ABSTRACT
Sepsis is a systemic inflammatory response to infection, with no preventative strategies. In this study, we identify a role for habitual physical activity in the prevention of adipose tissue inflammation induced by a model of sepsis, lipopolysaccharide (LPS). Male C57BL/6J mice (8 weeks old) were housed with access to voluntary wheel running (VWR) or sedentary (SED) for 10 weeks. Mice were then injected with LPS (2 mg/kg) or saline (SAL), and tissues were removed 6 hours post-injection. VWR attenuated body, epididymal adipose tissue (eWAT), and subcutaneous inguinal adipose tissue (iWAT) mass gain, improved glucose tolerance, increased markers of mitochondrial biogenesis in iWAT and eWAT, and increased UCP-1 protein content in iWAT. In iWAT, VWR attenuated the LPS induced increase in mRNA expression of TNF-α, MCP-1, and follistatin, along with phosphorylation of STAT3. In addition, VWR had a main effect for reducing iWAT mRNA expression of IL-1β, IL-6, and SOCS3. In eWAT, VWR had a main effect for reducing mRNA expression of IL-1β, MCP-1, IL-6, and follistatin. Further, VWR increased SOCS3 mRNA expression and phosphorylation of STAT3 in SAL mice, thus the relative change in response to LPS for these markers was attenuated. The protective effect of prior physical activity occurred in conjunction with increases in the protein content of a component of the LPS binding complex, MyD88. Overall, the results from this study demonstrate that habitual physical activity can attenuate the LPS induced inflammatory response in adipose tissue and this occurs to a greater extent in iWAT compare with eWAT.
KEYWORDS: adipose tissue, exercise, inflammation, lipopolysaccharide, physical activity
Introduction
Lipopolysaccharide (LPS) is a component of the cell wall of gram-negative bacteria and is used to model sepsis, which is a systemic inflammatory response to infectious stimuli.1,2 LPS induces an inflammatory cascade by binding to the toll like receptor 4 (TLR4) protein complex, which initiates a response through a myeloid differentiation primary response gene 88 (MyD88) dependent or independent pathway.3 This leads to rapid increases in systemic inflammatory factors such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and interleukin 1-β (IL-1β).4,5 This systemic pro-inflammatory environment induces increases in inflammation at the organ level, including in adipose tissue.6 As adipose tissue is a key contributor in the inflammatory response to numerous metabolic stimuli,7 it is important to understand the etiology of sepsis-induced inflammation in adipose tissue and if there are potential preventative strategies.
In this regard, regular physical activity is known to have numerous beneficial effects on the body,8 many of which occur to adipose tissue. Physical activity and exercise training increase indices of mitochondrial biogenesis in adipose tissue9-12 and induce a thermogenic gene program that is characterized by increases in uncoupling protein 1 (UCP-1) mRNA expression and/or protein content in some9,13,14 but not all studies.15,16 While physical activity can protect against adipose tissue inflammation induced by obesity,15,17 this beneficial anti-inflammatory effect is likely mediated, at least in part, through reducing the accretion of adipose tissue.18 Thus, as the inflammatory cascade induced by sepsis manifests without significant changes in the accretion of adipose tissue, it is important to determine if physical activity or exercise protect against sepsis induced inflammation in adipose tissue.
There is data to suggest that physical activity19-21 or acute exercise22 can modulate the response to LPS. For example, we have recently shown that mice given access to a voluntary running wheel, a model of physical activity, were protected against LPS induced inflammation in the liver.21 This effect was predominantly evident at 6 hours versus 12 hours post LPS, which is a time frame at which systemic levels of pro-inflammatory factors peak.5 Despite these studies,19-22 little is known if a similar protective effect is evident in adipose tissue. Recently, using a cross sectional design, it was reported that persons who were exercise trained (VO2max >55 ml·kg−1·min−1) had an altered response to a low dose (0.3 ng/kg) of LPS over 2 hours of exposure when compare with those who were untrained.6 In subcutaneous adipose tissue from exercise trained individuals, the induction of inducible nitric oxide synthase (iNOS) and TLR4 mRNA expression was decreased, while the induction of IL-6 mRNA expression was increased, and TNF-α mRNA expression was unaltered.6 While this study did not directly assess the protective effects of exercise training per se, the results indicate that training status may alter the response of adipose tissue to LPS-induced inflammation. As this dose of LPS does not elicit a peak inflammatory response, but is required for tolerability in humans, it is possible that a greater protective effect of physical activity or exercise against higher doses of LPS may be found.23 Further, whether there are differences in the effect of physical activity or exercise on the response of subcutaneous and visceral adipose tissue depots to LPS is not known.
In this report, our objective was to determine if habitual physical activity, via VWR, could protect against adipose tissue inflammation induced by LPS in C57BL/6J mice, and if this occurs in a depot specific manner. We hypothesized that both subcutaneous inguinal adipose tissue (iWAT) and visceral epididymal adipose tissue (eWAT) would be protected against LPS-induced inflammation. As it has been suggested that the beneficial effects of physical activity on metabolic health are modulated, at least in part, through alterations in iWAT,9 we postulated that a protective effect of prior physical activity would be more apparent in iWAT.
Methods
Ethics
All procedures in this study adhere to the guidelines of the Canadian Council on Animal Care and were approved by the University of Guelph Animal Care Committee.
Animals
C57BL/6J mice (8 weeks old, male, n = 40) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were individually housed with access to voluntary wheel running (VWR) or in standard cages as sedentary controls (SED) for 10 weeks. The mice used in this study represent a subset of a prior publication from our group.21 All mice had ad libitum access to standard rodent chow from Teklad (Madison, WI; Cat # 7004) and water, were on a 12-hour light dark cycle (9 A.M. to 9 P.M.), and were housed at ∼22°C. Running wheel distances were counted daily using a wheel mounted rotation counter (VDO M3 wired bike computer, Mountain Equipment Co-Op Vancouver, Canada). Food intake was measured weekly during the 10 week period using the amount consumed from the hopper.
Glucose tolerance test
During the final week of VWR, an intraperitoneal glucose tolerance test (GTT) was performed (10 A.M.) on 6 hour fasted mice using an i.p. weight adjusted bolus (2 g·kg−1) of D-glucose. Mice in the VWR group had the wheels removed from their cage at the time of fasting and did not have access to wheels during the GTT. It should be noted that this experimental design allowed the VWR mice access to the wheel from lights off until the time of fasting, and as exercise increases glucose uptake24 it is possible that there may be residual effects of this last bout of wheel running. Blood glucose was measured via tail snip immediately prior to the glucose injection and at 15, 30, 45, 60, 90, and 120 minutes post injection using a handheld Freestyle Lite glucometer and glucose strips (Abbott Laboratories; Abbott Park, IL.).
Lipopolysaccharide injection and tissue collection
LPS, purchased from Sigma-Aldrich (St. Louis, MO; Cat # L2630), was injected (i.p.) at a dose of 2 mg/kg into awake, non-anesthetized mice starting at 9 A.M. and control mice were injected with saline (SAL). This dose has been shown to elicit a near peak inflammatory response, at least in the liver,23 and has been used by other groups to induce an inflammatory response.25 VWR mice had wheels locked 24 hours before the LPS injection. After LPS injection, all mice had ab libitum access to food and water, and were monitored for signs of septic shock. At 6 hours post-LPS injection, a time frame at which this dose induces near peak inflammation in circulation,5 tail blood glucose was measured and then a 0.5 U·kg−1 dose of insulin was injected and blood glucose was monitored for 20 minutes.25 These results have been published, and demonstrate that VWR attenuated LPS induced impairments in insulin tolerance.21 Given this short exposure to insulin it seems unlikely that this would have influenced the changes in inflammatory markers in the current study. Mice were then anesthetized with sodium pentobarbital (∼5 mg·100 g bw−1) upon which iWAT and eWAT were removed and immediately frozen in liquid nitrogen, then stored at −80°C.
Western blotting
Homogenization of tissue was completed using a FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA) in a 3 x cocktail of cell lysis buffer supplemented with phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Sigma-Aldrich, Oakville, ON, Canada). Western blotting procedures were completed as previously described.26 Briefly, proteins were transferred onto a nitrocellulose membrane, incubated with indicated primary antibodies overnight, the corresponding secondary antibody for 1 hour, and imaged using enhanced chemiluminescence on a FluorChem HD imaging system (α Innotect, Santa Clara, CA). Antibodies used were purchased from AbCam (Toronto, ON, Canada; β-actin Cat # 8227; Citrate synthase (CS) Cat # ab129095; Anti-Ubiquinol-Cytochrome C Reductase (CORE1) Cat # 110252; Cytochrome C Oxidase Subunit IV (COXIV) Cat # ab16056; GAPDH Cat # 8245; uncoupling protein 1 (UCP-1) Cat # 10983), Millipore (Etobicoke, ON, Canada; Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) Cat # AB3242, Mitosciences (Toronto, ON, Canada; Cytochrome C (CytC) Cat # MSA06; Pyruvate dehydrogenase subunit E1α (PDH-E1α) Cat # MSP03); and Cell Signaling (Danvers, MA; MyD88 Cat # 4283; pSTAT3 Cat # 9138; STAT3 Cat # 8768). β-actin, GAPDH, and/or ponceau staining were used to verify equal loading (where depicted),27 whereas pSTAT3 was expressed relative to STAT3. In addition, it should be noted that the specificity of the PGC-1α antibody has been validated using samples from PGC-1α knockout mice.28
Real-time quantitative PCR
RNA was extracted using an RNeasy Mini Kit (Cat # 74104, QIAGEN Toronto, ON). cDNA was synthesized using the Superscript II Reverse Transcriptase kit (Cat # 18064–014, from Thermo Fisher Scientific Waltman, MA) and diluted 1:15 using RNase free water. PCR plates were prepared using PerfeCTa qPCR FastMix II Low ROX (Cat # 95120 Quantabio Beverly, MA), RNase free water, TaqMan Gene Expression Assays for each gene of interest (Cat # in Table 1 from Thermo Fisher Scientific Burlington, ON CAN) and 5 ul of cDNA. Analysis was completed using the 2−ΔΔCT method,29 with samples expressed relative to the endogenous control GAPDH, which did not change with VWR or LPS. Data was normalized to the SED SAL group and analyzed using a 2-way ANOVA (large figure). In addition, to account for the basal difference between SED and VWR mice, we calculated the relative change to LPS by normalizing the SED LPS and VWR LPS group to the respective SAL group (small figure).
Table 1.
TaqMan gene expression assays. Purchased from Thermo Fisher Scientific (Cat # 4331182).
| Target | TaqMan probe |
|---|---|
| Gapdh | Mm99999915_g1 |
| TNF-α | Mm00443258_m1 |
| IL-1β | Mm00434228_m1 |
| Ccl2 (MCP-1) | Mm00441242_m1 |
| IL-6 | Mm00446190_m1 |
| Socs3 | Mm00545913_s1 |
| Adgre1 (F4/80) | Mm00802529_m1 |
| Tlr4 | Mm00445273_m1 |
| FST | Mm00514982_m1 |
Statistical analysis
Data were analyzed using an unpaired t-test, 2-way ANOVA, or 2-way ANOVA with repeated measures. Data was first assessed for normality and homogeneity of variance, and when not met the data was transformed (log10) or for t-tests a Mann-Whitney U-test was used. The Fisher's LSD post-hoc test was used for 2-way ANOVA interactions. All data are presented as mean ± standard error at a significance level of p < 0.05. For two-way ANOVA results, significance is shown as main effect, or for post-hoc testing following a significant interaction. Statistical tests were completed using Sigma Plot version 11.0 (San Jose, CA) and graphing was done using Graph Pad version 6.2 (La Jolla, CA).
Results
VWR attenuates body mass gain, improves glucose tolerance, and induces indices of mitochondrial biogenesis in adipose tissue
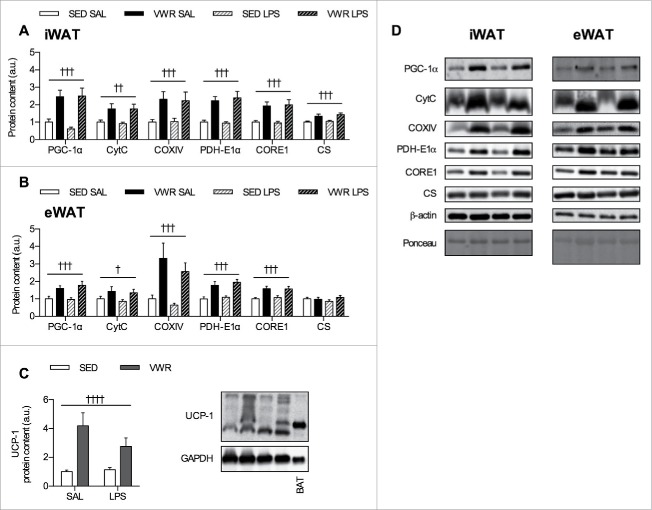
Mice in the VWR group ran an average of 3.68 ± 0.32 km/day over the 10-week period. Despite greater food intake, mice in the VWR group weighed less and had reduced eWAT and iWAT mass, along with improved glucose tolerance (Fig. 1A–E). As previous data from our group and that from others have shown that exercise training (swimming,11 treadmill running30) and VWR9 increase indices of mitochondrial biogenesis, we measured the protein content of PGC-1α, a transcriptional co-activator and master regulator of mitochondrial proteins,31 as well as other mitochondrial proteins in iWAT and eWAT. In iWAT, there was main effect of VWR for increasing the protein content of numerous markers of mitochondrial biogenesis including PGC-1α (p < 0.001), CytC (p < 0.01), COXIV (p < 0.001), PDH-E1α (p < 0.001), CORE1 (p < 0.001), and CS (p < 0.001) (Fig. 2A). In eWAT, there was a main effect of VWR for increasing the protein content of PGC-1α (p < 0.001), CytC (p < 0.05), COXIV (p < 0.001), PDH-E1α (p < 0.001), and CORE1 (p < 0.001) (Fig. 2B). Next, as exercise training and VWR are able to increase UCP-1 mRNA expression and/or protein content in iWAT,9,14,32 we measured the protein content of UCP-1. UCP-1 protein content was higher in iWAT with VWR (2-way ANOVA main effect p < 0.0001) (Fig. 2C). We were unable to detect UCP-1 in eWAT (data not shown). There was no effect of LPS injection in either depot for markers of mitochondrial biogenesis or UCP-1. Together, this demonstrates that VWR increases indices of mitochondrial biogenesis in iWAT and eWAT, and shows an effect of physical activity on adipose tissue in our VWR model.
Figure 1.
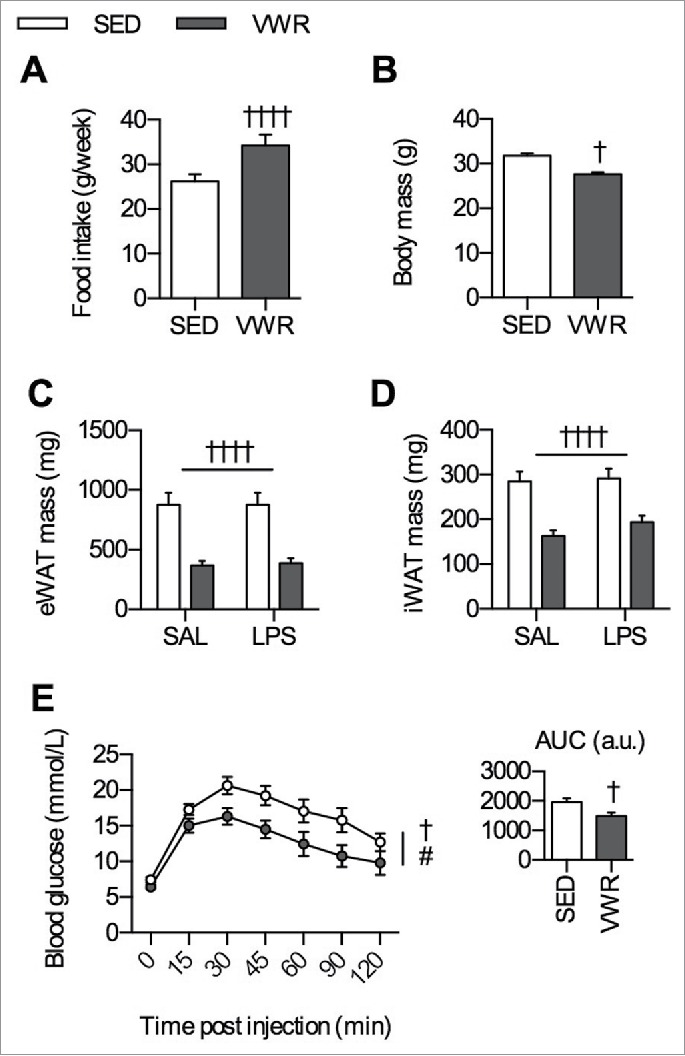
Effects of 10 weeks of VWR. A) Average food intake (g/week) and B) final body mass (g) (n = 20/group). C) eWAT mass (mg), D) iWAT mass (mg), and D) glucose tolerance test curve with inset indicating the area under the curve (n = 10/group). All data are presented as mean ± sem. Significance is displayed as †p < 0.05 and ††††p < 0.0001 for an effect of VWR and #p < 0.05 for an effect of time. Where a 2-way ANOVA was used a flat bar indicates a main effect.
Figure 2.
Effects of VWR on indices of mitochondrial biogenesis and adipose tissue browning. VWR induces increases in the protein content of markers of mitochondrial biogenesis in A) iWAT and B) eWAT. C) UCP-1 protein content in iWAT, with representative western blots including annotations for brown adipose tissue (BAT) as a positive control. D) Representative western blots for A) and B). Data is presented as mean ± sem (n = 7–10/group). A two-way ANOVA main effect of VWR (indicated by flat bar) has significance displayed as ††p < 0.01, †††p < 0.001, and ††††p < 0.0001.
VWR attenuates LPS induced increases in inflammatory marker gene expression to a greater degree in iWAT vs. eWAT
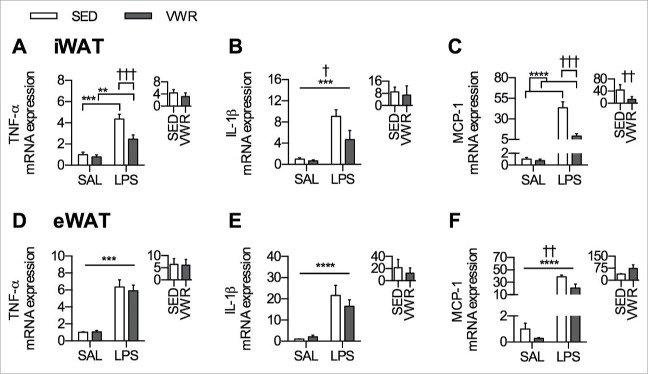
As TNF-α, IL-1β, and MCP-1 are markers of adipose tissue inflammatory status,33 changes in the mRNA expression of these genes were assessed in iWAT and in eWAT following SAL or LPS injection. In iWAT, VWR attenuated the LPS induced increase in TNF-α (p < 0.001) (Fig. 3A), and there was a main effect of VWR for reducing IL-1β (p < 0.05) with no differences in the response to LPS (Fig. 3B). In addition, there was an attenuation of the LPS induced increase in iWAT MCP-1 mRNA expression with VWR (p < 0.001), which was also significant when comparing the relative change within each group (p < 0.01) (Fig. 3C). In contrast to this anti-inflammatory effect of VWR observed in iWAT, there was no difference in the mRNA expression of TNF-α or IL-1β in eWAT (Fig. 3D and Fig. 3E), and only a main effect of VWR for reducing the mRNA expression of MCP-1 (p < 0.01) (Fig. 3F).
Figure 3.
Inflammatory gene expression in iWAT and eWAT. iWAT A) TNF-α mRNA expression, B) IL-1β mRNA expression, and C) MCP-1 mRNA expression. eWAT D) TNF-α mRNA expression, E) IL-1β mRNA expression, and F) MCP-1 mRNA expression. The inset figure in each panel indicates the relative change in mRNA expression induced by LPS within SED and VWR groups. Data is presented as mean ± sem (n = 5–8/group). A two-way ANOVA main effect (indicated by a flat bar), post-hoc testing of significant interactions (indicated by lines with ticks), or results of unpaired t-test (relative change inset) have significance displayed for LPS as **p < 0.01, ***p < 0.001, and ****p < 0.0001 and for VWR as †p < 0.05, ††p < 0.01, †††p < 0.001.
VWR alone, and in response to LPS, alters indices of IL-6 signaling in both iWAT and eWAT
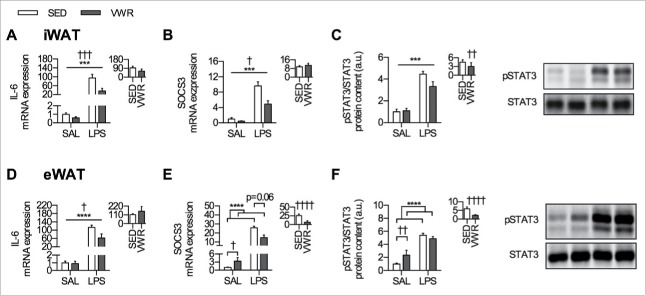
The IL-6 signaling pathway has been reported to be upregulated in adipose tissue in response to LPS,34 therefore we assessed the mRNA expression of IL-6 and SOCS3, along with the phosphorylation of STAT3 (downstream effector of IL-6 signaling). LPS induced increases in all indices of IL-6 signaling in both iWAT and eWAT. In iWAT, there was a main effect of VWR for reducing IL-6 and SOCS3 mRNA expression (p < 0.001 and p < 0.05, respectively) (Fig. 4A and Fig. 4B), and for attenuating the increase in phosphorylation of STAT3 when comparing the relative change within each group (Fig. 4C). In eWAT, there was a main effect of VWR for reducing IL-6 mRNA expression (p < 0.05) (Fig. 4D). VWR led to higher eWAT SOCS3 mRNA in SAL mice (p < 0.05), and only a trend for attenuating the LPS induced increase (p = 0.06) (Fig. 4E). When comparing the relative change of SOCS3 mRNA expression within each group, VWR mice had an attenuated response to LPS (p < 0.0001) (Fig. 4E). VWR led to an increase in phosphorylation of STAT3 in SAL injected mice (p < 0.01) but did not alter the absolute response to LPS, thus when comparing the relative change within each group the response to LPS was attenuated (p < 0.0001) (Fig. 4F).
Figure 4.
IL-6 signaling in eWAT and iWAT. iWAT mRNA expression of A) IL-6, B) SOCS3, along with the protein content of C) pSTAT3/STAT3. eWAT mRNA expression of D) IL-6, E) SOCS3, along with the protein content of F) pSTAT3/STAT3. Representative blots for pSTAT3 and STAT3 are shown beside each panel, and the inset figure indicates the relative change induced by LPS within SED and VWR groups. Data is presented as mean ± sem (n = 5–10/group). A two-way ANOVA main effect (indicated by a flat bar), post-hoc testing of significant interactions (indicated by lines with ticks), or results of unpaired t-test (relative change inset) have significance displayed for LPS as ***p < 0.001, and ****p < 0.0001 and for VWR as †p < 0.05, ††p < 0.01, †††p < 0.001, and ††††p < 0.0001.
VWR increases the protein content of MyD88, attenuates the LPS induced increase in FST mRNA expression in iWAT and eWAT, and reduces mRNA expression of F4/80 in eWAT
To identify potential mechanisms that may be mediating the altered inflammatory and IL-6 signaling in the VWR group in response to LPS, we measured components of the TLR4-MyD88 complex. The mRNA expression of TLR4 was not different in any group (Fig. 5A–B). Both VWR and LPS increased the protein content of MyD88, such that in iWAT and eWAT there was a main effect of VWR and LPS for increasing levels of MyD88 (p < 0.01 and p < 0.05 respectively) (Fig. 5C–D). There was no effect of VWR on the response to LPS.
Figure 5.
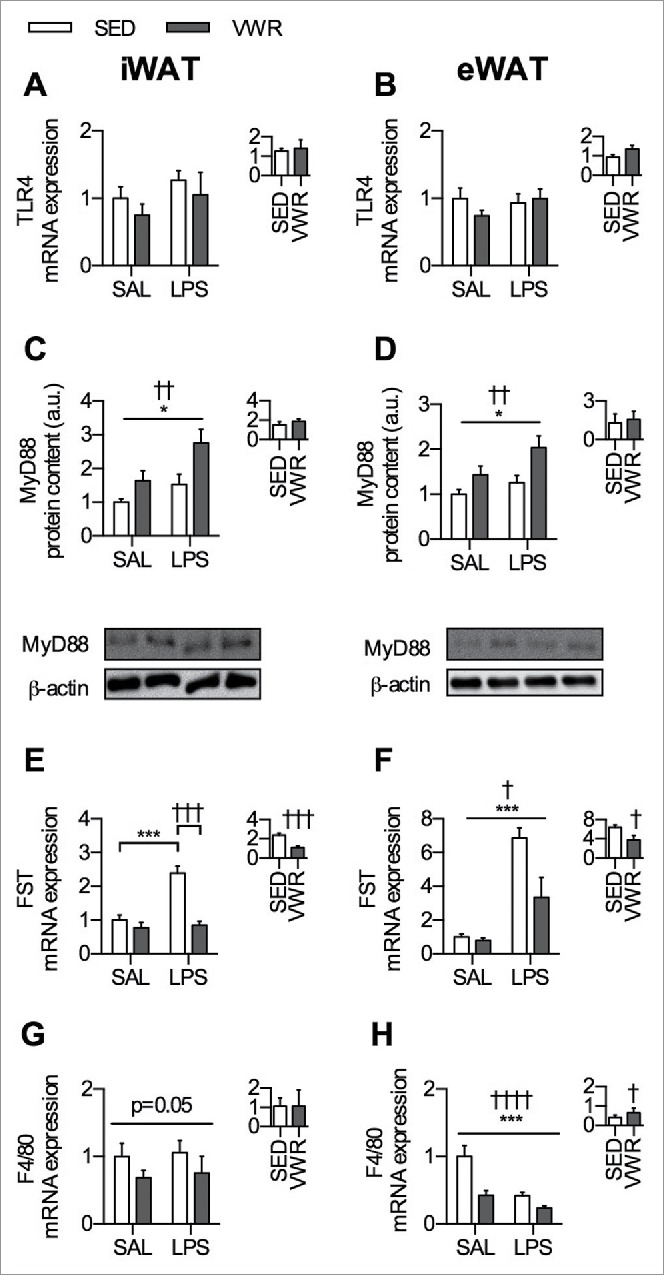
Potential molecular targets that may mediate the protective effects of VWR against LPS induced inflammation. TLR4 mRNA expression in A) iWAT and B) eWAT. MyD88 protein content in C) iWAT and D) eWAT with representative blots shown below. FST mRNA expression in E) iWAT and F) eWAT. F4/80 mRNA expression in G) iWAT and H) eWAT. The inset figure within each panel indicates the relative change induced by LPS within SED and VWR groups. Data is presented as mean ± sem (n = 6–10/group). A two-way ANOVA main effect (indicated by a flat bar) and post-hoc testing of significant interactions (indicated by lines with ticks) have significance displayed for LPS as *p < 0.05 and ***p < 0.001 and for VWR as †p < 0.05, ††p < 0.01, †††p < 0.001, or ††††.0001.
Follistatin (FST) is increased in circulation after LPS exposure21 and has been shown to inhibit inflammation,35 thus we wanted to determine if FST mRNA expression in adipose tissue was altered by LPS and/or VWR. In iWAT, LPS induced an increase in FST mRNA expression in SED mice (p < 0.001), whereas VWR completely attenuated this response (p < 0.001) (Fig. 5E). In eWAT, both SED and VWR mice had LPS induced increases in FST mRNA expression (p < 0.001), and there was a main effect for reductions in FST mRNA expression with VWR (p < 0.05) (Fig. 5F). When comparing the relative change within each group, VWR mice had an attenuated increase in eWAT FST mRNA expression (p < 0.05) (Fig. 5F).
LPS is known to activate macrophage-mediated inflammation36 and thus as an additional analysis, we assessed gene markers of macrophage content in adipose tissue. In iWAT, VWR led to overall lower mRNA expression of F4/80, however this was not significant (2 way ANOVA main effect of VWR, p = 0.05) (Fig. 5G). In eWAT, there was a main effect of VWR (p < 0.0001) and LPS (p < 0.001) to reduce F4/80 mRNA expression (Fig. 5H). When comparing the relative change in response to LPS between SED and VWR mice, VWR attenuated the LPS mediated reduction in F4/80 (p < 0.01) (Fig. 5H).
Discussion
We demonstrate that habitual physical activity, in the form of VWR, attenuates LPS induced inflammation in iWAT and eWAT of C57BL/6J mice. When VWR mice were challenged with LPS, the protective effects in iWAT appeared to be slightly more pronounced than those in eWAT. In iWAT, VWR was able to attenuate the LPS induced increase in TNF-α and MCP-1 mRNA expression, whereas this was not apparent in eWAT. We also assessed indices of IL-6 signaling and in iWAT there was a general trend for VWR mice to have lower levels of these markers, however in eWAT this signaling pathway appeared to be increased in VWR mice injected with SAL and attenuated in LPS injected mice. These depot specific effects do not appear to be explained by a lack of a physical activity effect in eWAT, as the induction of mitochondrial proteins was readily apparent in both adipose tissue depots. In our previous publication21 we measured the levels of systemic inflammatory markers in this sub-set of mice, and found that VWR only attenuated the LPS induced increase in IL-1β, but not IL-6, MCP-1, IFN-γ, TNF-α, or IL-10, suggesting that VWR altered the inflammatory response directly in adipose tissue. If this protective effect of VWR on adipose tissue inflammation occurs across multiple doses of LPS, or if it leads to improved mortality and beneficial effects on other clinical outcomes, is not known.
To our knowledge only a single study has assessed the protective effect of physical activity against LPS induced inflammation in adipose tissue of humans.6 In a cross-sectional study design, Olesen et al.6 observed an increase in IL-6 mRNA expression in response to LPS in subcutaneous adipose tissue of trained subjects, an effect that was not recapitulated, albeit in mice, in the current investigation. Although the results of our study and that of Olesen et al.6 are divergent, it is difficult to directly compare this study to ours due to differences in species examined.37 Further, we used a significantly larger dose of LPS (2 mg/kg vs 0.3 ng/kg), which at least in mice is known to induce near peak inflammation,23 and the time of assessment post–LPS treatment was also slightly different between studies.
In our study, we observed that iWAT from VWR mice appeared to be more protected against LPS inflammation than eWAT. The reason why this occurred is likely not explained by a lack of physical activity effect, as both depots had reductions in adiposity and increases in indices of mitochondrial biogenesis. Moreover, VWR increased MyD88 and reduced F4/80 expression to a similar degree in both depots. While we currently do not have an explanation for the depot specific protective effect of prior physical activity, others have reported greater beneficial effects of physical activity in iWAT compared with eWAT. For example, Stanford et al. found that the transplantation of iWAT but not visceral adipose tissue into the abdominal cavity improved glucose homeostasis in the recipient animals.9 Together these findings highlight an intriguing depot specific effect of physical activity on adipose tissue metabolism that warrants further investigation.
In support of our data, others have shown that 6 weeks of VWR38 and exercise training30 protect against acute stressors. For example, our group has shown that exercise training offers protection against adipose tissue inflammation induced by the β-3 adrenergic agonist CL 316, 243.30 In this study, protective effects were primarily observed in eWAT for indices of IL-6 signaling as marked by the mRNA expression of IL-6 and SOCS3, along with the mRNA expression of TNF-α, yet a similar effect did not occur in iWAT. Others38 have demonstrated that 6 weeks of VWR alters the response to acute tail shock stress in rats. They found that VWR led to an increase in the protein content of MCP-1, IL-6, and IL-10, and a decrease in the ratio of pro-inflammatory to anti-inflammatory proteins (e.g. IL-1β and TNF-α to IL-10). Together, these findings suggest that physical activity and exercise, offers protection against a variety of acute stressors.
The results of our study add to a large body of evidence suggesting that physical activity and exercise have anti-inflammatory properties.39 For instance, 12 weeks of VWR lowered the mRNA expression of inflammatory markers (TNF-α, IL-6, and MCP-1) in adipose tissue from obese rats, with a slightly greater effect reported in visceral compared with subcutaneous adipose tissue.15 As these effects occur in parallel with a reduction in adiposity, it is likely that at least a portion of the reductions in inflammatory markers are secondary to alterations in adipose tissue accretion.15,17 In this regard, a recent study from Welly and colleagues demonstrated that caloric restriction as a means to match the reduction in adiposity induced by VWR, led to similar effects on inflammatory gene expression when rats were fed a high fat diet.16 The results from our current study support and add to these previous studies, as we observed that VWR attenuated the LPS-mediated induction of TNF-α and MCP-1 mRNA expression in iWAT providing some evidence that the effects of VWR go beyond the attenuation of weight gain. Intriguingly, the effects of physical activity are transient as cessation of VWR for only 1 week leads to an increase in body mass and adiposity, along with promotion of a pro-inflammatory environment in adipose tissue.40
The potential mechanistic basis for the protective effects of VWR against LPS induced adipose tissue inflammation are likely multifaceted. We hypothesized that VWR would modulate the LPS binding complex, namely TLR4 and MyD88. We found that there were no differences in the mRNA expression of TLR4, but VWR led to increases in the protein content of MyD88 in both iWAT and eWAT. These results initially appear to be counter intuitive, as MyD88 null mice have a blunted response to endotoxin41 and are protected from hindlimb unloading induced glucose intolerance and inflammation.42 Furthermore, myeloid and endothelial cell specific deletion of MyD88 attenuates some of the deleterious effects of a high fat diet.43 However this is not always the case, as there is evidence to suggest MyD88 null mice have an exaggerated response to high fat diet-induced increases in leptin, insulin, cholesterol and indices of liver inflammation.44 To our knowledge, there is no other report of the effects of physical activity or exercise on the protein content of MyD88 in adipose tissue, and therefore the physiological relevance of the increased MyD88 in both iWAT and eWAT with VWR warrants future investigation. As a secondary assessment, we considered the role of FST as a potential molecular mediator as others have shown that FST increases in circulation after LPS exposure and can act to reduce inflammation.35 While we did not observe differences in the circulating levels of FST between SED and VWR mice after LPS (previously published21), we found an attenuated mRNA expression of FST in response to LPS in VWR mice, particularly in iWAT. As FST levels are increased by, and have been shown to protect against, LPS-induced inflammation35 we speculate that the blunted induction of FST mRNA expression is secondary to an attenuation of LPS-induced inflammation in adipose tissue from VWR mice.
In addition to the protective effects of physical activity against LPS induced inflammation, we demonstrate that VWR increases markers of mitochondrial biogenesis in both depots. Interestingly, this included an increase in the protein content of PGC-1α, a transcriptional co-activator that has been suggested to possess anti-inflammatory properties. In one report, 3 d of treadmill exercise were completed after LPS injection as a treatment approach.45 They found that this protected mice against the deleterious effects of LPS, and occurred in parallel with increases in PGC-1α. Similarly, loss of PGC-1α, albeit in muscle, potentiates the pro-inflammatory environment of aging.46 Therefore, as VWR increased PGC-1α in both iWAT and eWAT, it is possible that this may have mediated some of the protective effects we see against LPS induced inflammation. Clearly further work is needed to elucidate the mechanisms through which prior physical activity protects against LPS-induced inflammation.
Although we provide novel data for the protective effect of VWR against LPS induced inflammation in adipose tissue, we must note some potential limitations. First, the removal of running wheels 24 hours prior to LPS injection creates a possible stressful environment for mice, and as some have suggested that acute stress may lead to an immunoprotective effect,47 there is a possibility that this acts in combination with VWR to attenuate LPS induced inflammation. Second, while we measured the protein content of UCP-1 and PGC-1α in iWAT and observed that this was increased with VWR, we did not assess other measures of adipose tissue browning / thermogenesis or mitochondrial function. This would be an interesting avenue to explore as others have shown that transplantation of iWAT from trained into sedentary mice preserves body temperature upon exposure to cold,9 and that UCP-1 may be involved in the thermogenic response to LPS.48
Overall, we demonstrate that habitual physical activity, in the form of VWR, is able to protect against acute inflammation in adipose tissue of C57BL/J mice. This effect occurs with modest depot specificity, such that the effects are greater in iWAT vs. eWAT. This work, coupled with our previously published findings in the liver,21 suggests that VWR is able to protect against LPS induced inflammation across multiple tissues.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
DCW is a Tier II Canada Research Chair in Lipids, Metabolism, and Health and holds a National Sciences and Engineering Research Council of Canada Discovery Grant. REKM is supported by an Alzheimer Society postdoctoral fellowship. WTP is supported by a scholarship from the Guelph Food Technology Center (GFTC) and College of Biological Sciences (Guelph).
References
- [1].Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Med 2003; 31:1250-6; http://dx.doi.org/ 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- [2].Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al.. International study of the prevalence and outcomes of infection in intensive care units. Jama 2009; 302:2323-9; PMID:19952319; http://dx.doi.org/ 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- [3].Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42:145-51; PMID:18304834; http://dx.doi.org/ 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- [4].Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cellular Mol Physiol 2005; 288:L333-41; PMID:15475380; http://dx.doi.org/ 10.1152/ajplung.00334.2004 [DOI] [PubMed] [Google Scholar]
- [5].Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain, behavior, and immunity 2011; 25:1637-48; PMID:21704698; http://dx.doi.org/ 10.1016/j.bbi.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Olesen J, Bienso RS, Meinertz S, van Hauen L, Rasmussen SM, Gliemann L, Plomgaard P, Pilegaard H. Impact of training status on LPS-induced acute inflammation in humans. J Applied Physiol (Bethesda, Md : 1985) 2015; 118:818-29; http://dx.doi.org/ 10.1152/japplphysiol.00725.2014 [DOI] [PubMed] [Google Scholar]
- [7].Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860-7; PMID:17167474; http://dx.doi.org/ 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- [8].Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ : Canadian Medical Association J = J De l'Association Medicale Canadienne 2006; 174:801-9; PMID:16534088; http://dx.doi.org/ 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al.. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015; 64:2002-14; PMID:25605808; http://dx.doi.org/ 10.2337/db14-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol 1991; 261:E410-4; PMID:1653528 [DOI] [PubMed] [Google Scholar]
- [11].Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol 2009; 587:1607-17; PMID:19221126; http://dx.doi.org/ 10.1113/jphysiol.2008.165464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, et al.. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014; 63:2800-11; PMID:24622799; http://dx.doi.org/ 10.2337/db13-1234 [DOI] [PubMed] [Google Scholar]
- [13].Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14:324-38; PMID:21907139; http://dx.doi.org/ 10.1016/j.cmet.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al.. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463-8; PMID:22237023; http://dx.doi.org/ 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, Rector RS, Thyfault JP, Laughlin MH, Padilla J. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regulatory Integrative Comparative Physiol 2014; 306:R596-606; PMID:24523340; http://dx.doi.org/ 10.1152/ajpregu.00493.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Welly RJ, Liu TW, Zidon TM, Rowles JL 3rd, Park YM, Smith TN, Swanson KS, Padilla J, Vieira-Potter VJ. Comparison of diet versus exercise on metabolic function and Gut Microbiota in Obese Rats. Med Sci Sports Exerc 2016; 48:1688-98; PMID:27128671; http://dx.doi.org/ 10.1249/MSS.0000000000000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Haczeyni F, Barn V, Mridha AR, Yeh MM, Estevez E, Febbraio MA, Nolan CJ, Bell-Anderson KS, Teoh NC, Farrell GC. Exercise improves adipose function and inflammation and ameliorates fatty liver disease in obese diabetic mice. Obesity (Silver Spring, Md) 2015; 23:1845-55; PMID:26250514; http://dx.doi.org/ 10.1002/oby.21170 [DOI] [PubMed] [Google Scholar]
- [18].Thyfault JP, Wright DC. “Weighing” the effects of exercise and intrinsic aerobic capacity: are there beneficial effects independent of changes in weight? Applied Physiol Nutrition Metab = Physiologie appliquee Nutrition Et Metabolisme 2016; 41:911-6; PMID:27512815; http://dx.doi.org/ 10.1139/apnm-2016-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martin SA, Pence BD, Greene RM, Johnson SJ, Dantzer R, Kelley KW, Woods JA. Effects of voluntary wheel running on LPS-induced sickness behavior in aged mice. Brain Behavior Immunity 2013; 29:113-23; PMID:23277090; http://dx.doi.org/ 10.1016/j.bbi.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huey KA, Meador BM. Contribution of IL-6 to the Hsp72, Hsp25, and αβ-crystallin responses to inflammation and exercise training in mouse skeletal and cardiac muscle. J Applied Physiol 2008; 105:1830-6; PMID:18927263; http://dx.doi.org/ 10.1152/japplphysiol.90955.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Peppler WT, Anderson ZG, Sutton CD, Rector RS, Wright DC. Voluntary wheel running attenuates lipopolysaccharide induced liver inflammation in mice. Am J Physiol Regulatory Integrative Comparative Physiol 2016;310:R934-42; PMID:26887432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Browne CA, O'Brien FE, Connor TJ, Dinan TG, Cryan JF. Differential lipopolysaccharide-induced immune alterations in the hippocampus of two mouse strains: effects of stress. Neuroscience 2012; 225:237-48; PMID:22917616; http://dx.doi.org/ 10.1016/j.neuroscience.2012.08.031 [DOI] [PubMed] [Google Scholar]
- [23].Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatol (Baltimore, Md) 1994; 19:480-8; PMID:8294104; http://dx.doi.org/ 10.1002/hep.1840190229 [DOI] [PubMed] [Google Scholar]
- [24].Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013; 93:993-1017; PMID:23899560; http://dx.doi.org/ 10.1152/physrev.00038.2012 [DOI] [PubMed] [Google Scholar]
- [25].Schertzer JD, Tamrakar AK, Magalhaes JG, Pereira S, Bilan PJ, Fullerton MD, Liu Z, Steinberg GR, Giacca A, Philpott DJ, et al.. NOD1 activators link innate immunity to insulin resistance. Diabetes 2011; 60:2206-15; PMID:21715553; http://dx.doi.org/ 10.2337/db11-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buzelle SL, MacPherson RE, Peppler WT, Castellani L, Wright DC. The contribution of IL-6 to beta 3 adrenergic receptor mediated adipose tissue remodeling. Physiological Reports 2015; 3 pii:e12312; PMID:2571333225852189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Janes KA. An analysis of critical factors for quantitative immunoblotting. Sci Signal 2015; 8:rs2; PMID:25852189; http://dx.doi.org/ 10.1126/scisignal.2005966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gouspillou G, Sgarioto N, Norris B, Barbat-Artigas S, Aubertin-Leheudre M, Morais JA, Burelle Y, Taivassalo T, Hepple RT. The relationship between muscle fiber type-specific PGC-1alpha content and mitochondrial content varies between rodent models and humans. PLoS One 2014; 9:e103044; PMID:25121500; http://dx.doi.org/ 10.1371/journal.pone.0103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001; 25:402-8; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- [30].Castellani L, Root-Mccaig J, Frendo-Cumbo S, Beaudoin MS, Wright DC. Exercise training protects against an acute inflammatory insult in mouse epididymal adipose tissue. J Applied Physiol (Bethesda, Md : 1985) 2014; 116:1272-80; PMID:24674860; http://dx.doi.org/ 10.1152/japplphysiol.00074.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92:829-39; PMID:9529258; http://dx.doi.org/ 10.1016/S0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- [32].Ringholm S, Grunnet Knudsen J, Leick L, Lundgaard A, Munk Nielsen M, Pilegaard H. PGC-1alpha is required for exercise- and exercise training-induced UCP1 up-regulation in mouse white adipose tissue. PLoS One 2013; 8:e64123; PMID:23717545; http://dx.doi.org/ 10.1371/journal.pone.0064123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Et Biophys Acta 2014; 1842:446-62; PMID:23707515; http://dx.doi.org/ 10.1016/j.bbadis.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Starr ME, Saito M, Evers BM, Saito H. Age-Associated increase in cytokine production during systemic inflammation-II: The Role of IL-1beta in Age-Dependent IL-6 Upregulation in Adipose Tissue. J Gerontol Series A Biol Sci Med Sci 2015; 70:1508-15; PMID:25344820; http://dx.doi.org/ 10.1093/gerona/glu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A 2007; 104:16239-44; PMID:17911255; http://dx.doi.org/ 10.1073/pnas.0705971104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Martinez FO, Sica A Fau - Mantovani A, Mantovani A Fau - Locati M, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13:453-61. [DOI] [PubMed] [Google Scholar]
- [37].Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagnostic Lab Immunol 2005; 12:60-7; PMID:15642986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M. Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behavior Immunity 2014; 39:87-98; PMID:24246250; http://dx.doi.org/ 10.1016/j.bbi.2013.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Applied Physiol (Bethesda, Md : 1985) 2005; 98:1154-62; PMID:15772055; http://dx.doi.org/ 10.1152/japplphysiol.00164.2004 [DOI] [PubMed] [Google Scholar]
- [40].Ruegsegger GN, Company JM, Toedebusch RG, Roberts CK, Roberts MD, Booth FW. Rapid alterations in perirenal adipose tissue transcriptomic networks with cessation of voluntary running. PLoS One 2015; 10:e0145229; PMID:26678390; http://dx.doi.org/ 10.1371/journal.pone.0145229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999; 11:115-22; PMID:10435584; http://dx.doi.org/ 10.1016/S1074-7613(00)80086-2 [DOI] [PubMed] [Google Scholar]
- [42].Kwon OS, Tanner RE, Barrows KM, Runtsch M, Symons JD, Jalili T, Bikman BT, McClain DA, O'Connell RM, Drummond MJ. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am J Physiol Endocrinol Metab 2015; 309:E11-21; PMID:25968578; http://dx.doi.org/ 10.1152/ajpendo.00124.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yu M, Zhou H, Zhao J, Xiao N, Roychowdhury S, Schmitt D, Hu B, Ransohoff RM, Harding CV, Hise AG, et al.. MyD88-dependent interplay between myeloid and endothelial cells in the initiation and progression of obesity-associated inflammatory diseases. J Exp Med 2014; 211:887-907; PMID:24752299; http://dx.doi.org/ 10.1084/jem.20131314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One 2010; 5; pii e12537; PMID:2082409826953756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Irahara T, Sato N, Inoue K, Otake K, Ohtsuru S, Koike K, Fushiki T, Yokota H. Low-intensity exercise in the acute phase of lipopolysaccharide-induced sepsis improves lipid metabolism and survival in mice by stimulating PGC-1alpha expression. J Trauma Acute Care Surgery 2016; 80:933-40; PMID:26953756; http://dx.doi.org/ 10.1097/TA.0000000000001023 [DOI] [PubMed] [Google Scholar]
- [46].Sczelecki S, Besse-Patin A, Abboud A, Kleiner S, Laznik-Bogoslavski D, Wrann CD, Ruas JL, Haibe-Kains B, Estall JL. Loss of Pgc-1alpha expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab 2014; 306:E157-67; PMID:24280126; http://dx.doi.org/ 10.1152/ajpendo.00578.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16:300-17; PMID:19571591; http://dx.doi.org/ 10.1159/000216188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Riley CL, Dao C, Kenaston MA, Muto L, Kohno S, Nowinski SM, Solmonson AD, Pfeiffer M, Sack MN, Lu Z, et al.. The complementary and divergent roles of uncoupling proteins 1 and 3 in thermoregulation. J Physiol 2016; PMID:27647490; http://dx.doi.org/10.1113/JP2729 [DOI] [PMC free article] [PubMed] [Google Scholar]