Abstract
To investigate the (co)expression, interaction, and membrane location of multifunctional NAD(P)H dehydrogenase type 1 (NDH-1) complexes and their involvement in carbon acquisition, cyclic photosystem I, and respiration, we grew the wild type and specific ndh gene knockout mutants of Synechocystis sp PCC 6803 under different CO2 and pH conditions, followed by a proteome analysis of their membrane protein complexes. Typical NDH-1 complexes were represented by NDH-1L (large) and NDH-1M (medium size), located in the thylakoid membrane. The NDH-1L complex, missing from the ΔNdhD1/D2 mutant, was a prerequisite for photoheterotrophic growth and thus apparently involved in cellular respiration. The amount of NDH-1M and the rate of P700+ rereduction in darkness in the ΔNdhD1/D2 mutant grown at low CO2 were similar to those in the wild type, whereas in the M55 mutant (ΔNdhB), lacking both NDH-1L and NDH-1M, the rate of P700+ rereduction was very slow. The NDH-1S (small) complex, localized to the thylakoid membrane and composed of only NdhD3, NdhF3, CupA, and Sll1735, was strongly induced at low CO2 in the wild type as well as in ΔNdhD1/D2 and M55. In contrast with the wild type and ΔNdhD1/D2, which show normal CO2 uptake, M55 is unable to take up CO2 even when the NDH-1S complex is present. Conversely, the ΔNdhD3/D4 mutant, also unable to take up CO2, lacked NDH-1S but exhibited wild-type levels of NDH-1M at low CO2. These results demonstrate that both NDH-1S and NDH-1M are essential for CO2 uptake and that NDH-1M is a functional complex. We also show that the Na+/HCO3− transporter (SbtA complex) is located in the plasma membrane and is strongly induced in the wild type and mutants at low CO2.
INTRODUCTION
NAD(P)H dehydrogenase type 1 (NDH-1) complexes have been reported to have multiple functions both in cyanobacteria and plant chloroplasts. Common for both organisms seems to be the function in respiration (chlororespiration in chloroplasts) and in cyclic photosystem I (PSI) (Ogawa, 1991; Mi et al., 1992; Burrows et al., 1998; Munekage et al., 2004). The genome analysis has revealed 11 genes encoding NDH-1 subunits (NdhA to NdhK) in Synechocystis 6803 (Kaneko et al., 1996) as well as in chloroplasts of several plant species (Friedrich et al., 1995). Most of these genes are present as single copies in cyanobacterial genomes, except for the ndhD and ndhF genes, which in Synechocystis 6803 comprise small gene families of six and three members, respectively (Kaneko et al., 1996; Shibata et al., 2001). Reverse genetics has been essential in revealing the roles of specific Ndh subunits (Price et al., 1998; Ogawa and Kaplan, 2003). NDH-1 complexes containing NdhD1 and NdhD2 proteins, together with NdhF1, have been postulated to function in PSI-associated cyclic electron flow as well as in cellular respiration (Mi et al., 1992, 1995; Ohkawa et al., 2000a). NDH-1 complexes with other NdhD and NdhF gene products have been suggested to have additional functions in carbon concentrating mechanisms in cyanobacteria (Ohkawa et al., 1998, 2000a; Price et al., 1998; Klughammer et al., 1999; Shibata et al., 2001; Maeda et al., 2002). These mechanisms are important in aquatic organisms to overcome the low affinity of their ribulose-1,5-bisphosphate carboxylase/oxygenase to CO2 (Volokita et al., 1984; Kaplan and Reinhold, 1999; Badger and Spalding, 2000; Price et al., 2002).
Four distinct inorganic carbon (Ci) acquisition systems have been identified in cyanobacteria by reverse genetics approaches (Ogawa and Kaplan, 2003). Two of them are specialized in CO2 uptake (Ohkawa et al., 2000a; Shibata et al., 2001; Maeda et al., 2002). One is a constitutively expressed low-affinity CO2 uptake system, and the other one is a high-affinity CO2 uptake system induced at limiting CO2 conditions. Reverse genetics with cyanobacteria has demonstrated that the inducible CO2 uptake system involves the NdhD3 and NdhF3 proteins, whereas the constitutively expressed CO2 uptake system involves NdhD4 and NdhF4 proteins (Ohkawa et al., 2000a; Shibata et al., 2001; Maeda et al., 2002). Moreover, the two homologous proteins CupA and CupB are essential for inducible and constitutive CO2 uptake, respectively (Shibata et al., 2001; Maeda et al., 2002). Function of both these CO2 uptake systems has been suggested to occur via specialized NDH-1 complexes (Ohkawa et al., 2000a). The other two Ci acquisition systems in cyanobacteria are involved in bicarbonate transport. The more important one in Synechocystis 6803 is a Na+-dependent HCO3− transporter, which is strongly inducible under Ci limitation. This transporter is encoded by the sbtA gene and appears to operate as a Na+/HCO3− symporter (Shibata et al., 2002). The ATP binding cassette transporter, BCT1 (Omata and Ogawa, 1986; Omata et al., 1999), on the other hand, has been shown to have only little, if any, effect on HCO3− transport activity in Synechocystis 6803 (Shibata et al., 2002).
Although considerable progress has been made during the past few years in elucidating the functions of the NDH-1 complexes in cyanobacteria and chloroplasts, the structural bases and cooperation of various complexes still requires elaborate research. One major obstacle in this elucidation has been the lack of profound knowledge on protein level of the multiplicity and the composition of the complexes participating in different functions. To understand the structural and functional basis of cyanobacterial membrane complexes, we have started a functional proteomics project with Synechocystis 6803. Recently, we reported the presence of four distinct complexes containing ndh gene products in Synechocystis 6803 membrane: NDH-1L (large), NDH-1M (medium size), NDH-1S1 (small1), and NDH-1S2 (small2) (Herranen et al., 2004). Here, we have investigated the function, cooperation and subunit composition of these complexes by performing membrane proteomics of specific ndh gene knockout mutants of Synechocystis 6803 grown under high and low CO2 and pH conditions. We provide evidence that the thylakoid membrane–associated low CO2 inducible complex NDH-1S, composed of NdhD3, NdhF3, CupA, and Sll1735, is functionally active in CO2 uptake when coexpressed with the NDH-1M complex. NDH-1M complex contains all known single copy ndh gene products but lacks the NdhD and NdhF subunits. This complex is efficient in P700+ rereduction, implying the function in PSI cyclic electron flow. The NDH-1L complex closely resembles in subunit composition the NDH-1 complex of Escherichia coli and the chloroplast thylakoid NDH-1 complex (Friedrich and Scheide, 2000). This complex is constitutively expressed and particularly important when cellular energy production relies on respiration.
RESULTS
Growth of the Wild Type and ndh Mutant Strains at Different pH and CO2 Conditions
To address the vitality of cells by induction of Ci acquisition systems (and/or various Ndh-containing complexes) at low CO2, we first cultured Synechocystis wild type and Ci acquisition mutant strains M55 (ΔNdhB), ΔNdhD3, ΔNdhD4, ΔNdhD3/D4, and ΔNdhD3/D4/SbtA at 3% CO2, pH 7.5, and then shifted the cells to low (air level) CO2, pH 7.5 or 8.3 (in former pH the Ci species are depleted in HCO3−, whereas at alkaline pH Ci is mostly present as HCO3−). Although the Ndh mutants used in this study have previously been characterized in their capacity for CO2 uptake, respiration and cyclic PSI electron flow under some of the growth conditions described above (Ohkawa et al., 2000a), we found it crucial to compare their growth capacities at CO2 downshift both at pH 7.5 and 8.3, the conditions that induced differential expression of the Ci acquisition and Ndh complexes in different Synechocystis strains (see later). It is also important to note that the cells were always cultured in liquid medium because it is known from previous studies that the Ci acquisition systems might be displayed differently when cells are grown on plates directly in contact with air (Ohkawa et al., 2000b).
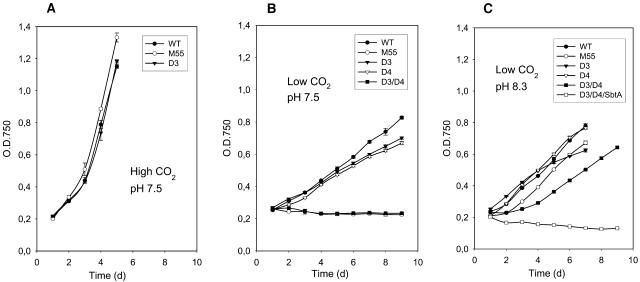
As shown in Figure 1A, the wild type, M55, and ΔndhD3 grew equally well at high CO2 (as also the ΔNdhD4, ΔNdhD3/D4, and ΔNdhD3/D4/SbtA strains; data not shown). At low CO2, only ΔNdhD3, ΔNdhD4, and the wild type were capable of growing at pH 7.5, however, with a substantially slower rate than at high CO2 (Figure 1B). The mutants M55 (deficient in NdhB protein; Ogawa, 1991) and ΔNdhD3/D4 did not grow under these conditions. Moreover, M55 became bleached and died after a few days of incubation (as also the triple mutant ΔNhdD3/D4/SbtA; data not shown), whereas ΔNdhD3/D4 remained green, yet the growth of this mutant was suppressed. When the cells were shifted from high CO2 to low CO2 and pH 8.3 (Figure 1C), both M55 and the ΔNhdD3/D4 double mutant were capable of growing, though the latter one exhibiting a retarded growth rate compared with the other strains. Only the triple mutant ΔNhdD3/D4/SbtA could not sustain viability and became bleached upon culturing at low CO2 and pH 8.3.
Figure 1.
Growth Curves of Wild-Type Synechocystis 6803 Cells and M55, ΔNdhD3, ΔNdhD4, ΔNdhD3/D4 Double Mutant, and ΔNdhD3/D4/SbtA Triple Mutant.
(A) Growth in BG-11 medium, pH 7.5, 3% CO2.
(B) Growth in BG-11 medium, pH 7.5, air level of CO2.
(C) Growth in BG-11 medium, pH 8.3, air level of CO2.
Identification and Diversity of the Ndh-Containing Complexes and the SbtA Complex in M55 Strain as Compared with Wild-Type Cells
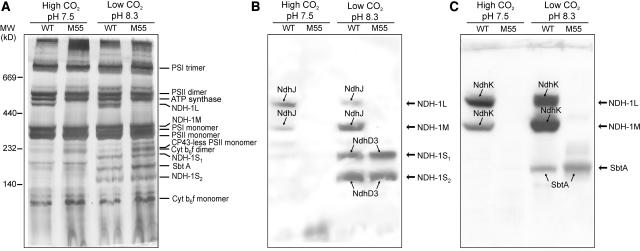
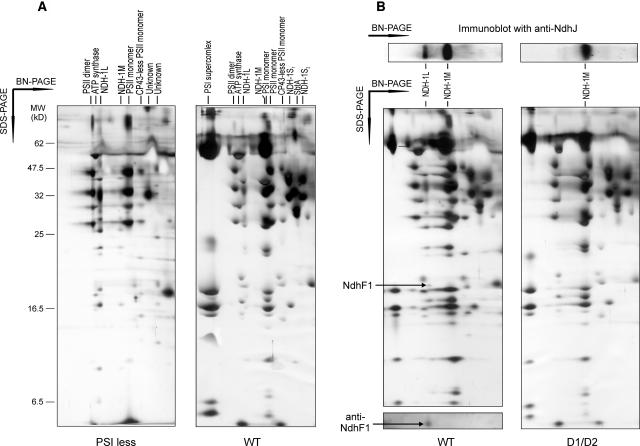
Crude thylakoid membrane fractions were isolated from wild-type and M55 mutant cells grown in liquid cultures either at high CO2, pH 7.5, or at low CO2, pH 8.3. The membranes were then subjected to blue-native (BN)-PAGE separation of the intrinsic membrane protein complexes (Figure 2). The cytochrome b6f complex as monomers and dimers, PSII as monomers and dimers, ATP synthase, and PSI monomers and trimers were well distinguished in all membranes (Figure 2A) (for matrix-assisted laser-desorption ionization time of flight [MALDI-TOF] identification, see Herranen et al., 2004). The NdhJ- and NdhK-specific antibodies were used in immunoblotting to identify the NDH-1 complexes. These antibodies reacted with two membrane protein complexes, NDH-1L and NDH-1M (Figures 2B and 2C, respectively). These complexes were present in wild-type cells under both growth conditions tested here but absent from M55 membranes independently of the growth conditions. It is noteworthy that in wild-type membranes under high CO2 conditions the NDH-1L complex was more abundant than the NDH-1M complex, whereas at low CO2 and pH 8.3 the NDH-1M complex was clearly the more dominant one. After immunoblotting with the NdhJ and NdhK antibodies, the membranes were probed with the NdhD3-, NdhF3-, and SbtA-specific antibodies. Two considerably smaller protein complexes were detected with the NdhD3 antibody (Figure 2B) and also with the NdhF3 antibody (data not shown). These complexes, NDH-1S1 and NDH-1S2, appeared in both wild-type and M55 cells grown in low CO2 and were absent in high CO2. The SbtA antibody reacted with a protein complex between the two NDH-1S complexes (Figure 2C). The SbtA complex appeared only when the cells were grown at low CO2, showing a similar expression profile as the NDH-1S complexes. The apparent molecular mass of the SbtA complex was estimated to be ∼160 kD.
Figure 2.
Membrane Protein Complexes of Synechocystis 6803 Wild Type and the M55 Strain.
Wild-type and M55 cells were grown at 3% CO2 in BG-11 medium at pH 7.5 or at air level of CO2 in BG-11 medium at pH 8.3. Cells were then harvested and crude thylakoid membranes isolated as described in Methods. After solubilization of membranes with 1.5% n-dodecyl-β-d-maltoside, the protein complexes were separated by BN-PAGE.
(A) The BN-gel was stained with silver. Molecular markers are indicated to the left, and the assignment of the major protein complexes is given to the right.
(B) Protein complexes were electroblotted to a polyvinylidene difluoride (PVDF) membrane, and the membrane was first probed with anti-NdhJ to identify the NDH-1 complexes (NDH-1L and NDH-1M) and subsequently with anti-NdhD3, which recognized the NDH-1S1 and NDH-1S2 complexes.
(C) PVDF membrane was first probed with anti-NdhK and subsequently with anti-SbtA to recognize the Na+/HCO3− transporter. Protein bands indicated by arrows interacted with respective antibodies.
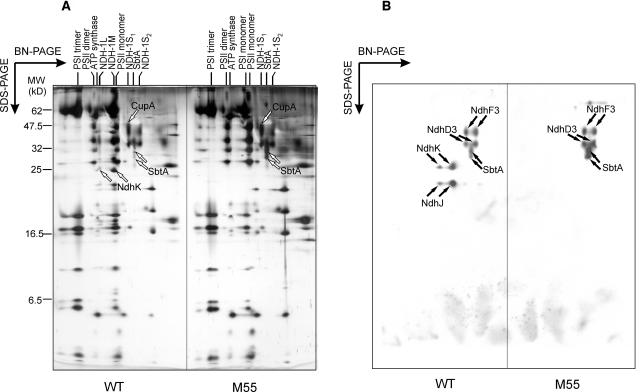
To study the subunit diversity of Ndh-containing complexes in more detail, the BN gel lanes of the wild type and M55 (low CO2, pH 8.3) (Figure 2) were subjected to SDS-PAGE in the second dimension (Figure 3A). General features of Synechocystis 6803 membrane proteome were previously described in Herranen et al. (2004). The two-dimensional (2-D) gels were analyzed by sequential immunoblotting with the NdhJ, NdhK, NdhD3, NdhF3, and SbtA antibodies. Immunoblots clearly indicated the absence of NdhD3 and NdhF3 proteins from the NDH-1L and NDH-1M complexes, and conversely, the complete absence of NdhJ and NdhK proteins from the NDH-1S complexes (Figure 3B). It is also noteworthy that neither NdhJ nor NdhK were found as free proteins in the thylakoid membrane. Distinct of wild-type cells grown at low CO2 and high pH (8.3) was an abundant NDH-1M complex (Figures 3A and 3B). Both the NDH-1L and NDH-1M complexes, composed of at least 10 different subunits (Figure 3), were absent from M55 cells deficient in the NdhB protein. As already noted from one-dimensional BN gels, both wild-type and M55 cells, grown at low CO2, exhibited considerable amounts of both the NDH-1S and SbtA complexes. The identities of NdhK, CupA, and SbtA proteins, present in NDH-1L/M, NDH-1S1, and SbtA complexes, respectively, were further verified by MALDI-TOF mass spectrometry (Figure 4).
Figure 3.
Two-Dimensional Analysis of Synechocystis 6803 Wild Type and M55 Membrane Protein Complexes from Cells Grown at Low CO2, pH 8.3.
After separation of the protein complexes in the BN gel, the lane was cut out, solubilized with Laemmli buffer, and subjected to SDS-PAGE.
(A) Silver stained gels of wild-type and M55 membranes (crude thylakoid preparations).
(B) Gels were electroblotted to a PVDF membrane and probed sequentially with NdhK, NdhJ, NdhD3, NdhF3, and SbtA antibodies revealing the presence or absence, as well as the positions, of the NDH-1L, NDH-1M, NDH-1S complexes (S1 and S2), and the SbtA complex.
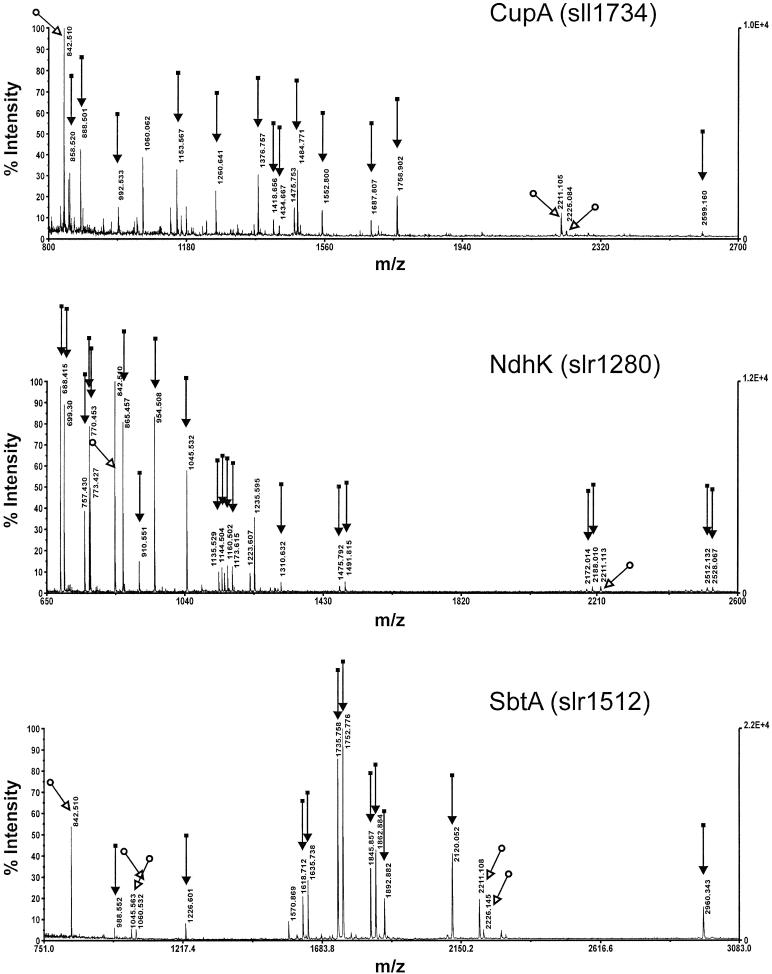
Figure 4.
Identification by MALDI-TOF Mass Spectrometry of the NdhK Protein Present in the NDH-1L and NDH-1M Complexes, the CupA Protein Present in the NDH-1S1 Complex, and the SbtA Protein in the HCO3− Transporter Complex.
Protein spots for identification were taken from the gel in Figure 3. Essentially similar SbtA spectra were obtained from three different partially overlaying spots. m/z, mass-to-charge ratio.
In the SbtA complex, several protein bands with slightly different mobility in SDS-PAGE were detected in silver stained gels (Figure 3A). Analysis by two independent methods, MALDI and immunoblotting, identified them as SbtA, suggesting an involvement of posttranslational modifications of this protein.
Coexpression of the Ndh-Containing and SbtA Complexes in Wild-Type and Ci Acquisition Mutants as Influenced by Growth Conditions
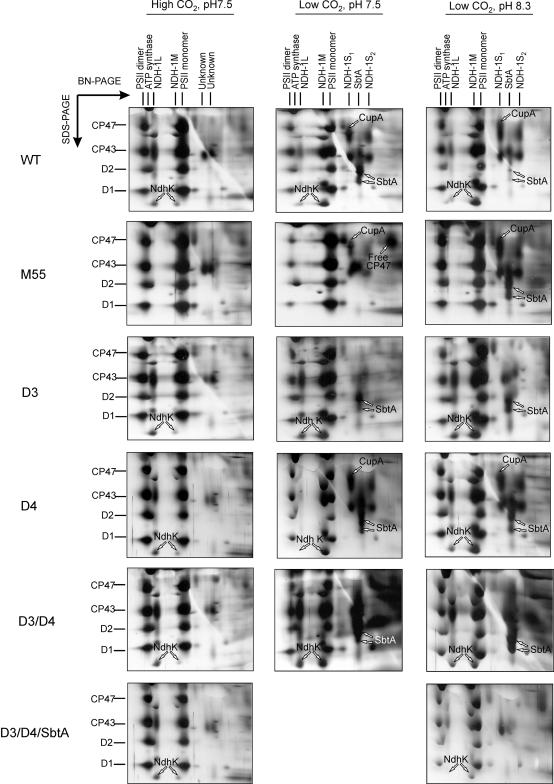
To get further insights into the induction, coexpression, and function of the Ndh-containing and SbtA complexes, we subjected the wild type and different ndh mutant strains to proteome analysis before and after the CO2 downshift at pH 7.5 and 8.3 for 24 h, if not otherwise indicated (conditions corresponding to those in Figure 1). In addition to the ΔNdhB mutant M55, the membrane protein complexes of several other Ci acquisition mutants, ΔNdhD3, ΔNdhD4, ΔNdhD3/D4, and ΔNdhD3/D4/SbtA, were subjected to 2-D BN/SDS-PAGE analysis. Sections of silver-stained gels in Figure 5, enclosing the major components of the Ndh-containing complexes and the SbtA complex, make it possible to evaluate the coexpression of these protein complexes with each other.
Figure 5.
Sections of Silver-Stained 2-D BN/SDS-PAGE Gels from Synechocystis Wild Type and M55, ΔNdhD3, ΔNdhD4, ΔNdhD3/D4 Double Mutant, and ΔNdhD3/D4/SbtA Triple Mutant, Enclosing the Major Components of the Carbon Acquisition Complexes and the PSII Monomer and Dimer Complexes.
Major PSII proteins CP47, CP43, D2, and D1 present in PSII monomers and dimers, identified by MALDI (data not shown), are indicated to the left. Membranes were isolated from cells grown under high CO2, pH 7.5, and after a CO2 downshift at pH 7.5 and 8.3 for 24 h. The NdhK protein in NDH-1L and NDH-1M complexes, CupA in the NDH-1S1 complex, and SbtA are indicated by arrows. CP47 released from the PSII monomer in M55 cells shifted to low CO2, pH 7.5, was identified with mass spectrometry (data not shown) and is likewise indicated by an arrow.
Under high CO2 conditions, the presence of a dominant NDH-1L complex was distinct to the membranes of all strains except M55 (Figure 5, left panel), and all other complexes harboring ndh gene products, as well as the SbtA complex, were either absent or present only in very low amounts (NDH-1M).
At low CO2 and pH 7.5, the Ci acquisition and NDH-1 complexes showed somewhat varying patterns between the different mutant strains (Figure 5, middle panel). Typical for wild-type cells was a strong expression of NDH-1M, NDH-1S1, and NDH-1S2 as well as of the bicarbonate transporter SbtA. Both NDH-1L and NDH-1M were absent from M55. The membrane proteome of M55 showed initially, within 24 h after CO2 downshift, some transient upregulation of the NDH-1S complexes (Figure 5). However, already after 48 h of incubation at low CO2, the newly synthesized NDH-1S complexes were degraded (data not shown). Another distinct feature of the M55 cells at low CO2 pH 7.5 was the lack of accumulation of the SbtA transporter, in sharp contrast with that observed in the wild-type cells. It is also noteworthy that free CP47 protein (identified by MALDI-TOF; data not shown) of photosystem II (PSII) accumulated in M55 membranes under these conditions, referring to oxidative stress and destruction of the PSII complexes (Aro et al., 2005). The ΔNdhD3 and ΔNdhD3/D4 mutants showed yet a different membrane proteome with regard to Ci transporters and NDH-1 complexes when incubated at low CO2, pH 7.5. The NDH-1S complex was absent but particularly the NDH-1M complex accumulated heavily. Intriguingly, the NdhD3/D4 double mutant showed a prominent expression of the SbtA transporter as well (Figure 5).
Low CO2 at pH 8.3 induced different patterns of Ci acquisition and NDH-1 complexes compared with that at pH 7.5, especially in the M55 strain (Figure 5, right panel). At elevated pH (8.3), M55 showed an evident upregulation of both the NDH-1S and SbtA complexes, which at pH 7.5 remained at a low level or were completely unexpressed, respectively (Figure 5). Patterns of the carbon acquisition complexes of the wild type, ΔNdhD3, ΔNdhD4, and ΔNdhD3/D4, on the other hand, did not qualitatively differ from those occurring at low CO2 at pH 7.5.
The membrane proteome of the ΔNdhD4 mutant did not significantly differ from that of the wild-type cells (Figure 5). It seems that our proteome approach did not recognize the constitutively expressed CO2 uptake system composed of NdhD4/NdhF4/CupB (Ohkawa et al., 2000a), possibly because of a low abundance of this complex.
The growth capacity and the expression of various NDH-1 and Ci acquisition complexes of the wild type and the Ci acquisition mutants at high CO2 and after CO2 downshift (pH 7.5 and 8.3) are summarized in Table 1.
Table 1.
Summary of the Growth and Expression of the Ndh and SbtA Containing Protein Complexes in the Wild Type and Different Carbon Acquisition Mutants of Synechocystis 6803
| Growth Conditions
|
||||
|---|---|---|---|---|
| Strain | Growth/Complex | High CO2, pH 7.5 | Low CO2, pH 7.5 | Low CO2, pH 8.3 |
| Wild type | Growth | ++ | + | + |
| NDH-1L | ++ | +/− | + | |
| NDH-1M | +/− | ++ | ++ | |
| NDH-1S | − | ++ | ++ | |
| SbtA | − | + | + | |
| M55 | Growth | ++ | − | + |
| NDH-1L | − | − | − | |
| NDH-1M | − | − | − | |
| NDH-1S | − | +/− | ++ | |
| SbtA | − | − | +++* | |
| D3 | Growth | ++ | + | + |
| NDH-1L | ++ | + | + | |
| NDH-1M | +/− | ++ | ++ | |
| NDH-1S | − | − | − | |
| SbtA | − | + | + | |
| D4 | Growth | ++ | + | + |
| NDH-1L | ++ | +/− | + | |
| NDH-1M | +/− | ++ | ++ | |
| NDH-1S | − | ++ | ++ | |
| SbtA | − | + | + | |
| D3/D4 | Growth | ++ | +/− | + |
| NDH-1L | ++ | ++ | ++ | |
| NDH-1M | +/− | ++ | ++ | |
| NDH-1S | − | − | − | |
| SbtA | − | ++ | ++ | |
| D3/D4/SbtA | Growth | ++ | − | − |
| NDH-1L | ++ | n.a. | +/− | |
| NDH-1M | +/− | n.a. | + | |
| NDH-1S | − | n.a. | − | |
| SbtA | − | n.a. | − | |
+++, Exceptionally strong; ++, strong; +, moderate; +/−, poor; −, none (cell bleached); n.a., not analyzed; *, see Figure 9.
Expression of NDH-1 Complexes in PSI-Less and ΔNdhD1/D2 Mutants of Synechocystis 6803
To further clarify the functional role(s) of the NDH-1L and NDH-1M complexes, we investigated the membrane proteomes of yet two other strains, the PSI-less mutant, which cannot perform cyclic PSI electron transfer, and the ΔNdhD1/D2 mutant, which is not capable of photoheterotrophic growth (Ohkawa et al., 2000a; confirmed here, data not shown).
The PSI-less mutant grew only heterotrophically in the presence of glucose. Thus, the energy for cell growth and metabolism was likely to be derived from respiration. NDH-1L was by far the most dominating NDH-1 complex in this strain, and only traces of NDH-1M were detected (Figure 6A). This was in sharp contrast with wild-type cells grown under similar conditions in the presence of glucose, having only small amounts of NDH-1L as compared with the dominating NDH-1M complex (Figure 6A). Moreover, the NDH-1S and SbtA complexes present in the wild type were completely missing in the PSI-less mutant.
Figure 6.
Proteomes of the Membrane Protein Complexes of the PSI-Less Mutant and the ΔNdhD1/D2 Mutant as Compared with the Wild-Type Strain Grown under Similar Conditions.
(A) The wild type and the PSI-less mutant were grown in BG-11 medium supplemented with 5 mM glucose at low CO2, pH 7.5, 5 μmol photons m−2 s−1. The crude thylakoid membrane fraction was isolated and subjected to 2-D BN/SDS-PAGE, and the gel was stained with silver.
(B) The wild type and the ΔNdhD1/D2 mutant were grown at low CO2, pH 7.5, and 50 μmol photons m−2 s−1. On the top of the silver-stained 2-D gels is shown an immunoblot of one-dimensional BN gel probed with anti-NdhJ to demonstrate the locations and abundances of the NDH-1L and NDH-1M complexes in silver-stained gels below, prepared after 2-D BN/SDS-PAGE. Below wild-type membranes is shown an NdhF1 immunoblot after 2-D BN/SDS-PAGE. Arrows indicate the spot reacting with anti-NdhF1.
The ΔNdhD1/D2 mutant, on the other hand, was capable of growing in normal BG-11 medium at low CO2 and pH 7.5, the conditions sustaining the expression of all Ndh-containing complexes in wild-type cells (Figure 6B). The ΔNdhD1/D2 mutant, however, lacked the NDH-1L complex (Figure 6B) but interestingly enough, had a relatively high level of the NDH-1M complex, similar to that in the wild type. Shift of the ΔNdhD1/D2 mutant to high CO2 drastically decreased the amount of NDH-1M (data not shown). Addition of glucose and DCMU to the growth medium induced bleaching of the ΔNdhD1/D2 mutant as well as M55, whereas wild-type cells continued growing and upregulated the expression of the NDH-1L complex (data not shown). The NDH-1L complex thus appeared to be a prerequisite for photoheterotrophic growth and could not be replaced by the NDH-1M complex.
As evidenced by the ΔNdhD1/D2 mutant, the NdhD1 (or D2) subunit is clearly missing from the NDH-1M complex. The other difference between the NDH-1L and NDH-1M complexes was the absence of the NdhF1 subunit from the NDH-1M complex (Figure 6B). Besides recognition of NdhF1 by protein-specific antibody, the identity of the NdhF1 subunit missing from NDH-1M was further proven by N-terminal sequencing, which gave similar results to those by Prommeenate et al. (2004). It is noteworthy that the NdhF1 antibody did not recognize any smaller complexes or free NdhF1 proteins in the thylakoid membrane.
To obtain insights into the function of the NDH-1M complex, we next measured the cyclic PSI electron transfer by monitoring the reduction rate of P700+ in darkness after illumination of cells with far red light. As shown in Table 2, the half-time of P700+ rereduction in the ΔNdhD1/D2 mutant was similar to that in wild-type cells, in accordance with similar amounts of the NDH-1M complex in these two strains under the given growth conditions. For comparison, the cyclic electron transfer rates for wild-type and M55 cells, grown at high CO2, were also measured (Table 2). The wild type showed two times and M55 10 times slower rereduction of P700+ in darkness as compared with wild-type cells grown at low CO2 conditions. This slow rate of P700+ rereduction was accompanied with a small amount of NDH-1M in wild-type cells at high CO2 and a complete lack of NDH-1M (and NDH-1L) in M55 cells (Figures 2 and 5).
Table 2.
Half-Time of P700+ Reduction in Darkness after Far Red Light Illumination of Wild-Type, ΔNdhD1/D2, and M55 (ΔNdhB) Mutants Grown at High and Low CO2
| Strain | Growth Conditions | t1/2 of P700+ Reduction |
|---|---|---|
| Wild type | Low CO2 | 252 ms ± 29 ms |
| ΔNdhD1/D2 | Low CO2 | 220 ms ± 32 ms |
| Wild type | High CO2 | 529 ms ± 31 ms |
| M55 | High CO2 | 2162 ms ± 154 ms |
Measurements were made at 26.5 W m−2 far red light with maximum at 715 nm. Results are a mean ± se of at least three independent cultures.
Ndh-Containing Complexes in Thermosynechococcus elongatus
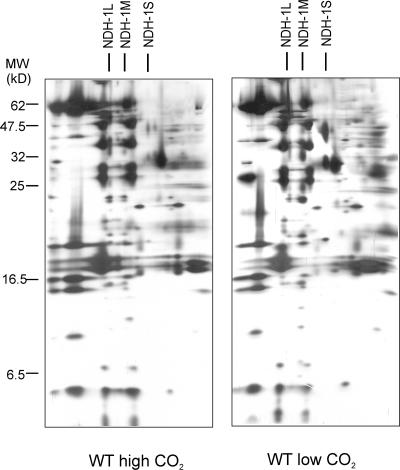
The diversity and integrity of the thylakoid Ndh protein complexes was also tested with T. elongatus, a cyanobacterial strain known for the stability of the membrane protein complexes and, therefore, generally used for structural analysis and crystallization of membrane complexes. The proteome of the thylakoid membrane protein complexes, isolated from cells grown at high and low CO2, pH 8.3, is shown in Figure 7. Compared with Synechocystis 6803 (Figures 3 to 6), a distinct feature was the integrity of the NDH-1S complex—only one NDH-1S complex, corresponding to NDH-1S1, was detected in T. elongatus. Also importantly, both the NDH-1M and NDH-1L complexes were present (Figure 7). Moreover, the regulation of the abundances of these complexes by CO2 was similar to that in Synechocystis 6803, the NDH-1M complex being the dominating one under low CO2 conditions.
Figure 7.
Two-Dimensional Analysis of Membrane Protein Complexes from T. elongatus Cells Grown at High and Low CO2, pH 8.3.
NDH-1L, NDH-1M, and NDH-1S complexes are marked on the top of the gel.
Localization of SbtA and the Ndh Proteins to the Plasma and the Thylakoid Membrane
To localize different NDH-1 complexes and the SbtA proteins to the thylakoid and the plasma membrane of Synechocystis 6803, we took advantage of the two-phase partitioning system in purification of the two membrane compartments. As a criterion for the purity of the plasma and thylakoid membranes, we probed the membrane fractions with the plasma membrane–specific NrtA and the thylakoid membrane–specific CP43 antibodies (Norling et al., 1998). As shown in Figure 8, the obtained membrane fractions were pure, and indeed the marker proteins were exclusively present in the plasma membrane (NrtA) or the thylakoid membrane (CP43). All tested Ndh proteins, NdhD3, NdhF3, NdhJ, and NdhK, were detected only in the thylakoid membrane fraction, as previously reported also for the NdhH protein (Ohkawa et al., 2001). On the other hand, SbtA was recorded solely from the plasma membrane fraction. Thus, SbtA observed in the crude thylakoid preparation both here and in our previous article (Herranen et al., 2004) is because of cytoplasma membrane contamination. Indeed, there seems to be a strict compartmentalization of all Ndh proteins to the thylakoid membrane and the Na+/HCO3− transporter SbtA to the plasma membrane.
Figure 8.
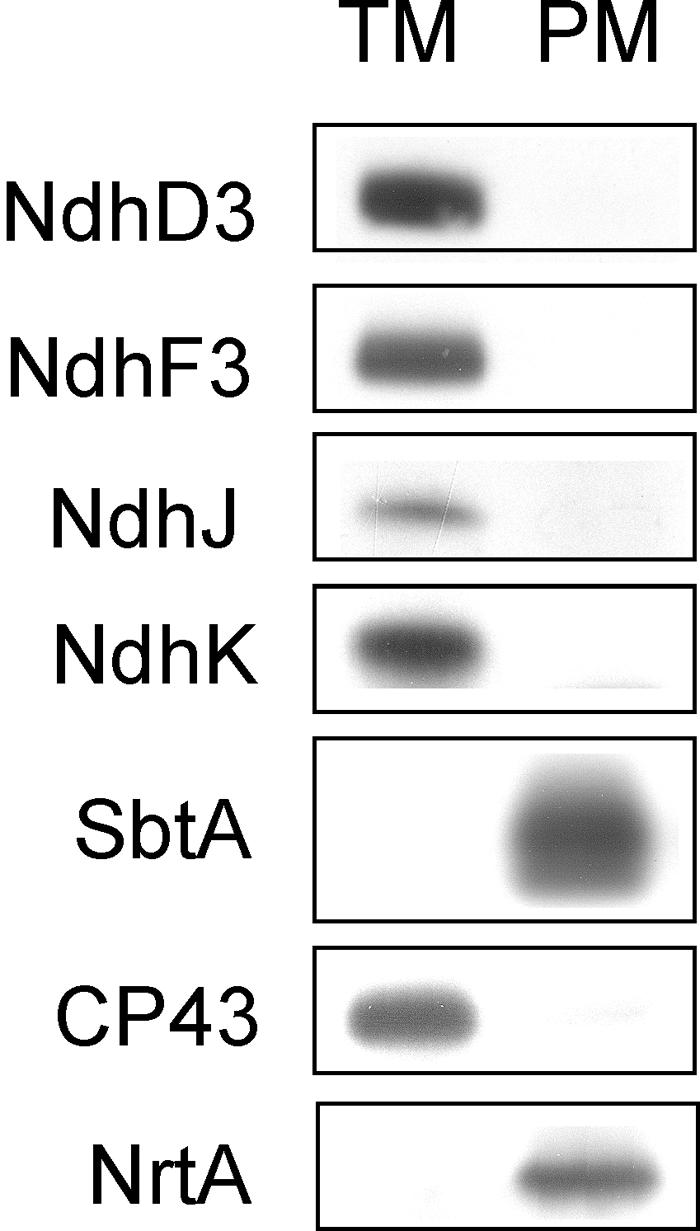
Location of the Various Carbon Acquisition Systems and NDH-1 Complexes in the Thylakoid Membrane and the Plasma Membrane.
The purified membrane fractions were obtained by sucrose density fractionation and subsequent purification of the thylakoid and plasma membranes in the two-phase partitioning system composed of dextran and polyethylene glycol. CP43 and NrtA were used as markers for the purity of the plasma and the thylakoid membrane fractions, respectively. Anti-NdhD3 and anti-NdhF3 were used to localize the marker proteins of the NDH-1S complexes and anti-NdhJ and anti-NdhK the marker proteins of the NDH-1L and NDH-1M complexes to the thylakoid membrane, whereas Anti-SbtA localizes the Na+/HCO3− transporter to the plasma membrane.
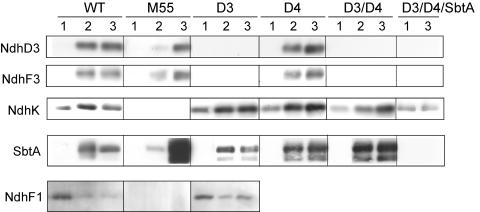
Abundance of Distinct Ndh Proteins and SbtA in Synechocystis Membranes of Different Strains in Response to Growth Conditions
Because of different membrane locations of the Ndh and SbtA proteins, the comparison of the abundances of these proteins in various Synechocystis 6803 strains acclimated to high and low CO2, pH either 7.5 or 8.3, was performed using total (thylakoid plus plasma) membrane fractions isolated from the wild type, M55, ΔNdhD3, ΔNdhD4, ΔNdhD3/D4, and ΔNdhD3/D4/SbtA. Immunoblots (Figure 9) were made based on the assumption that NdhD3/F3 proteins represent the NDH-1S complex, NdhF1 the NDH-1L complex, NdhK stands for both the NDH-1L and NDH-1M complexes, and SbtA for the HCO3− transporter in cellular membranes.
Figure 9.
Immunoblots Demonstrating the Accumulation of the NdhD3, NdhF3, NdhF1, NdhK, and SbtA Proteins in the Total Membrane Fractions of Synechocystis Wild Type and Several Ci Acquisition Mutant Strains.
The cells were first grown at high CO2 (lane 1) and then shifted to low CO2 at pH 7.5 (lane 2) or 8.3 (lane 3) for 24 h before isolation of the total membrane fractions of the cells. To work on the linear region of the immunoresponse with different antibodies, 5 μg of membrane proteins were loaded in the well for detection with SbtA antibody, 20 μg protein for detection with the NdhK, NdhD3, and NdhF3 antibodies, and 40 μg protein for detection with the NdhF1 antibody.
When the strains were grown at high CO2, pH 7.5 (Figure 9, lane 1), NdhK was the only protein detected in all strains except for M55. Upon CO2 downshift (Figure 9, lanes 2 and 3), the strains reacted in different ways. In wild-type cells, the expression of NdhD3, NdhF3, NdhK, and SbtA was enhanced irrespectively of the pH of the growth medium (Figure 9, wild type, lanes 2 and 3), whereas a drastic decrease in the NdhF1 subunit took place upon CO2 downshift.
In M55, the response to low CO2 was strongly dependent on pH. The induction of NdhD3, NdhF3, and SbtA was weak but detectable at pH 7.5 (Figure 9, M55, lane 2), in contrast with pH 8.3, where NdhD3 and NdhF3 were upregulated to wild-type levels while the accumulation of SbtA was exceptionally strong (Figure 9, M55, lane 3). The ΔNdhD3 and ΔNdhD4 mutants behaved upon CO2 downshift in a pH-independent manner showing an upregulation of NdhK and SbtA and a downregulation of NdhF1 similar to the wild type (Figure 9, D3 and D4, lanes 2 and 3). NdhD3 and NdhF3 proteins, which belong to the same NDH-1S complex, were both absent from the ΔNdhD3 mutant (Figure 9, D3, lanes 2 and 3) but present in the ΔNdhD4 mutant on wild-type levels (Figure 9, D4, lanes 2 and 3). The ΔNdhD3/D4 double mutant, lacking the NdhD3 and NdhF3 proteins, showed a lower level of NdhK accumulation at pH 7.5 compared with pH 8.3 and a more abundant accumulation of SbtA at CO2 downshift as compared with wild-type membranes (Figure 9, D3 and D4, lanes 2 and 3). The triple mutant ΔNdhD3/D4/SbtA showed a response only with the NdhK antibody without any upregulation at CO2 downshift (Figure 9, D3/D4/SbtA), and this mutant eventually bleached and died in the course of incubation at low CO2 concentration.
DISCUSSION
Composition and Location of the Ndh- and SbtA-Containing Protein Complexes in Synechocystis 6803
Based on extensive reverse genetics studies, it has been postulated that different forms of NDH-1 complexes reside in Synechocystis 6803 membranes (Ohkawa et al., 1998; Price et al., 1998; Klughammer et al., 1999). These NDH-1 complexes were hypothesized to contain the same Ndh single copy gene products but different members of the NdhD/F family. NdhD3 and NdhF3 as well as NdhD4 and NdhF4 were hypothesized to form, together with the single copy gene products, two specific NDH-1 complexes functioning in inducible and constitutive CO2 transport, respectively. NdhD1/D2 and NdhF1, on the other hand, were postulated to be components of the NDH-1 complexes involved in respiration and cyclic electron flow around PSI (Ohkawa et al., 2000a). However, the structural basis of the Ndh-containing complexes has turned out to be much more diverse (Herranen et al., 2004), which prompted us to investigate distinct ndh gene deletion mutants to specify the diversity of the NDH-1 complexes and their coexpression with Ci acquisition complexes in Synechocystis 6803 membranes under different growth conditions.
Four different Ndh-containing complexes, all localized to the thylakoid membrane, were present in Synechocystis 6803 cells grown at low CO2: the NDH-1L (large, ∼490 kD), NDH-1M (medium size, ∼350 kD), and the NDH-1S1 and NDH-1S2 (small, ∼200 and 140 kD, respectively) complexes (Figure 2, the mass estimations were based on the known mass of the PSII, PSI, cytochrome b6f, and ATPase). Analysis of the protein complexes in the second dimension by SDS-PAGE (Figures 3A and 3B) revealed more than 10 protein spots in NDH-1L and NDH-1M, and their patterns imply structural similarity between these two complexes. The presence of the NdhH, NdhI, NdhJ, and NdhK proteins in these complexes was verified (Figures 2 and 3; Herranen et al., 2004). Therefore, both NDH-1L and NDH-1M contain the subunits homologous to NuoB, -C, -D, and -I comprising the interconnecting module of the E. coli NDH-1 complex (Leif et al., 1995; Holt et al., 2003), which is presumed to connect the membrane module of the complex with catalytically active subunits still unknown in cyanobacteria. Furthermore, the absence of both NDH-1L and NDH-1M from M55 strain lacking the functional ndhB gene (Figures 2A to 2C, 3A, and 3B) implies that the membrane protein NdhB (NuoN) is also an intrinsic subunit of both NDH-1L and NDH-1M. Two other hydrophobic membrane subunits, NdhD1(D2) (NuoM) and NdhF1 (NuoL), on the other hand, were present only in the NDH-1L complex (Figure 6B). Our NDH-1L complex most probably corresponds to the NDH-1 complex recently isolated from Synechocystis 6803 cells with 10 identified Ndh subunits (including NdhD1 and the C terminus of NdhF1) and two previously unidentified subunits (Prommeenate et al., 2004). The NDH-1M complex, on the other hand, lacks the NdhD1(D2) and the NdhF1 subunits but otherwise seems to be identical in the subunit composition with the NDH-1L complex (N. Battchikova, P. Zhang, S. Rudd, T. Ogawa, and E.-M. Aro, unpublished data). In overstained gels, we detected 10 subunits in NDH-1M, and they all were present also in NDH-1L, probably corresponding to NdhA, B, C, G, H, I, J, K, and two novel subunits, as reported by Prommeenate et al. (2004).
Contrary to NDH-1M and NDH-1L, NDH-1S1 is a small complex with a simple protein composition comprising NdhD3 (Figure 2B), NdhF3, CupA (Figure 4), and Sll1735 proteins, whereas NDH-1S2 is composed of only NdhD3 and NdhF3 (Herranen et al., 2004). The presence of only the NDH-1S1 complex in T. elongatus strongly suggests that the NDH-1S2 complex easily disassembles from NDH-1S1 in Synechocystis 6803. Hereafter we refer to these two complexes in Synechocystis 6803 collectively as the NDH-1S complex. This complex functions in CO2 uptake and is strongly induced by low CO2 conditions (Figures 2 and 5).
Another low CO2-induced membrane complex of ∼160 kD (Figures 2A and 2C) was localized to the plasma membrane and was composed of SbtA proteins of slightly varying molecular masses, which might be an indication of protein posttranslational modifications. Also, the ABC type bicarbonate transporter (BCT1) has been localized to the plasma membrane (Omata and Ogawa, 1986). It is therefore evident that from the inducible Ci acquisition complexes, the bicarbonate transporters function in the plasma membrane, whereas the inducible CO2 uptake system (NDH-1S) and the typical multisubunit NDH-1 complexes (NDH-1L and NDH-1M) are specific for the thylakoid membrane.
Both NDH-1S and NDH-1M Complexes Are Involved in Inducible CO2 Uptake
In wild-type cells, the expression of the NDH-1S complex starts at CO2 downshift and closely coincides with the upregulation of the NDH-1M complex. Such coexpression might suggest also functional cooperation between the NDH-1S and NDH-1M complexes. Under particular conditions, the NDH-1S complex was, however, expressed also independently of both NDH-1L and NDH-1M. This was unambiguously demonstrated in M55 cells, which despite the absence of both the NDH-1L and NDH-1M complex, accumulated NDH-1S upon CO2 downshift (Figure 5). Conversely, a strong expression of the NDH-1M complex was characteristic to the ΔNdhD3/D4 mutant completely devoid of the NDH-1S complex (Figure 5). Common to both the M55 and ΔNdhD3/D4 mutant was a suppression of growth at low CO2, pH 7.5 (Figure 1) as a result of inefficient CO2 uptake (Shibata et al., 2001). Thus, both the NDH-1S and NDH-1M complexes are essential for inducible CO2 uptake and cell survival at low CO2 and low pH. At high pH, on the other hand, Ci mainly occurs in a form of bicarbonate ions that are efficiently taken up by the SbtA transporter, which was strongly induced both in M55 and ΔNdhD3/D4 mutants making the growth possible.
It was recently demonstrated by a genome-wide DNA microarray analysis that the ndhF3/ndhD3/cupA operon and the sbtA gene are both upregulated as a result of inactivation of the ndhR gene, a LysR family regulator of Ci uptake (Wang et al., 2004). It is conceivable that Ci availability and associated pH homeostasis under given growth conditions regulate the ndhR gene expression, which in turn coordinately controls the expression of both the CO2 and HCO3− uptake systems. In this regard, it is interesting that the single copy ndh genes, whose products constitute the NDH-1M complex, are not under a direct control of NdhR (Wang et al., 2004), thus possibly allowing, when appropriate, an independent expression of the CO2 uptake (NDH-1S) complex from NDH-1M. Generally at CO2 downshift, the upregulation is particularly prominent for genes directly involved in Ci acquisition (the ndhF3/ndhD3/cupA operon and the sbtA gene) but also occurs for the single copy ndh genes (Wang et al., 2004), whose products constitute the NDH-1M complex, thus being in accordance with the abundance of both the NDH-1S and NDH-1M complexes at low CO2 conditions (Figure 5). Interestingly, the ndhD1(D2) and ndhF1 genes rather respond negatively to CO2 downshift (Wang et al., 2004), which in turn is reflected in a low abundance of the NdhF1 protein (Figure 9) and the NDH-1L complex at low CO2 conditions (Figure 5).
NDH-1M and NDH-1L Are Different Protein Complexes with Distinct Functions
As discussed above, the transfer of Synechocystis cells from high to low CO2 induced an upregulation of the NDH-1M complex, particularly in the wild type and the ΔNdhD3, ΔNdhD4, and ΔNdhD3/D4 mutants (Figures 2 and 5, Table 1), whereas the contents of NDH-1L were downregulated. It has been reported previously that the shift of cyanobacterial cells to low CO2 also increases cyclic electron flow around PSI (Deng et al., 2003; Table 2 for wild-type cells). NDH-1–mediated cyclic PSI electron transfer was first reported in cyanobacteria (Ogawa, 1991; Mi et al., 1995), and recently this pathway was shown to be essential for efficient photosynthesis also in plant chloroplasts (Munekage et al., 2004). It is conceivable that in Synechocystis 6803 the NDH-1M complex, which showed distinct upregulation at low CO2, is specifically involved in cyclic PSI. Therefore, we analyzed the capacity for PSI cyclic electron flow (rereduction of P700+ in darkness) in the ΔNdhD1/D2 mutant lacking the NDH-1L complex but having a prominent NDH-1M complex at low CO2 (Figure 6B). The ΔNdhD1/D2 mutant showed wild-type rates of P700+ rereduction at low pH and low CO2 (Table 2), and the same mutant was previously shown also to have wild-type levels of CO2 uptake under similar growth conditions (Ohkawa et al., 2000a). Thus, the upregulation of PSI cyclic electron transfer is likely to be energetically important for inducible CO2 uptake systems in cells grown at low CO2 (Tchernov et al., 2001).
In the absence of the NDH-1M and NDH-1L complexes (M55 strain), the cells died upon the CO2 downshift at pH 7.5. This was probably because of oxidative stress resulting from the failure of the cells in CO2 uptake in the absence of NDH-1M (Figure 5), thereby limiting the intracellular contents of the terminal photosynthetic electron acceptor CO2 and hence inducing the production of active oxygen species. It is conceivable that the function of NDH-1M is important for upregulation and function of NDH-1S complexes at low pH (7.5), whereas at elevated pH (8.3) the upregulation of both the SbtA and NDH-1S complexes occurs independently of NDH-1M, as was shown here with the M55 cells. The function of NDH-1M elevates cytosolic pH, which seems to be essential for upregulation of Ci transporters (Shibata et al., 2002) at neutral pH of the growth medium, whereas the growth of cells at higher pH probably modulates the intracellular pH independently of the function of the NDH-1M and NDH-1L complexes, resulting in sustained growth of also the M55 cells. Thus, the NDH-1M–supported cyclic electron flow around PSI is probably essential for CO2 uptake both in modifying the cytosolic pH suitable for upregulation of NDH-1S at low pH of the growth medium and for providing energy for the function of the Ci acquisition systems.
Proteome studies of the wild type and various ndh gene knockout mutants of Synechocystis 6803 demonstrated that the NDH-1L complex is generally expressed under all growth conditions (Figure 5). The ΔNdhD1/D2 mutant, lacking the NDH-1L complex (Figure 6B), exhibits wild-type levels of cyclic electron flow (Table 2) and reduced rates of respiration and is not capable of photoheterotrophic growth in the presence of glucose and DCMU (Ohkawa et al., 2000a; confirmed by us, data not shown). These phenotypes of the NdhD1/D2 mutant strongly suggest the role of the NDH-1L complex in cellular respiration. Because the respiratory pathways in Synechocystis 6803 are probably very complex (e.g., Cooley and Vermaas, 2001), the exact function, either direct or indirect, of the NDH-1L complex in cellular respiration is difficult to assess. Studies with the PSI-less mutant further supported the involvement of NDH-1L in cellular respiration. This mutant, capable of only heterotrophic growth in the presence of glucose (Shen et al., 1993), thus strongly relying on respiration, showed a distinguished expression of the NDH-1L complex in the thylakoid membrane (Figure 6A). It is presently not clear whether the NdhD1(D2) and NdhF1 subunits as such confer the specificity of NDH-1L and NDH-1M to respiratory and cyclic electron flow, respectively, or whether posttranslational modifications of the Ndh subunits are possibly involved as well.
We conclude that the specific low CO2-inducible CO2 uptake complex in Synechocystis 6803, composed of the NdhD3, NdhF3, CupA, and Sll1735 proteins, is located exclusively in the thylakoid membrane and is functionally dependent on the NDH-1M complex. The NDH-1M complex contains single gene copy Ndh proteins, components of both the hydrophilic and the membrane domains of the NDH-1 complexes, whereas NDH-1L additionally comprises the NdhD1/D2 and the NdhF1 subunits. Both NDH-1M and NDH-1L are located in the thylakoid membrane. NDH-1M is capable of fast rereduction of P700+ in darkness and is strongly coexpressed with the NDH-1S complex, suggesting that NDH-1M fuels the thylakoid-associated CO2 uptake systems. NDH-1M might function as a ferredoxin plastoquinone oxidoreductase with less complicated structural requirements as compared with respiratory NDH-1 complexes using NAD(P)H as an electron source (Sapra et al., 2003). Only the NDH-1L complex, composed of the most complete set of ndh gene products, also including the NdhD1/D2 protein and the NdhF1 subunit, is capable of supporting photoheterotrophic growth of Synechocystis 6803, yet the electron donation domain still remains unknown. It will be interesting to find out whether any differentiation of the NDH-1 complexes occurs in plant chloroplasts, possibly being differentially involved in cyclic PSI electron flow and chlororespiration.
METHODS
Cell Culture Conditions
Synechocystis 6803 glucose tolerant strain (wild type) and the ndh gene inactivation mutants ΔndhB (M55), ΔndhD3, ΔndhD4, ΔndhD3/ndhD4, ΔndhD3/D4/sbtA, and ΔndhD1/ndhD2 (Ogawa, 1991; Ohkawa et al., 2000a; Shibata et al., 2002) were grown in BG-11 medium (Williams, 1988) at 32°C under 50 μmol photons m−2 s−1 in 200-mL batch cultures under gentle agitation. The ΔPSI mutant (Shen et al., 1993) was grown in BG-11 medium supplemented with 5 mM glucose at 32°C under 5 μmol photons m−2 s−1. The mutant strains were grown in the presence of appropriate antibiotics. The experimental conditions used for culturing Synechocystis wild-type and mutant strains were as follows: high CO2 (3% CO2 in air) at pH 7.5 (buffered with 20 mM Hepes-NaOH) and low CO2 (air level) both at pH 7.5 and 8.3 (buffered with 20 mM Tes-KOH).
Thermosynechococcus elongatus BP1 was grown in BG-11 medium at 50°C under 50 μmol photons m−2 s−1.
Isolation of Cyanobacterial Membranes
Isolation of the Total Membrane Fraction
The cells (1 liter cultures) were harvested when the cultures had reached the optical density of 1.2 at 730 nm and were washed and resuspended in 3 mL of disruption buffer (20 mM potassium phosphate, pH 7.8). Glass beads (150 to 212 μm) were added to the cell suspension, and the cells were broken by vortexing three times at the highest speed for 2 min with 1 min cooling on ice between the runs. To remove the glass beads, the sample was centrifuged at 2000g for 10 min, and the membranes were subsequently collected by ultracentrifugation at 150,000g for 40 min.
Isolation of Crude Thylakoid Membranes
The cell cultures (200 mL) were harvested at the logarithmic phase and washed twice by suspending in 20 mL of washing buffer (50 mM Hepes-NaOH, pH 7.5, and 30 mM CaCl2), and the thylakoids were isolated according to Gombos et al. (1994) as follows. The cells suspended in 2 mL of isolation buffer (50 mM Hepes-NaOH, pH 7.5, 30 mM CaCl2, 800 mM sorbitol, and 1 mM ɛ-amino-n-caproic acid) were supplemented by glass beads and disrupted by vortexing eight times at the highest speed for 1 min at 4°C with 1 min cooling on ice between the runs. The crude extract was centrifuged at 3000g for 5 min to remove the glass beads and unbroken cells. Membranes were pelleted by centrifugation at 17,000g for 20 min and resuspended in storage buffer (50 mM Tricine-NaOH, pH 7.5, 600 mM sucrose, 30 mM CaCl2, and 1 M glycinebetaine).
Aqueous Polymer Two-Phase Partitioning of the Plasma and Thylakoid Membranes
Plasma and thylakoid membranes were isolated from Synechocystis 6803 cells by aqueous polymer two-phase partitioning. In this process, the total Synechocystis membrane pellet (isolated as described above) was first fractionated by sucrose density gradient centrifugation and thereafter, according to the surface properties of the membrane fractions, by two-phase partitioning using the polymers Dextran T-500 and PEG 3350 (Norling et al., 1998; Jansén et al., 2002). CP43 and NrtA proteins were used as markers of the purity of the thylakoid and the plasma membranes (Norling et al., 1998).
Electrophoresis and Immunoblotting
The BN-PAGE of Synechocystis 6803 membranes was performed basically as described earlier (Kügler et al., 1997) with modifications from Cline and Mori (2001) and Herranen et al. (2004).
Isolated membranes were prepared for BN-PAGE as follows. Membranes were washed with 330 mM sorbitol, 50 mM Bis-Tris, pH 7.0, and 250 μg/mL of pefabloc and subsequently suspended in 20% glycerol (w/v), 25 mM Bis-Tris, pH 7.0, 10 mM MgCl2, and 0.01 unit/μL RNase-Free DNase RQ1 (Promega, Madison, WI) at the final concentration of 20 μg protein/μL. The samples were incubated on ice for 10 min, and the equal volume of 3% n-dodecyl-β-d-maltoside was added. Solubilization was performed for 10 min on ice followed by incubation at room temperature for 20 min. Insoluble material was removed by centrifugation at 18,000g for 15 min. The collected supernatant was mixed with one-tenth volume of 0.1 M EDTA and one-tenth volume of sample buffer (5% Serva blue G, 200 mM Bis-Tris, pH 7.0, 75% sucrose, and 1 M ɛ-amino-n-caproic acid) and applied to 0.75-mm-thick 5 to 12.5% acrylamide gradient gel (Hoefer Mighty Small mini-vertical unit; San Francisco, CA). Samples were loaded on an equal protein basis of 150 μg per well. Electrophoresis was performed at 4°C by increasing voltage gradually from 50 V up to 200 V during the 5.5-h run.
For electrophoresis in the second dimension, a lane of the BN gel was cut out and incubated in Laemmli SDS sample buffer containing 5% β-mercaptoethanol and 6 M urea for 1 h at 23°C. The lane was then laid onto a 1-mm-thick 14% SDS-PAGE gel with 6 M urea (Laemmli, 1970). After electrophoresis, the proteins were visualized by silver staining (Blum et al., 1987).
For immunoblotting, the proteins were electrotransferred to a PVDF membrane (Immobilon P; Millipore, Bedford, MA) and detected by protein-specific antibodies using the CDP-Star chemiluminescent detection kit (New England Biolabs, Beverly, MA). The NdhD3 antibody was prepared against amino acids 185 to 196 and 346 to 359, the NdhF3 antibody against amino acids 28 to 41 and 439 to 453, and the NdhF1 antibody against amino acids 495 to 509 and 610 to 624 of the respective proteins of Synechocystis 6803 (Eurogentec, Seraing, Belgium). SbtA antibody was prepared against amino acids 184 to 203. The antibody for NrtA (a subunit of an ABC-type nitrate transporter located in the plasma membrane) was provided by B. Norling (Stockholm University, Sweden). The antibody for CP43 (a chlorophyll a binding protein located in the thylakoid membrane) was obtained from R. Barbato (University of Piemonte Orientale, Alessandria, Italy) and the NdhJ and NdhK antibodies from J. Appel (Institute of Botany, Kiel, Germany) and P. Nixon (Imperial College London, UK), respectively.
Identification of Proteins by MALDI-TOF
Silver-stained protein spots were excised from gels and digested with modified porcine trypsin (Promega) according to Shevchenko et al. (1996). Trypsin digests were concentrated and purified from salts using self-made reverse-phase POROS R3 (Perseptive Biosystems, Framingham, MA) microcolumns (Gobom et al., 1999). Peptides were eluted from a column directly onto the MALDI plate using a solution of α-cyano-4-hydroxycinnamic acid (10 mg/mL) in 60% acetonitrile and 0.3% trifluoroacetic acid. MALDI-TOF analysis was performed in reflector mode on the Voyager-DE PRO mass spectrometer (Applied Biosystems, Foster City, CA). Calibration of spectra was based on masses of trypsin autodigestion products (842.510, 1045.564, and 2211.105 D). Proteins were identified by searching in the National Center for Biotechnology Information database using Mascot (www.matrixscience.com). The search parameters allowed for carbamidomethylation of cystein, one miscleavage of trypsin, and 50 ppm mass accuracy.
Determination of Protein Concentration
Protein was determined using a DC (detergent-compatible) protein assay kit (Bio-Rad, Hercules, CA).
Measurement of P700+ Rereduction Rate
P700+ reduction was measured as an absorption change at 820 nm as described by Appel et al. (2000). The first order kinetics of P700+ rereduction was determined in the dark after illumination of cells with 26.5 W m−2 of far red light with a maximum at 715 nm.
Acknowledgments
We thank Wim Vermaas for the ΔPSI mutant. Financial support was obtained from the Academy of Finland and the Nordic Joint Committee for Agricultural Research.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Eva-Mari Aro (evaaro@utu.fi).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026526.
References
- Appel, J., Phunpruch, S., Steinmuller, K., and Schultz, R. (2000). The bi-directional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch. Microbiol. 173, 333–338. [DOI] [PubMed] [Google Scholar]
- Aro, E.-M., Suorsa, M., Rokka, A., Allahverdiyeva, Y., Paakkarinen, V., Saleem, A., Battchikova, N., and Rintamäki, E. (2005). Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot., in press. [DOI] [PubMed]
- Badger, M.R., and Spalding, M.H. (2000). CO2 acquisition, concentration and fixation in cyanobacteria and algae. In Advances in Photosynthesis: Physiology and Metabolism, Vol. 9, R.C. Leegood, T.D. Sharkey, and S. von Caemmerer, eds (Dordrecht: Kluwer Acadademic Publishers), pp. 399–434.
- Blum, H., Beier, H., and Gross, J.H. (1987). Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8, 93–99. [Google Scholar]
- Burrows, P.A., Sazanov, L.A., Svab, Z., Maliga, P., and Nixon, P.J. (1998). Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 17, 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., and Mori, H. (2001). Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC–Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 154, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley, J.W., and Vermaas, W. (2001). Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. PCC 6803: Capacity comparisons and physiological functions. J. Bacteriol. 183, 4251–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y., Ye, J., and Mi, H. (2003). Effect of low CO2 on NAD(P)H dehydrogenase, a mediator of cyclic electron transport around photosystem I in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 44, 534–540. [DOI] [PubMed] [Google Scholar]
- Friedrich, T., and Scheide, D. (2000). The respiratory complex I of bacteria, archea and eukarya and its module common with membrane-bound multisubunit hydrogenases. FEBS Lett. 479, 1–5. [DOI] [PubMed] [Google Scholar]
- Friedrich, T., Steinmuller, K., and Weiss, H. (1995). The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett. 367, 107–111. [DOI] [PubMed] [Google Scholar]
- Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R., and Roepstorff, P. (1999). Sample purification and preparation technique based on nano-scale reverse-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34, 105–116. [DOI] [PubMed] [Google Scholar]
- Gombos, Z., Wada, H., and Murata, N. (1994). The recovery of photosynthesis from low-temperature photoinhibition is accelerated by the unsaturation of membrane lipids: A mechanism of chilling tolerance. Proc. Natl. Acad. Sci. USA 91, 8787–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranen, M., Battchikova, N., Zhang, P., Graf, A., Sirpiö, S., Paakkarinen, V., and Aro, E.-M. (2004). Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiol. 134, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, P.J., Morgan, D.J., and Sazanov, L.A. (2003). The location of NuoL and NuoM subunits in the membrane domain of the Escherichia coli complex 1: Implications for the mechanism of proton pumping. J. Biol. Chem. 278, 43114–43120. [DOI] [PubMed] [Google Scholar]
- Jansén, T., Kanervo, E., Aro, E.-M., and Mäenpää, P. (2002). Localisation and processing of the precursor form of photosystem II protein D1 in Synechocystis 6803. J. Plant Physiol. 159, 1205–1211. [Google Scholar]
- Kaneko, T., et al. (1996). Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3, 109–136. [DOI] [PubMed] [Google Scholar]
- Kaplan, A., and Reinhold, L. (1999). CO2-concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 539–570. [DOI] [PubMed] [Google Scholar]
- Klughammer, B., Sültemeyer, D., Badger, M.R., and Price, G.D. (1999). The involvement of NAD(P)H dehydrogenase subunits, NdhD3 and NdhF3, in high-affinity CO2 uptake in Synechococcus sp. PCC 7002 gives evidence for multiple NDH-1 complexes with specific roles in cyanobacteria. Mol. Microbiol. 32, 1316–1332. [DOI] [PubMed] [Google Scholar]
- Kügler, M., Jänsch, L., Kruft, V., Schmitz, U.K., and Braun, H.P. (1997). Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosyn. Res. 53, 35–44. [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Leif, H., Sled, V.D., Ohnishi, T., Weiss, H., and Friedrich, T. (1995). Isolation and characterization of the proton-translocating NADH: Ubiquinone oxidoreductase from Escherichia coli. Eur. J. Biochem. 230, 538–548. [DOI] [PubMed] [Google Scholar]
- Maeda, S., Badger, M.R., and Price, G.D. (2002). Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol. Microbiol. 43, 425–435. [DOI] [PubMed] [Google Scholar]
- Mi, H., Endo, T., Ogawa, T., and Asada, K. (1995). Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediated cyclic electron transport in the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 36, 661–668. [Google Scholar]
- Mi, H., Endo, T., Schreiber, U., Ogawa, T., and Asada, K. (1992). Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 33, 1233–1237. [Google Scholar]
- Munekage, Y., Hashimoto, M., Miyake, C., Tomizawa, K.-I., Endo, T., Tasaka, M., and Shikanai, T. (2004). Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429, 579–582. [DOI] [PubMed] [Google Scholar]
- Norling, B., Zak, E., Andersson, B., and Pakrasi, H. (1998). 2D-isolation of pure plasma and thylakoid membranes from cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 436, 189–192. [DOI] [PubMed] [Google Scholar]
- Ogawa, T. (1991). A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC 6803. Proc. Natl. Acad. Sci. USA 88, 4275–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, T., and Kaplan, A. (2003). Inorganic carbon acquisition systems in cyanobacteria. Photosyn. Res. 77, 105–115. [DOI] [PubMed] [Google Scholar]
- Ohkawa, H., Pakrasi, H.B., and Ogawa, T. (2000. a). Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J. Biol. Chem. 275, 31630–31634. [DOI] [PubMed] [Google Scholar]
- Ohkawa, H., Price, G.D., Badger, M.R., and Ogawa, T. (2000. b). Mutation of ndh genes leads to inhibition of CO2 uptake rather than HCO3- uptake in Synechocystis sp. strain PCC6803. J. Bacteriol. 182, 2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa, H., Sonoda, M., Katoh, H., and Ogawa, T. (1998). The use of mutants in the analysis of the CCM in cyanobacteria. Can. J. Bot. 76, 1025–1034. [Google Scholar]
- Ohkawa, H., Sonoda, M., Shibata, M., and Ogawa, T. (2001). Localization of NAD(P)H dehydrogenase in the cyanobacterium Synechocystis sp. PCC 6803. J. Bacteriol. 183, 4938–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata, T., and Ogawa, T. (1986). Biosynthesis of a 42KD polypeptide in the cytoplasmic membrane of the cyanobacterium Anacystis nidulans strain R2 during adaptation to low CO2 concentration. Plant Physiol. 80, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata, T., Price, G.D., Badger, M.R., Okamura, M., Gohta, S., and Ogawa, T. (1999). Identification of an ABC-Type bicarbonate transporter of the cyanobacterium Synechococcus sp. strain PCC 7942. Proc. Natl. Acad. Sci. USA 96, 13571–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, G.D., Klughammer, B., Ludwig, M., and Badger, M.R. (1998). The functioning of the CO2 concentrating mechanism in several cyanobacterial strains: A review of general physiological characteristics, genes, proteins and recent advances. Can. J. Bot. 76, 973–1002. [Google Scholar]
- Price, G.D., Maeda, S., Omata, T., and Badger, M.R. (2002). Modes of active inorganic carbon uptake in the cyanobacterium, Synechococcus sp. PCC 7942. Funct. Plant Biol. 29, 131–149. [DOI] [PubMed] [Google Scholar]
- Prommeenate, P., Lennon, A.M., Markert, C., Hippler, M., and Nixon, P.J. (2004). Subunit composition of NDH-1 complexes of Synechocystis sp. PCC 6803: Identification of two new ndh gene products with nuclear-encoded homologues in the chloroplast Ndh complex. J. Biol. Chem. 279, 28165–28173. [DOI] [PubMed] [Google Scholar]
- Sapra, R., Bagramyan, K., and Adams, M.W.W. (2003). A simple energy-conserving system: Proton reduction coupled to proton translocation. Proc. Natl. Acad. Sci. USA 100, 7545–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, G., Boussiba, S., and Vermaas, W.F. (1993). Synechocystis sp. PCC 6803 strains lacking photosystem I and phycobilisome function. Plant Cell 5, 1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858. [DOI] [PubMed] [Google Scholar]
- Shibata, M., Katoh, H., Sonoda, M., Ohkawa, H., Shimoyama, M., Fukuzawa, H., Kaplan, A., and Ogawa, T. (2002). Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: Function and phylogenetic analysis. J. Biol. Chem. 277, 18658–18664. [DOI] [PubMed] [Google Scholar]
- Shibata, M., Ohkawa, H., Kaneko, T., Fukuzawa, H., Tabata, S., Kaplan, A., and Ogawa, T. (2001). Distinct constitutive and low CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. USA 98, 11789–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov, D., Helman, Y., Keren, N., Luz, B., Ohad, I., Reinhold, L., Ogawa, T., and Kaplan, A. (2001). Passive entry of CO2 and its intracellular conversion to HCO3- in cyanobacteria are driven by a photosystem I-generated ΔμH+. J. Biol. Chem. 276, 23450–23455. [DOI] [PubMed] [Google Scholar]
- Volokita, M., Zenvirth, D., Kaplan, A., and Reinhold, L. (1984). Nature of the inorganic carbon species actively taken up by the cyanobacterium Anabaena variabilis. Plant Physiol. 76, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.-L., Postier, B.L., and Burnap, R.L. (2004). Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 279, 5739–5751. [DOI] [PubMed] [Google Scholar]
- Williams, J.K.G. (1988). Construction of specific mutations in PSII photosynthetic reaction center by genetic engineering. Methods Enzymol. 167, 766–778. [Google Scholar]