Abstract
This study aimed to investigate antibiotic resistance genes in the multidrug-resistant (MDR) Acinetobacter baumannii (A. baumanii) strain, MDR-SHH02, using whole-genome sequencing (WGS). The antibiotic resistance of MDR-SHH02 isolated from a patient with breast cancer to 19 types of antibiotics was determined using the Kirby-Bauer method. WGS of MDR-SHH02 was then performed. Following quality control and transcriptome assembly, functional annotation of genes was conducted, and the phylogenetic tree of MDR-SHH02, along with another 5 A. baumanii species and 2 Acinetobacter species, was constructed using PHYLIP 3.695 and FigTree v1.4.2. Furthermore, pathogenicity islands (PAIs) were predicted by the pathogenicity island database. Potential antibiotic resistance genes in MDR-SHH02 were predicted based on the information in the Antibiotic Resistance Genes Database (ARDB). MDR-SHH02 was found to be resistant to all of the tested antibiotics. The total draft genome length of MDR-SHH02 was 4,003,808 bp. There were 74.25% of coding sequences to be annotated into 21 of the Clusters of Orthologous Groups (COGs) of protein terms, such as 'transcription' and 'amino acid transport and metabolism'. Furthermore, there were 45 PAIs homologous to the sequence MDRSHH02000806. Additionally, a total of 12 gene sequences in MDR-SHH02 were highly similar to the sequences of antibiotic resistance genes in ARDB, including genes encoding aminoglycoside-modifying enzymes [e.g., aac(3)-Ia, ant(2″)-Ia, aph33ib and aph(3′)-Ia], β-lactamase genes (bl2b_tem and bl2b_tem1), sulfonamide-resistant dihydropteroate synthase genes (sul1 and sul2), catb3 and tetb. These results suggest that numerous genes mediate resistance to various antibiotics in MDR-SHH02, and provide a clinical guidance for the personalized therapy of A. baumannii-infected patients.
Keywords: Acinetobacter baumannii, antibiotic resistance gene, whole-genome sequencing
Introduction
Acinetobacter baumannii (A. baumanii) is a notable pathogen that causes hospital-acquired infections among immune-compromised patients, accounting for 5% of Gram-negative infections (1). Due to a strong resistance to desiccation and multiple antibacterial agents, the widespread dissemination of multidrug-resistant (MDR) A. baumanii strains has been a threat to hospitalized patients in recent years (2).
Antibiotic resistance determinants play pivotal roles in whether or not A. baumannii will flourish in the host (3). For instance, the expression of β-lactamase genes [e.g., oxacillinase (OXA)-235 gene, blaOXA-51 and blaTEM-1] has been shown to be involved in antibiotic resistance (4–6). An 86-kb region AbaR resistant to heavy metal and antibiotics has been found in a MDR isolate AYE (7), indicating the important role of AbaR in the spread of A. baumannii in hospitals (8,9). Furthermore, other resistance determinants, such as macrolide (msrA/msrB), aminoglycoside (e.g., aacC1, armA, and aphA1) and tetracycline [e.g., tet (39), tet (A), and tet (B)] have been identified in various A. baumannii isolates (10).
Bacterial whole-genome sequencing (WGS) has enhanced our ability to evaluate antibiotic resistance determinants. For example, a AbaR-type genomic resistance island, AbaR22, has been identified in the MDR A. baumannii strain, MDR-ZJ06, via WGS (11). A whole-genome comparison detected 18 putative single nucleotide polymorphisms (SNPs) between 2 pre- and post-therapy A. baumannii isolates (12). Furthermore, 10 types of AbaR resistance islands were identified in 2 A. baumannii isolates using WGS (13). Despite increased research on antibiotic resistance determinants in A. baumannii, the molecular mechanisms of antibiotic resistance in MDR A. baumannii are not yet fully understood, and various antibiotic resistance genes have not been detected.
In the present study, we applied WGS to obtain the whole genomic sequence of the MDR A. baumannii strain, MDR-SHH02, isolated from a patient with breast cancer. Furthermore, the antibiotic resistance of MDR-SHH02 to multiple antibiotics was determined, and potential antibiotic resistance genes in MDR-SHH02 were predicted. The results of our study may enhance our understanding of the molecular mechanisms of antibiotic resistance in MDR A. baumannii, and provide a clinical guidance for the therapy of A. baumannii-infected patients.
Materials and methods
Isolation and identification of A. baumanii strain
The clinical MDR A. baumanii strain, named MDR-SHH02, was isolated from the blood obtained from a 65-year-old woman with terminal-stage breast cancer at Shanghai Sixth People's Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China. This patient had received a double mastectomy and nearby lymph node excision. After being discharged from the hospital, this patient was hospitalized again due to symptoms of fever, cough (lasting for days) and shortness of breath. During her second hospital administration, she received several antimicrobial therapies, including maxipime, impenem, methylprednisolone, levofloxacin and cefoperazone-sulbactam sodium. A. baumanii was positive in the blood culture and sputum culture. The results of the antimicrobial susceptibility test revealed that the A. baumanii strain was resistant to multiple commonly used antibiotics. This study was approved by the Shanghai Health and Family Planning Commission Foundation (Shanghai, China), and informed consent was obtained from the patient.
The isolated strain was inoculated onto blood agar plates and then incubated in an atmosphere of 5% CO2 at 35°C for 48 h. Afterwards, this strain was identified using morphological and biochemical tests according to standard methods (14). Colonies with typical morphological and biochemical characteristics of Acinetobacter were cultivated on 5% sheep blood agar and identified using an automated Microscan® system (Dade Behring, Inc., West Sacramento, CA, USA). The A. baumanii strain was stored at −70°C in skim milk for further analyses.
Antibiotic resistance test for A. baumanii MDR-SHH02
According to the Clinical and Laboratory Standards Institute (CLSI) guidelines (15), disc diffusion assay (DDA) with dry wafers saturated by 19 types of antibiotics, including gentamicin (10 µg/wafer), tobramycin (30 µg/wafer), amikacin (30 µg/wafer), ampicillin-sulbactam (10/10 µg/wafer), ceftazidime (30 µg/wafer), ciprofloxacin (5 µg/wafer), levofloxacin (5 µg/wafer), imipenem (10 µg/wafer), meropenem (10 µg/wafer), piperacillin/tazobactam (100/10 µg/wafer), ticarcillin/clavulanic acid (75/10 µg/wafer), cefepime (30 µg/wafer), cefotaxime (30 µg/wafer), ceftriaxone (30 µg/wafer), doxycycline (30 µg/wafer), minocyline (30 µg/wafer), tetracycline (30 µg/wafer), piperacillin (100 µg/wafer) and trimethoprim-sulfamethoxazole (1.25/23.75 µg/wafer) (all from Oxoid, Ltd., Basingstoke, UK), were carried out using the Kirby-Bauer (KB) method, as previously described (16). Briefly, Mueller-Hinton agar (Oxoid, Ltd.) plates were overlaid with the inocula of the clinical A. baumanii strain, and the turbidity of the inocula was equivalent to the 0.5 McFarland standard. Subsequently, dry wafers saturated by antibiotics were placed on the surface of the agar, and plates were placed in an atmosphere of 5% CO2 at 35°C. Following 24 h of culture, the diameter of the inhibition zone around each wafer was measured according to the CLSI criteria (15). In this test, Escherichia coli ATCC 25922, ATCC 35218 and Pseudomonas aeruginosa ATCC 27853 obtained from the Clinical Laboratory Center of the Ministry of Health were used as reference strains.
DNA preparation, library construction and sequencing
The genomic DNA of A. baumanii MDR-SHH02 was extracted using a bacterial genomic DNA purification kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's instructions. The Illumina sequencing library was then prepared using the Nextera™ DNA Sample Preparation kit (Illumina®-Compatible). Paired-end dual index 2×90 bp sequencing was fulfilled following the Illumina HiSeq 2000. Sequencing was performed by Beijing Genomics Institute (BGI; Shenzhen, China). The sequencing data were uploaded to the public database the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/) under the BioProject PRJNA256112 with BioSample accession no. SAMN02991371.
Quality control
For the raw sequencing data, the reads were cleaned by removing the empty reads, adapter sequences and reads with n≥10% using the SeqPrep program (https://github.com/jstjohn/SeqPrep) and Sickle (https://github.com/najoshi/sickle). In addition, the reads were trimmed by discarding the reads containing >30% bases with a Q-value ≤20 in the 3′ terminal, as well as reads with adaptor sequences (the length of overlapping sequences between adaptor and read was at least >15 bp, and the number of mismatch bases was <3 bp).
Genome assembly
The clean reads were assembled using the short oligonucleotide analysis package SOAPdenovo (version 2.04; http://soap.genomics.org.cn/). To determine whether the GC content has a significant effect on sequencing randomness or not, the GC content and average depth of the genomic sequence were calculated without repetition as a unit of 500 bp.
Genome annotation
Genes in the assembled genomic sequence were predicted using Glimmer 3.0 (http://www.cbcb.umd.edu/software/glimmer/) (17), which is a system for identifying genes in DNA sequences of microorganism, particularly bacteria, archaea and viruses.
Furthermore, tRNA and rRNA (5S, 16S, and 23S rRNA) in the genomic sequence were searched using tRNAscan-SE (http://lowelab.ucsc.edu/tRNAscan-SE/) (18) and RNAmmer 1.2 (http://www.cbs.dtu.dk/services/RNAmmer/) (19), respectively.
Additionally, tandem repeat sequences and clustered regularly interspaced short palindromic repeats (CRISPR) in the genomic sequence were predicted using the Tandem Repeat Finder (http://tandem.bu.edu/trf/trf.html) and CRISPR Finder (http://crispr.u-psud.fr/Server/) software, respectively. Insertion sequences (ISs) were characterized using the IS Finder database (https://www-is.biotoul.fr//), and the parameter -e was set as 1e-5, identity set as 35%. Besides, protein domains associated with the genomic sequence were predicted using the InterPro database (https://www.ebi.ac.uk/interpro/), and the parameter was set as -appl PfamA.
Functional annotation of genes
Sequence alignment of the amino acid sequences of genes to the Cluster of Orthologous Groups (COGs) of proteins database (http://www.ncbi.nlm.nih.gov/COG/) (20) was performed using the Basic Local Alignment Search Tool (BLASTP; version 2.0) program from NCBI (E-value ≤10–4) (21). We also performed sequence alignment of the amino acid sequences of genes to the NCBI non-redundant (NR) database (E-value ≤10–10, identity score ≥35%, and coverage length ≥80%). If the amino acid sequence of a gene was aligned to multiple sequences in the databases, the optimal result was retained.
Construction of phylogenetic tree
Based on the NCBI 16S rRNA gene database, 16S rRNA gene sequences of 5 A. baumanii strains, including ATCC 17978, ATCC 19606, CIP 70.34, DSM 30007 and A. baumanii JCM 68415, as well as 2 species belonging to Acinetobacter (A. haemolyticus ATCC 17906 and A. bereziniae ATCC 17924), were used to construct the phylogenetic tree, along with MDR-SHH02. Briefly, multiple sequence alignment was performed using ClustalW-2.1 (22). Subsequently, the software package PHYLIP 3.695 (http://evolution.genetics.washington.edu/phylip.html), along with the bootstrap algorithm, was used to construct the maximum likelihood phylogenetic tree, and the phylogenetic tree was visualized by FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Prediction of pathogenicity islands (PAIs)
The Pathogenicity Island database (PAIDB; http://www.paidb.re.kr/about_paidb.php), which is a web-based user-friendly resource and widely used for detecting PAIs in newly sequenced genomes (23), was utilized to predict PAIs in the genomic sequence of MDR-SHH02.
Identification of antibiotic resistance genes
To identify potential antibiotic resistance genes in the genomic sequence of MDR-SHH02, sequence alignment of the protein sequences of antibiotic resistance genes in the Antibiotic Resistance Genes database (ARDB; http://ardb.cbcb.umd.edu/) (24) and MDR-SHH02 genomic sequence was conducted using BLASTP (E-value ≤10–10, identity score ≥90%, and coverage length ≥80%).
Results
Antibiotic-resistance of A. baumanii MDR-SHH02
The antibiotic-resistance assay revealed that the diameter of the inhibition zone for 17 types of antibiotics on MDR-SHH02 plates was 6 mm, apart from levofloxacin and minocyline (diameter, 10 mm) (Table I). According to the CLSI criteria, MDR-SHH02 was resistant to all of the tested antibiotics.
Table I.
The results of antibiotic-resistance assay for A. baumannii MDR-SHH02.
| Antibiotic name | Diameter of inhibition zone on MDR-SHH02 plate (mm) | Antibiotic-resistance of MDR-SHH02 |
|---|---|---|
| Ampicillin-sulbactam | 6 | R |
| Ceftazidime | 6 | R |
| Ciprofloxacin | 6 | R |
| Levofloxacin | 10 | R |
| Imipenem | 6 | R |
| Meropenem | 6 | R |
| Gentamicin | 6 | R |
| Tobramycin | 6 | R |
| Amikacin | 6 | R |
| Piperacillin/tazobactam | 6 | R |
| Ticarcillin/clavulanic acid | 6 | R |
| Cefepime | 6 | R |
| Cefotaxime | 6 | R |
| Ceftriaxone | 6 | R |
| Doxycycline | 6 | R |
| Minocyline | 10 | R |
| Tetracycline | 6 | R |
| Piperacillin | 6 | R |
| Trimethoprim-sulfamethoxazole | 6 | R |
Diameter of inhibition zone <15 mm is determined as drug-resistant (R); diameter of inhibition zone between 15 and 17 mm is determined as intermediate (I); and diameter of inhibition zone >17 mm is determined as drug-sensitive (S). A. baumannii, Acinetobacter baumannii.
Assembly and annotation of the genomic sequence of A. baumanii MDR-SHH02
During the genome assembly, a total of 85 scaffolds were generated, and the scaffold N50 length was 131,822 bp. The total draft genome length of MDR-SHH02 was 4,003,808 bp, with 38.99% of GC content. There were 3,787 coding sequences, 62 tRNA sequences and 3 rRNA sequences in the genomic sequence. Moreover, 2 CRISPR and 36 tandem repeat sequences, as well as 29 ISs were predicted in the genomic sequence (Table II).
Table II.
The results of genome assembly and annotation of A. baumannii MDR-SHH02.
| Feature | Statistics |
|---|---|
| Length of total draft genome length (bp) | 4,003,808 |
| No. of scaffolds | 85 |
| Length of scaffold N50 (bp) | 131,822 |
| GC content (%) | 38.99 |
| No. of coding sequences | 3,787 |
| No. of tRNAs | 62 |
| No. of rRNAs | 3 |
| No. of CRISPR | 2 |
| No. of tandem repeat sequences | 36 |
| No. of insertion sequences | 29 |
A. baumannii, Acinetobacter baumannii; CRISPR, clustered regularly interspaced short palindromic repeats.
Furthermore, numerous protein domains were predicted in the genomic sequence of MDR-SHH02, such as the AcrB/AcrD/AcrF family (Table III).
Table III.
The top 10 predicted protein domains in the genomic sequence of A. baumannii MDR-SHH02.
| Gene ID | Protein domain | P-value |
|---|---|---|
| MDRSHH02002231 | AcrB/AcrD/AcrF family | <1.0E-300 |
| MDRSHH02000985 | Monomeric isocitrate dehydrogenase | <1.0E-300 |
| MDRSHH02003056 | AcrB/AcrD/AcrF family | <1.0E-300 |
| MDRSHH02000840 | AcrB/AcrD/AcrF family | <1.0E-300 |
| MDRSHH02003594 | AcrB/AcrD/AcrF family | <1.0E-300 |
| MDRSHH02000667 | AcrB/AcrD/AcrF family | <1.0E-300 |
| MDRSHH02000153 | Phosphoenolpyruvate carboxylase | 2.70E-292 |
| MDRSHH02000199 | Urocanase | 5.20E-287 |
| MDRSHH02000831 | Phosphoenolpyruvate carboxykinase | 2.70E-279 |
| MDRSHH02003134 | AcrB/AcrD/AcrF family | 5.50E-266 |
A. baumannii, Acinetobacter baumannii.
Functional annotation of the genomic sequence of A. baumanii MDR-SHH02
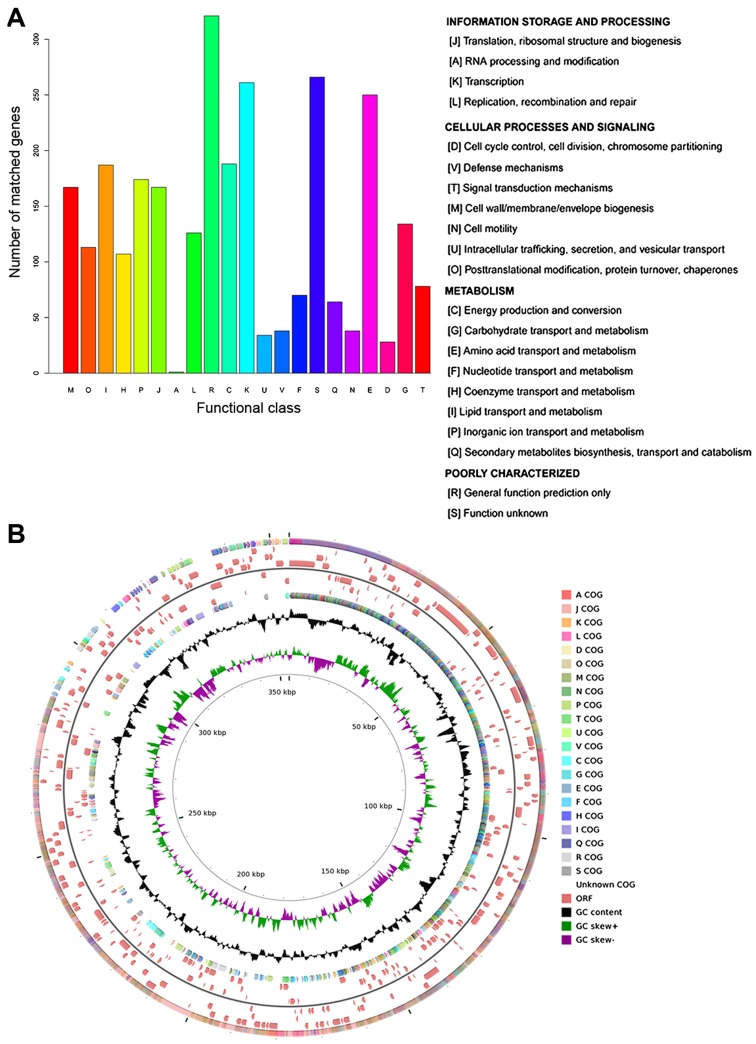
According to the COG annotation, 74.25% (2,812/3,787) of coding sequences were annotated into 21 COG terms, which were divided into 3 categories: information storage of processing, cellular processes and signaling, and metabolism. Apart from the category of poorly characterized, most o the coding sequences were annotated into 'transcription' (number of coding sequences, 261) and 'amino acid transport and metabolism' (number of coding sequences, 250) (Fig. 1A). The distribution of COG categories in the genomic sequence of A. baumannii MDR-SHH02 is shown in Fig. 1B.
Figure 1.
The functional annotation of the genomic sequence of Acinetobacter baumannii (A. baumannii) MDR-SHH02 using the Cluster of Orthologous Groups (COGs) of proteins database. (A) The bar diagram displaying the COG categories. The x-coordinate represents the COG categories, and y-coordinate represents the number of matched genes; (B) the annotation circle displaying the distribution of COG categories, open reading frames (ORFs) and GC content in the genomic sequence of A. baumannii MDR-SHH02. The outermost circle (circle 1) displays the distribution of COG categories on positive strand, and each kind of color represents one COG term, which is annotated as that in (A). Circles 2–4 and 5–7 display the distribution of ORFs on positive and negative strands. Circle 8 displays the distribution of COG categories on negative strand. Circle 9 displays the GC content. Circle 10 displays the GC skew (G-C)/(G+C) (green bars represent positive values, purple bars represent negative values). Circle 11 dislays the the scale in kbp.
Analysis of the phylogenetic tree
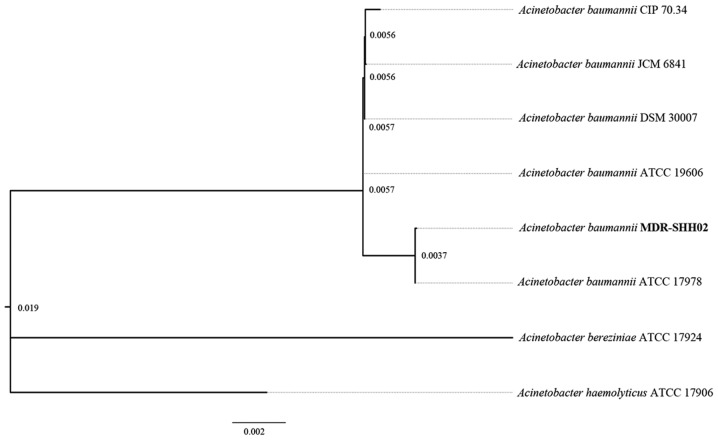
Based on the 16S rRNA gene sequences of A. baumanii in NCBI, the phylogenetic tree revealed that the genomic sequence of MDR-SHH02 was most similar to the 16S rRNA gene sequence of A. baumanii ATCC 17978 (Fig. 2).
Figure 2.
Whole-genome phylogeny of the genomes of Acinetobacter baumannii (A. baumannii) MDR-SHH02 and 5 sequenced A. baumannii genomes. The phylogenetic tree was rooted with 2 genomes of Acinetobacter species.
Analysis of PAIs
During the process of bacterial infection, PAIs play pivotal roles in the evolution of pathogens and the development of diseases. In the genomic sequence of MDR-SHH02, a total of 45 PAIs homologous to the sequence MDRSHH02000806 were detected (Table IV). Most of the PAIs were previously identified from Escherichia coli [e.g., locus of enterocyte effacement (LEE)] and Pseudomonas (e.g., PAPI-1 and T-PAI).
Table IV.
Pathogenicity islands homologous to a region in the genomic sequence of A. baumannii MDR-SHH02.
| Gene ID | Start | End | Size (bp) | no. of ORFs | PAIs homologous to this region |
|---|---|---|---|---|---|
| MDRSHH02000806 | 332 | 21071 | 20740 | 18 | PAPI-1 (Pseudomonas aeruginosa PA14) |
| PAGI-2(C) (Pseudomonas aeruginosa C) | |||||
| PAGI-3(SG) (Pseudomonas aeruginosa SG17M) | |||||
| PAGI-5 (Pseudomonas aeruginosa PSE9) | |||||
| PPHGI-1 (Pseudomonas syringae pv. phaseolicola 1302A) | |||||
| SPI-7 (Salmonella enterica subsp. enterica serovar Typhi str. CT18) | |||||
| SPI-7 (Salmonella enterica subsp. enterica serovar Typhi Ty2) | |||||
| AbaR25 (Acinetobacter baumannii BJAB07104) | |||||
| AbaR26 (Acinetobacter baumannii BJAB0868) | |||||
| Hrp PAI (Erwinia amylovora 321) | |||||
| S-PAI (Pseudomonas cichorii 83-1) | |||||
| Hrp PAI (Pseudomonas syringae pv. tomato DC3000) | |||||
| Hrp PAI (Pseudomonas syringae pv. tomato str. DC3000) | |||||
| T-PAI (Pseudomonas viridiflava LP23.1a) | |||||
| T-PAI (Pseudomonas viridiflava PNA3.3a) | |||||
| S-PAI (Pseudomonas viridiflava RMX23.1a) | |||||
| S-PAI (Pseudomonas viridiflava ME3.1b) | |||||
| S-PAI (Pseudomonas viridiflava RMX3.1b) | |||||
| Not named (Corynebacterium urealyticum DSM 7109) | |||||
| LEE (Citrobacter rodentium DBS100) | |||||
| PAI I 536 (Escherichia coli 536) | |||||
| LEE (Escherichia coli E2348/69) | |||||
| LEE (Escherichia coli O157:H7 str. EDL933 ATCC43895) | |||||
| LEE (Escherichia coli 71074) | |||||
| LEE (Escherichia coli 83/39) | |||||
| LEE (Escherichia coli REPEC 84/110-1) | |||||
| LEE (Escherichia coli RW1374) | |||||
| LEE (Escherichia coli RDEC-1) | |||||
| LEE (Escherichia coli O157:H7 EDL933) | |||||
| LEE (Escherichia coli O157:H7 str. Sakai) | |||||
| LEE (Escherichia coli O103:H2 str. 12009) | |||||
| LEE (Escherichia coli O26:H11 str. 11368) | |||||
| LEE (Escherichia coli O111:H-str. 11128) | |||||
| LEE II (Escherichia coli 413/89-1) | |||||
| AGI-3 (Escherichia coli BEN2908) | |||||
| TAI (Escherichia coli O157:H7 EDL933) | |||||
| OI-122 (Escherichia coli O157:H7 EDL933) | |||||
| PAI I CFT073 (Escherichia coli CFT073) | |||||
| Not named (Escherichia coli UMN026) | |||||
| SESS LEE (Salmonella enterica subsp. salamae serovar Sofia S1296) | |||||
| SESS LEE (Salmonella enterica subsp. salamae serovar Sofia S1635) | |||||
| SHI-1 (Shigella flexneri 2a str. 301) | |||||
| SHI-1 (Shigella flexneri 2a str. 2457T) | |||||
| SRL (Shigella flexneri 2a YSH6000) | |||||
| Not named (Yersinia pestis CO92) |
A. baumannii, Acinetobacter baumannii; ORF, open reading frame; PAIs, pathogenicity islands.
Screening of antibiotic resistance genes
To reveal the genes relevant to the antibiotic resistance of MDR-SHH02, sequence alignment of the protein sequences of antibiotic resistance genes in ARDB and MDR-SHH02 genomic sequence was performed. Based on the selection criteria, a total of 12 gene sequences (e.g., MDRSHH02002408, MDRSHH02000600 and MDRSHH02000597) in MDR-SHH02 were highly similar to the sequences of antibiotic resistance genes in ARDB, such as aac(3)-Ia, aac(6′)-Ib, ant(2″)-Ia and aph(3′)-Ia. According to the antibiotics that were resistant by the 12 gene sequences, MDR-SHH02 was resistant to multiple antibiotics, such as gentamicin, amikacin, tobramycin, spectinomycin, streptomycin and neomycin (Table V), which was partly consistent with the aforementioned results of antibiotic-resistance assay.
Table V.
The potential gene sequences in A. baumannii MDR-SHH02 relevant to antibiotic resistance.
| Gene ID | Resistance gene type from ARDB | Resistance gene class | Antibiotics |
|---|---|---|---|
| MDRSHH02002408 | aac(3)-Ia | Aminoglycoside N-acetyltransferase, which modifies aminoglycosides by acetylation |
Astromicin, gentamicin, sisomicin |
| MDRSHH02000600 | aac(6′)-Ib | Aminoglycoside N-acetyltransferase, which modifies aminoglycosides by acetylation |
Amikacin, dibekacin, isepamicin, netilmicin, sisomicin, tobramycin |
| MDRSHH02000597 | ant(2″)-Ia | Aminoglycoside O-nucleotidylyltransferase, which modifies aminoglycosides by adenylylation |
Dibekacin, gentamicin, kanamycin, sisomicin, tobramycin |
| MDRSHH02000611 | ant(3″)-Ia | Aminoglycoside O-nucleotidylyltransferase, which modifies aminoglycosides by adenylylation |
Spectinomycin, streptomycin |
| MDRSHH02001946 | aph33ib | Aminoglycoside O-phosphotransferase, which modifies aminoglycosides by phosphorylation |
Streptomycin |
| MDRSHH02002406 | aph(3′)-Ia | Aminoglycoside O-phosphotransferase, which modifies aminoglycosides by phosphorylation |
Gentamincin b, kanamycin, neomycin, paromomycin, lividomycin, ribostamycin |
| MDRSHH02001945 | aph(6)-Id | Aminoglycoside O-phosphotransferase, which modifies aminoglycosides by phosphorylation |
streptomycin |
| MDRSHH02000608 | bl2b_tem, bl2b_tem1 | Class A β-lactamase. This enzyme breaks the β-lactam antibiotic ring open and deactivates the molecule's antibacterial properties | Cephalosporin, penicillin, cephalosporin i, cephalosporin ii |
| MDRSHH02000599 | catb3 | Group B chloramphenicol, acetyltransferase which can inactivate chloramphenicol. Also referred to as xenobiotic acetyltransferase | Chloramphenicol |
| MDRSHH02000610 | sul1 | Sulfonamide-resistant dihydropteroate synthase, which can not be inhibited by sulfonamide | Sulfonamide |
| MDRSHH02001738 | sul2 | Sulfonamide-resistant dihydropteroate synthase, which can not be inhibited by sulfonamide | Sulfonamide |
| MDRSHH02001941 | tetb | Major facilitator superfamily transporter, tetracycline efflux pump | Tetracycline |
A. baumannii, Acinetobacter baumannii; ARDB, Antibiotic Resistance Genes Database; DDA, disc diffusion assay. The antibiotics in bold are resisted by A. baumanii MDR-SHH02 in the DDA.
Discussion
In the present study, we reported the draft genomic sequence of the clinical MDR A. baumannii strain, MDR-SHH02, and predicted one gene seuqence homologous to 45 PAIs and 12 potential gene sequences relevant to antibiotic resistance. The antibiotic-resistance assay and the high similarity between the 12 gene sequences in MDR-SHH02 and the sequences of antibiotic resistance genes in ARDB, revealed that MDR-SHH02 was resistant to multiple antibiotics.
According to the prediction of PAIs, the gene sequence MDRSHH02000806 was homologous to 45 PAIs, such as LEE and PAPI-1. LEE PAIs were previously identified from multiple enteropathogenic Escherichia coli strains, and they are highly conserved in gene order and nucleotide sequence (25,26). PAPI-1 was previously identified from the P. aeruginosa strain, PA14, and it contributes directly and synergistically along with PAPI-2 to the virulence of PA14 (27). Therefore, the virulence of MDR-SHH02 may be due to the presence of MDRSHH02000806 homologous to these PAIs.
In this study, we discovered a set of gene sequences that were highly similar to the sequences of multiple genes encoding aminoglycoside-modifying enzymes (AMEs), including 2 aminoglycoside N-acetyltransferase genes [aac(3)-Ia and aac(6′)-Ib], 2 aminoglycoside O-nucleotidylyltransferase genes [ant(2″)-Ia and ant(3″)-Ia], and 3 aminoglycoside O-phosphotransferase genes [aph33ib, aph(3′)-Ia and aph(6)-Id]. The expression of AMEs enables bacteria to catalyze the modification of amino and hydroxyl groups on sugar moieties, such as aminoglycosides (28), which is a major cause of aminoglycoside resistance in many bacteria (29). The majority of aminoglycoside-resistant Acinetobacter isolates have the ability of enzymatic modification of aminoglycosides by acetyltransferases, nucleotidyltransferases and/or phosphotransferases (30). Previous studies have reported the prevalence of multiple AME genes [e.g., aac(3)-Ia, and aac(6′)-Ib] in a set of A. baumannii isolates that are resistant to various aminogl ycosides (e.g., amikacin, gentamicin and tobramycin) (31,32). Besides, Aph(6)-Id and ant(3″)-Ia have been detected in the A. baumannii strain, MRSN 12227, which is resistant to various antibiotics, such as amikacin, tobramycin and cefotaxime (33). Another study reported that ant(2″)-Ia present in a group of A. baumannii isolates (62.6%) was associated with resistance to the tested aminoglycosides (amikacin, tobramycin and gentamicin) (34). Furthermore, in this study, we found that MDR-SHH02 was resistant to 19 antibiotics, such as several types of aminoglycosides (amikacin, gentamicin and tobramycin), indicating that the resistance of MDR-SHH02 to aminoglycosides likely resulted from the coding sequences highly similar to AME genes. However, aph33ib has not been previously detected in A. baumannii isolates, and it is worthy of further study. For example, following knockout and complementation of the gene sequence that is highly similar to aph33ib, the resistance of MDR-SHH02 to aminoglycosides is determined.
In this study, several gene sequences in MDR-SHH02 had a high similarity to class A β-lactamase genes (bl2b_tem and bl2b_tem1), group B chloramphenicol acetyltransferase gene (catb3), sulfonamide-resistant dihydropteroate synthase genes (sul1 and sul2) and tetracycline efflux pump gene (tetb). The gene bl2b_tem has been detected in Staphylococcus aureus (35), and bl2b_tem1 was detected in a series of marine bacteria, such as Pelagibacter, Polaribacter and Roseobacter (36) . However, there is no evidence to support that bl2b_tem and bl2b_tem1 are carried by A. baumannii isolates. All other genes (catb3, sul1, sul2 and tetb) have been found in A. baumannii isolates (37–40).
Despite the aforementioned results, there were still several limitations to this study. The association of MDRSHH02000806 with the virulence of MDR-SHH02 needs to be validated in further studies. Besides, the associations between the 12 gene sequences similar to AME genes and the antibiotic resistance of MDR-SHH02 are also needs to be confirmed in further studies. We aim to conduct such experiments in our future studies.
In conclusion, this study fulfilled the draft genomic sequence of the clinical MDR A. baumannii strain, MDR-SHH02, and 12 gene sequences in MDR-SHH02 had a highly similarity to the sequences of genes encoding AMEs [e.g., aac(3)-Ia, ant(2″)-Ia, aph33ib and aph(3′)-Ia], β-lactamase genes (bl2b_ tem and bl2b_tem1), sulfonamide-resistant dihydropteroate synthase genes (sul1 and sul2), catb3 and tetb. Of these genes, aph33ib, bl2b_tem and bl2b_tem1 were potential new antibiotic resistance genes. Furthermore, the antibiotic-resistance assay revealed that MDR-SHH02 was resistant to multiple antibiotics, such as amikacin, gentamicin and tobramycin. These findings were expected to enrich the data of antibiotic resistance genes in MDR A. baumannii, and provide a clinical guidance for the personalized therapy of A. baumannii-infected patients.
Acknowledgments
This study was supported by a project supported by Shanghai Health and Family Planning Commission Foundation, China (grant no. 20134010) and a project supported by the Natural Science Foundation of Shanghai, China (grant no. 15ZR1436100).
References
- 1.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–3359. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visca P, Seifert H, Towner KJ. Acinetobacter infection - an emerging threat to human health. IUBMB Life. 2011;63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen BL, Skaar EP. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell Microbiol. 2012;14:1336–1344. doi: 10.1111/j.1462-5822.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins PG, Pérez-Llarena FJ, Zander E, Fernández A, Bou G, Seifert H. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zander E, Nemec A, Seifert H, Higgins PG. Association between β-lactamase-encoding bla DiversiLab rep-PCR-based typing of Acinetobacter (OXA-51) variants and baumannii isolates. J Clin Microbiol. 2012;50:1900–1904. doi: 10.1128/JCM.06462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krizova L, Poirel L, Nordmann P, Nemec A. TEM-1 β-lactamase as a source of resistance to sulbactam in clinical strains of Acinetobacter baumannii. J Antimicrob Chemother. 2013;68:2786–2791. doi: 10.1093/jac/dkt275. [DOI] [PubMed] [Google Scholar]
- 7.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramírez MS, Vilacoba E, Stietz MS, Merkier AK, Jeric P, Limansky AS, Márquez C, Bello H, Catalano M, Centrón D. Spreading of AbaR-type genomic islands in multidrug resistance Acinetobacter baumannii strains belonging to different clonal complexes. Curr Microbiol. 2013;67:9–14. doi: 10.1007/s00284-013-0326-5. [DOI] [PubMed] [Google Scholar]
- 9.Šeputienė V, Povilonis J, Sužiedėlienė E. Novel variants of AbaR resistance islands with a common backbone in Acinetobacter baumannii isolates of European clone II. Antimicrob Agents Chemother. 2012;56:1969–1973. doi: 10.1128/AAC.05678-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taitt CR, Leski T, Stockelman MG, Craft DW, Zurawski DV, Kirkup BC, Vora GJ. Antimicrobial resistance determinants in Acinetobacter baumannii isolates taken from military treatment facilities. Antimicrob Agents Chemother. 2014;58:767–781. doi: 10.1128/AAC.01897-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Zhang T, Yu D, Pi B, Yang Q, Zhou J, Hu S, Yu Y. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob Agents Chemother. 2011;55:4506–4512. doi: 10.1128/AAC.01134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornsey M, Loman N, Wareham DW, Ellington MJ, Pallen MJ, Turton JF, Underwood A, Gaulton T, Thomas CP, Doumith M, et al. Whole-genome comparison of two Acinetobacter baumannii isolates from a single patient, where resistance developed during tigecycline therapy. J Antimicrob Chemother. 2011;66:1499–1503. doi: 10.1093/jac/dkr168. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Liu F, Zhang Y, Wang X, Zhao C, Chen H, Zhang F, Zhu B, Hu Y, Wang H. Evolution of carbapenem-resistant Acinetobacter baumannii revealed through whole-genome sequencing and comparative genomic analysis. Antimicrob Agents Chemother. 2015;59:1168–1176. doi: 10.1128/AAC.04609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atlas RM, Brown AE, Parks LC. Laboratory Manual of Experimental Microbiology. Mosby; St. Louis, MO: 1995. pp. 119–127. [Google Scholar]
- 15.Watts JL. Performance Standards For Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals: Approved Standard. Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 16.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 17.Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33(Web Server):W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagesen K, Hallin P, Rødland EA, Staerfeldt H-H, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, Krylov DM, Mazumder R, Mekhedov SL, Nikolskaya AN, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mount DW. Using the basic local alignment search tool (BLAST) CSH Protoc. 2007;2007:pdb. top17. doi: 10.1101/pdb.top17. [DOI] [PubMed] [Google Scholar]
- 22.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon SH, Park Y-K, Lee S, Choi D, Oh TK, Hur C-G, Kim JF. Towards pathogenomics: a web-based resource for pathogenicity islands. Nucleic Acids Res. 2007;35(Database):D395–D400. doi: 10.1093/nar/gkl790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Pop M. ARDB - Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009;37(Database):D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pósfai G, Koob MD, Kirkpatrick HA, Blattner FR. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol. 1997;179:4426–4428. doi: 10.1128/jb.179.13.4426-4428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauschek M, Strugnell RA, Robins-Browne RM. Characterization and evidence of mobilization of the LEE pathogenicity island of rabbit-specific strains of enteropathogenic Escherichia coli. Mol Microbiol. 2002;44:1533–1550. doi: 10.1046/j.1365-2958.2002.02968.x. [DOI] [PubMed] [Google Scholar]
- 27.Harrison EM, Carter ME, Luck S, Ou H-Y, He X, Deng Z, O'Callaghan C, Kadioglu A, Rajakumar K. Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain A14. Infect Immun. 2010;78:1437–1446. doi: 10.1128/IAI.00621-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemec A, Dolzani L, Brisse S, van den Broek P, Dijkshoorn L. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J Med Microbiol. 2004;53:1233–1240. doi: 10.1099/jmm.0.45716-0. [DOI] [PubMed] [Google Scholar]
- 31.Cho YJ, Moon DC, Jin JS, Choi CH, Lee YC, Lee JC. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn Microbiol Infect Dis. 2009;64:185–190. doi: 10.1016/j.diagmicrobio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Ploy MC, Denis F, Courvalin P, Lambert T. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob Agents Chemother. 2000;44:2684–2688. doi: 10.1128/AAC.44.10.2684-2688.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterman PE, McGann P, Snesrud E, Clifford RJ, Kwak YI, Munoz-Urbizo IP, Tabora-Castellanos J, Milillo M, Preston L, Aviles R, et al. Bacterial peritonitis due to Acinetobacter baumannii sequence type 25 with plasmid-borne new delhi metallo-β-lactamase in Honduras. Antimicrob Agents Chemother. 2013;57:4584–4586. doi: 10.1128/AAC.00275-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akers KS, Chaney C, Barsoumian A, Beckius M, Zera W, Yu X, Guymon C, Keen EF, III, Robinson BJ, Mende K, et al. Aminoglycoside resistance and susceptibility testing errors in Acinetobacter baumannii-calcoaceticus complex. J Clin Microbiol. 2010;48:1132–1138. doi: 10.1128/JCM.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostić T, Ellis M, Williams MR, Stedtfeld TM, Kaneene JB, Stedtfeld RD, Hashsham SA. Thirty-minute screening of antibiotic resistance genes in bacterial isolates with minimal sample preparation in static self-dispensing 64 and 384 assay cards. Appl Microbiol Biotechnol. 2015;99:7711–7722. doi: 10.1007/s00253-015-6774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatosy SM, Martiny AC. The ocean as a global reservoir of antibiotic resistance genes. Appl Environ Microbiol. 2015;81:7593–7599. doi: 10.1128/AEM.00736-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang-Tai Z, Yang L, Zhong-Yi H, Chang-Song Z, Yin-Ze K, Yong-Ping L, Chun-Lei D. High frequency of integrons related to drug-resistance in clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol. 2011;29:118–123. doi: 10.4103/0255-0857.81784. [DOI] [PubMed] [Google Scholar]
- 38.Martí S, Fernández-Cuenca F, Pascual A, Ribera A, Rodríguez-Baño J, Bou G, Miguel Cisneros J, Pachón J, Martínez-Martínez L, Vila J, Grupo de Estudio de Infección Hospitalaria (GEIH) Prevalence of the tetA and tetB genes as mechanisms of resistance to tetracycline and minocycline in Acinetobacter baumannii clinical isolates. Enferm Infecc Microbiol Clin. 2006;24:77–80. doi: 10.1157/13085012. In Spanish. [DOI] [PubMed] [Google Scholar]
- 39.Post V, Hall RM. AbaR5, a large multiple-antibiotic resistance region found in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:2667–2671. doi: 10.1128/AAC.01407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agersø Y, Petersen A. The tetracycline resistance determinant Tet 39 and the sulphonamide resistance gene sulII are common among resistant Acinetobacter spp. isolated from integrated fish farms in Thailand. J Antimicrob Chemother. 2007;59:23–27. doi: 10.1093/jac/dkl419. [DOI] [PubMed] [Google Scholar]