenetic protein receptors Ia (ALK3) and Ib (ALK6) in early embryonic patterning and neural crest development. BMC Dev Biol 2016; 16:1-17; PMID:26780949; http://dx.doi.org/ 10.1186/s12861-016-0101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wilson SI, Rydström A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 2001; 411:325-30; PMID:11357137; http://dx.doi.org/ 10.1038/35077115 [DOI] [PubMed] [Google Scholar]
- [61].Stern CD. Neural induction: Old problem, new findings, yet more questions. Development 2005; 132:2007-21; PMID:15829523; http://dx.doi.org/ 10.1242/dev.01794 [DOI] [PubMed] [Google Scholar]
- [62].Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol 2002; 245:187-99; PMID:11969265; http://dx.doi.org/ 10.1006/dbio.2002.0641 [DOI] [PubMed] [Google Scholar]
- [63].Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn 2004; 229:668-76; PMID:14991722; http://dx.doi.org/ 10.1002/dvdy.10491 [DOI] [PubMed] [Google Scholar]
- [64].Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 2008; 136:73-83; PMID:19060333; http://dx.doi.org/ 10.1242/dev.025890 [DOI] [PubMed] [Google Scholar]
- [65].Ragland JW, Raible DW. Signals derived from the underlying mesoderm are dispensable for zebrafish neural crest induction. Dev Biol 2004; 276:16-30; PMID:15531361; http://dx.doi.org/ 10.1016/j.ydbio.2004.08.017 [DOI] [PubMed] [Google Scholar]
- [66].Shi J, Severson C, Yang J, Wedlich D, Klymkowsky MW. Snail2 controls mesodermal BMP/Wnt induction of neural crest. Development 2011; 138:3135-45; PMID:21715424; http://dx.doi.org/ 10.1242/dev.064394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chang C, Hemmati-Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development 1999; 126:3347-57; PMID:10393114 [DOI] [PubMed] [Google Scholar]
- [68].Délot E, Kataoka H, Goutel C, Yan YL, Postlethwait J, Wittbrodt J, Rosa FM. The BMP-related protein radar: A maintenance factor for dorsal neuroectoderm cells? Mech Dev 1999; 85:15-25; PMID:10415343; http://dx.doi.org/ 10.1016/S0925-4773(99)00026-X [DOI] [PubMed] [Google Scholar]
- [69].Reichert S, Randall RA, Hill CS. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development 2013; 140:4435-44; PMID:24089471; http://dx.doi.org/ 10.1242/dev.098707 [DOI] [PubMed] [Google Scholar]
- [70].Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol 2010; 26:581-603; PMID:19575671; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Selleck MA, García-Castro MI, Artinger KB, Bronner-Fraser M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development 1998; 125:4919-30; PMID:9811576 [DOI] [PubMed] [Google Scholar]
- [72].Carmona-Fontaine C, Acuña G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev Biol 2007; 309:208-21; PMID:17669393; http://dx.doi.org/ 10.1016/j.ydbio.2007.07.006 [DOI] [PubMed] [Google Scholar]
- [73].Betters E, Liu Y, Kjaeldgaard A, Sundström E, García-Castro MI. Analysis of early human neural crest development. Dev Biol 2010; 344:578-92; PMID:20478300; http://dx.doi.org/ 10.1016/j.ydbio.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Patton MA, Afzal AR. Robinow syndrome. J Med Genet 2002; 39:305-10; PMID:12011143; http://dx.doi.org/ 10.1136/jmg.39.5.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Schwabe GC, Trepczik B, Süring K, Brieske N, Tucker AS, Sharpe PT, Minami Y, Mundlos S. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev Dyn 2004; 229:400-10; PMID:14745966; http://dx.doi.org/ 10.1002/dvdy.10466 [DOI] [PubMed] [Google Scholar]
- [76].Asher JH, Sommer A, Morell R, Friedman TB. Missense mutation in the paired domain of PAX3 causes craniofacial-deafness-hand syndrome. Hum. Mutat. 1996; 7:30-35; PMID:8664898; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [77].Tassabehji M, Read AP, Newton VE, Harris R, Balling R, Gruss P, Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature 1992; 355:635-36; PMID:1347148; http://dx.doi.org/ 10.1038/355635a0 [DOI] [PubMed] [Google Scholar]
- [78].Wollnik B, Tukel T, Uyguner O, Ghanbari A, Kayserili H, Emiroglu M, Yuksel-Apak M. Homozygous and heterozygous inheritance of PAX3 mutations causes different types of Waardenburg syndrome. Am. J. Med. Genet. A 2003; 122A:42-45; PMID:12949970; http://dx.doi.org/ 10.1002/ajmg.a.20260 [DOI] [PubMed] [Google Scholar]
- [79].Milunsky JM, Maher TA, Zhao G, Roberts AE, Stalker HJ, Zori RT, Burch MN, Clemens M, Mulliken JB, Smith R, et al.. TFAP2A mutations result in branchio-oculo-facial syndrome. Am J Hum Genet 2008; 82:1171-77; PMID:18423521; http://dx.doi.org/ 10.1016/j.ajhg.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jabs EW, Müller U, Li X, Ma L, Luo W, Haworth IS, Klisak I, Sparkes R, Warman ML, Mulliken JB, et al.. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell 1993; 75:443-50; PMID:8106171; http://dx.doi.org/ 10.1016/0092-8674(93)90379-5 [DOI] [PubMed] [Google Scholar]
- [81].Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, Wall SA, Chrzanowska KH, Maxson RE Jr.. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet 2000; 24:387-90; PMID:10742103; http://dx.doi.org/ 10.1038/74224 [DOI] [PubMed] [Google Scholar]
- [82].van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet 2000; 24:342-43; PMID:10742093; http://dx.doi.org/ 10.1038/74155 [DOI] [PubMed] [Google Scholar]
- [83].van den Boogaard M-J, Créton M, Bronkhorst Y, van der Hout A, Hennekam E, Lindhout D, Cune M, Ploos van Amstel HK. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J Med Genet 2012; 49:327-31; PMID:22581971; http://dx.doi.org/ 10.1136/jmedgenet-2012-100750 [DOI] [PubMed] [Google Scholar]
- [84].Elliott AM, Evans JA. Genotype-phenotype correlations in mapped split hand foot malformation (SHFM) patients. Am. J. Med. Genet. A 2006; 140:1419-27; PMID:16688749; http://dx.doi.org/ 10.1002/ajmg.a.31244 [DOI] [PubMed] [Google Scholar]
- [85].Elliott AM, Evans JA. The association of split hand foot malformation (SHFM) and congenital heart defects. Birth Defects Res A Clin Mol Teratol 2008; 82:425-34; PMID:18383509; http://dx.doi.org/ 10.1002/bdra.20452 [DOI] [PubMed] [Google Scholar]
- [86].Shamseldin HE, Faden MA, Alashram W, Alkuraya FS. Identification of a novel DLX5 mutation in a family with autosomal recessive split hand and foot malformation. J Med Genet 2012; 49:16-20; PMID:22121204; http://dx.doi.org/ 10.1136/jmedgenet-2011-100556 [DOI] [PubMed] [Google Scholar]
- [87].Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 2009; 20:343-55; PMID:19897402; http://dx.doi.org/ 10.1016/j.cytogfr.2009.10.007 [DOI] [PubMed] [Google Scholar]
- [88].Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113:685-700; PMID:12809600; http://dx.doi.org/ 10.1016/S0092-8674(03)00432-X [DOI] [PubMed] [Google Scholar]
- [89].Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149:1192-1205; PMID:22682243; http://dx.doi.org/ 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- [90].Rao TP, Kühl M. An updated overview on Wnt signaling pathways: A prelude for more. Circ Res 2010; 106:1798-1806; PMID:20576942; http://dx.doi.org/ 10.1161/CIRCRESAHA.110.219840 [DOI] [PubMed] [Google Scholar]
- [91].Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: Requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol 2004; 2:E92; PMID:15138495; http://dx.doi.org/ 10.1371/journal.pbio.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]