Synopsis
Myelodysplastic syndrome (MDS) is a heterogeneous, clonal stem cell disorder of the blood and marrow typically diagnosed based on the presence of persistent cytopenia(s), dysplastic cells, and genetic markers. Common issues that arise in the clinical management include difficulty confirming MDS diagnosis, lack of a standard approach with novel agents in MDS, and few prospective long-term, randomized-controlled MDS clinical studies to guide allogeneic blood and marrow transplant. With the recent genetic characterization of MDS, certain aspects of these issues will be better addressed by integrating genetic data into clinical study design and clinical practice.
Keywords: Myelodysplastic syndrome, anemia, azacitidine, therapy-related myelodysplastic syndrome, blood and marrow transplant
Introduction
In this review article, I discuss anemia caused by underlying myelodysplastic syndrome (MDS), a hematologic malignancy associated with widely varying clinical presentations, mutation patterns, and patient outcomes. “Myelo” means marrow and “dysplasia” means abnormal development. MDS is characterized by low blood cell counts, abnormal blood cell development, clonal genetic markers, and increased propensity towards acute myeloid leukemia (AML). The general incidence in the US population is about 4.8 per 100,000 per year but is as high as 30 to 60 per 100,000 per year in people over 70 years old (Surveillance, Epidemiology and End Results, Cancer Statistics Review, 1975–2010. National Cancer Institute; 2013). There is a slight male to female predominance (1.26 to 1). Risk-adapted monitoring and therapy are essential cornerstones of MDS clinical management. Over the past 5 years, advances in massive parallel sequencing technology have enabled genetic characterization of MDS to a point that the focus has now shifted towards translating these findings to improve patient outcomes. This will require, for example, integration of genetic data and disease behavior to help choose/design better therapies, exploitation of synthetic lethality based on mutational exclusivity, and defining the genetic and biologic determinants of the good-responders versus poor-responders to various existing therapies. The use of genetic markers to predict and monitor risk for disease relapse may improve the selection of conditioning regimens and maintenance therapies in the setting of allogeneic blood and marrow transplant (BMT). The availability of molecular genetic testing has also helped with the diagnosis of MDS and other pre-diagnostic conditions, especially when dysplasia is not overtly present or WHO criteria are not met based on absolute cut-offs (e.g., >10% dysplasia in a cell lineage). I will present these discussions points using clinical vignettes that broadly represent common situations. I will integrate the latest research in MDS. A more targeted, in-depth review covering the genetics of MDS, prognostic systems, treatment options, allogeneic BMT, and drugs in the pipeline are available in recent excellent review articles as cited. The discussion points will focus on integrating genetic data into diagnostic and prognostic considerations, managing patients who “fail” DNA methyltransferase (DNMT) inhibitors, and evaluating patients for allogeneic BMT. I will discuss potential future directions in clinical management and translational research in MDS.
Clinical vignette #1: Diagnostic considerations in MDS
A previously healthy 37 year old male presented with progressive fatigue and dyspnea on exertion over the course of a few weeks. He was found to have a hypoproliferative anemia with hemoglobin level of 6.3 g/dL and reticulocyte of 0.15%. His platelet count, white blood cell count (WBC), and WBC differential were unremarkable. His blood smear was otherwise unremarkable. There was no laboratory evidence of hemolysis. He also reported intermittent fevers and diffuse body aches. His bone marrow biopsy showed hypercellular marrow (100% cellularity), marked myeloid hyperplasia, mild dysplasia in granulocytes (dyspoeisis in less than 10%), mild 1+ reticulin fibrosis, 3% blasts enumerated by morphology, slightly increased megakaryocytes with occasional clusters and small forms, scant erythroid hematopoiesis, and plasma cells focally increased but overall 2%. He had a normal male karyotype and BCR-ABL and JAK2-V617F were not detected. He was requiring frequent red cell transfusions to maintain hemoglobin above 7.0 g/dL. He was referred for discussion of treatment recommendations for newly diagnosed MDS.
Assessment of clinical vignette #1
Patient is relatively young with rapid onset of symptoms mainly due to symptomatic anemia. The median age of MDS diagnosis is 76 years old1 and the typical clinical presentation tends to be indolent and progressive over months not weeks. Approximately 6% of cases of MDS are diagnosed in people under 50 years old.1 The patient does not have the standard risk factors for MDS including, for example, advanced age, hereditary marrow failure syndromes, industrial benzene or other solvent exposure, or prior chemotherapy and radiation.2 In addition to cytopenias, the CBC and a blood smear in an MDS patient may also reveal bilobed (pseudo- Pelger Huet), hypersegmented, or hypogranulated neutrophils, unexplained macrocytosis (>100 fL) and oval-shaped RBCs (macro-ovalocytes), elevated red cell distribution width (RDW), and giant or hypogranulated platelets.
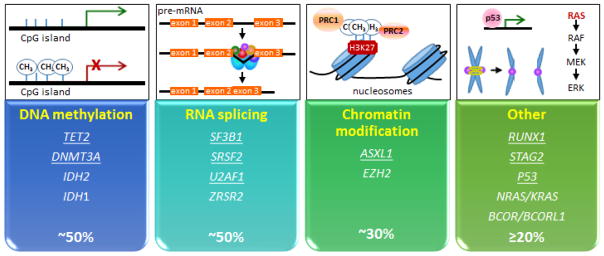
The patient did not have any of these findings. Thus, even before looking closer at his outside bone marrow biopsy slides and ordering molecular testing on his blood, I had already considered other possible diagnoses of anemia. The patient’s marrow is abnormal but the findings are non-specific. At 37 years old, the patient’s marrow cellularity, the hematopoietic cellular component expressed as a percentage relative to fatty tissue, should be around 50% based on the general approximation that a typical normal bone marrow cellularity percentage is 100-age.3 Referring to the 2008 WHO criteria,4 the following are required for a diagnosis of MDS: persistent cytopenias(s), ≥10% dysplasia in one or more cell lineage, and MDS-associated clonal cytogenetic or molecular marker. In this case, the cytopenia (anemia) has been present for only a few weeks since diagnosis, the dysplasia is not apparently a prominent finding (granulocytic dyspoiesis in less than 10% of cells), and the lack of a clonal cytogenetic marker was not helpful for a diagnosis of MDS. Approximately 50% of cases of de novo MDS (cases of MDS not associated with prior chemotherapy, radiation, or antecedent hematologic malignancy) are associated with a cytogenetic marker. 5,6 Therefore, not having a cytogenetic marker does not exclude the possibility of a diagnosis of MDS. It is also not uncommon to come across a pathology report that lacks qualitative and/or quantitative description of the degree of dysplasia, which is inherently important for evaluating the WHO criteria for diagnosing MDS. Some of the morphologic manifestations of MDS are less specific for MDS (e.g., increased megaloblastoid changes or cytoplasmic vacuolization) and some are more specific for MDS including nuclear hypolobation in granulocytes and multiple separated nuclei in megakaryocytes.4,7 Thus, so far, the patient does not appear to meet the criteria for a conclusive diagnosis of MDS. Furthermore, sequencing of a 42-gene hematologic malignancy panel from a blood sample returned without any mutations. It has been estimated that more than 90% of MDS will harbor mutations in at least one of the genes listed in Figure 1.8,9 In my clinical practice, if a patient has suspected MDS but does not have a conclusive diagnosis of MDS by WHO criteria and has no mutation in this gene panel, I will reconsider other differential diagnoses for anemia and proceed with work-up accordingly.
Figure 1. Categories of commonly mutated genes in myelodysplastic syndrome.
Approximately 90% of patients with MDS will have at least one mutation in one of the genes involved in DNA methylation, RNA splicing, chromatin modification, or in the other category. The other category include genes involved in DNA damage and stress response pathway, transcriptional regulation, RAS/RAF/MEK/ERK pathway, and sister chromatid separation (cohesion complex). Each of the genes underlined are typically reported as being present in 10% or more cases of MDS.
The patient does not fulfill the basic criterion for one of the pre-diagnostic stages associated with MDS and other hematologic malignancies (Table 1) because he does not have a clonal molecular or cytogenetic marker. These pre-diagnostic stages were only recently defined with the aid of high throughput, more cost-effective massive parallel sequencing of samples from existing cohort studies.10–13 Given the patient’s clinical presentation was highly unusual for MDS and he had symptoms suggestive of systemic inflammation, I checked an erythrocyte sedimentation rate, which was profoundly elevated at 120 mm/hr (normal range 0–15 mm/hr). I considered a diagnosis of immune-mediated pure red cell aplasia given the scant erythroid hematopoiesis. This is a condition associated with the destruction of precursors of red cells but without the typical laboratory findings of hemolytic anemia. I had ruled out aplastic anemia because he had preserved WBC and platelets and a hypercellular marrow. A paroxysmal nocturnal hemoglobinuria screen by flow cytometry was negative. Of note HIV antibody screen, EBV quantitation by PCR, and parvovirus B19 by PCR were negative. Thus, I treated the patient with prednisone (60mg daily for 2 weeks then rapidly tapered him off) and cyclosporine (trough level 200–250 ng/ml). Within 3 weeks, he had a brisk erythropoiesis with a hemoglobin rise to 10.3 g/dL without transfusion support then achieved a normal hemoglobin level after 5 weeks of treatment. He was treated with cyclosporine alone for one year then tapered off and has maintained normal blood counts for more than two years.
Table 1.
Prediagnostic stages toward myelodysplastic syndrome.
| ICUS | NRXi w/o mutation | NRXi w/mutation | CHIP† | CCUS | Low risk MDS | High risk MDS | |
|---|---|---|---|---|---|---|---|
| Cytopenia(s) | + | − | − | − | + | + | + |
| Dysplasia | − | − | − | − | − | +/− | + |
| Molecular drivers | − | − | + | + | + | + | + |
| Non-random X-inactivation | +/− | + | + | +/− | + | + | + |
| Neoplastic transformation |
|
||||||
ICUS: idiopathic cytopenia of unknown significance; NRXi: non-random X-inactivation (determined by differentiating polymorphic alleles of HUMARA or G6PD genes on X chromosome); CHIP: clonal hematopoiesis of indeterminate potential; CCUS: clonal cytopenia of undetermined significance; MDS: myelodysplastic syndrome.
The vast majority of mutaWons associated with CHIP involve ASXL1, DNTM3A, or TET2.
In summary, this case highlights the importance of carefully considering the clinical presentation, degree and type of “dysplasia” present, lack of a clonal genetic marker, and other causes of anemia in those patients without a conclusive diagnosis of MDS by WHO criteria.
Clinical vignette #2: Evaluating patients that fail hypomethylating agents
A 78 year old female was diagnosed with low-risk myelodysplasia (International Prognostic Scoring System ([IPSS] score of 0; estimated median OS of 5.7 years).14 She initially presented with a hemoglobin of 5.8 g/dL. Her WBC and platelets were in the normal range. Review of her bone marrow biopsy findings confirmed the WHO criteria diagnosis of MDS. Her marrow was markedly hypercellular at 90%. She had mild megaloblastoid changes in the erythroid lineage and prominent bilobed and unilobed megakaryocytic forms. Blasts were 3–4% by morphology and she had a normal female karyotype. JAK2-V617F and BCR-ABL were negative. No other molecular studies were done. The patient was highly dependent on regular red cell transfusions. Because of the degree of anemia, she was treated with azacitidine 75mg/m2/day subcutaneously on days 1–7 of every 28-day cycle. After the 2nd cycle, her hemoglobin rose to 11.3 g/dL and she achieved transfusion independence. She proceeded with the 3rd and 4th cycle as scheduled and was referred at the end of her 4th cycle due to persistent pancytopenia, with a clinical determination of azacitidine failure and disease progression. At her first consult visit, she had a fever in the setting of severe neutropenia and was promptly admitted to the hospital for further evaluation.
Assessment of clinical vignette #2
This patient had low risk MDS using the original IPSS scoring system. If we compare her risk group using a revised IPSS (IPSS-R) scoring system incorporating the degree of anemia as a variable her score is 3.5 (intermediate risk, median OS of 3 years).15 Other risk scoring systems and common cytogenetic findings observed in MDS are listed in Table 2. Several low risk scoring systems have emerged and may better delineate patients within the low risk group.16–18 Generally, the standard approach is to consider azacitidine for treatment of high risk patients (intermediate-2 or high risk IPSS categories) given the survival benefit of median 9.5 months observed in a multicenter, randomized phase 3 trial comparing azacitidine versus best supportive care.19 Azacitidine and decitabine are cytosine nucleoside analogs that inhibit DNA methyltransferase at lower dose ranges. DNA methyltransferase catalyzes methylation of cytosines in CpG islands involved in transcriptional regulation of genes. Azacitidine is also considered in low risk patients (low or intermediate-1 risk IPSS categories) who display significant cytopenias and/or have failed other treatment options and has been studied in a large phase II study using various dosing strategies.20 MDS treatment options for various clinical aspects of MDS are listed in Table 3. For example, for anemia, treatment might include danazol, erythropoiesis-stimulating agents (ESAs), immunosuppression, and/or lenalidomide. However, given the patient’s high EPO level of >500 ng/ml and degree of anemia and transfusion dependence, it was unlikely that she would have an adequate, if any, response to ESAs.21,22 Thus, she was treated with azacitidine (FDA-approved in 2004 for MDS) with the hope that this would significantly improve her anemia.
Table 2.
Prognostic scoring systems in myelodysplastic syndrome.
| System | Risk factors evaluated | Validated application |
|---|---|---|
|
IPSS (IWG-PM) Blood. 1997;89: 2079. |
|
|
|
WPSS (WHO)
J Clin Oncol. 2005;23:7594. |
|
|
|
MDACC (MD Anderson)
Cancer. 2008;113:1351. |
|
|
|
IPSS-R (IWG-PM)
Blood. 2012;120:2454. |
|
|
- Very good: -Y, del(11q).
- Good: Normal, del(5q), del(12p), del(20q), double including del(5q).
- Intermediate: del(7q), +8, +19, i(17q), any other single or double independent clones.
- Poor: -7, inv(3)/t(3q)/del(3q), double including -7/del(7q), complex: 3 abnormalities.
- Very poor: Complex: >3 abnormalities.
Table 3.
Treatment options for various issues encountered in myelodysplastic syndrome.
| Condition | Treatment |
|---|---|
| Anemia |
|
| Thrombocytopenia |
|
| Neutropenia |
|
| Infection prevention |
|
| Bleeding prevention |
|
| Disease-modifying therapies |
|
| Disease burden reduction |
|
Although azacitidine was studied with the dose/schedule used in this patient, there are no consensus guidelines on how to manage patients on azacitidine specifically. Therefore, I highlight some important considerations and provide a “how I evaluate and treat” guideline that I routinely apply in my patients (Figure 2). The guideline covers some key principles and is not meant to replace clinical assessment and judgment. There are specific disease/patient characteristics to consider when managing patients with MDS, including active comorbidities, overall health, goals of care, caregiver support, and access to clinic/infusion services. In this guideline, I have considered the existing data on azacitidine in low risk MDS, the fact that older patients have increased toxicity and altered drug metabolism, the kinetics of clinical responses with azacitidine in clinical trial studies, and the molecular and hematologic markers that predict clinical responses. Hematologic responses are higher in those patients harboring TET223,24 and DNMT3A mutations.25,26 Approximately 90% of clinical responses occur within 4 cycles of a 28- day cycle.20 This is a point when I re-evaluate risks/benefits with my patients, especially those who have experienced significant quality of life decrement and/or side effects or toxicities. If there has been no appreciable response after 6 cycles, the patient is unlikely to achieve a response with additional cycles but not impossible. Decitabine (FDA-approved in 2006 for MDS) is not my first choice in patients with intermediate-2 and high risk MDS mainly due to the lack of a phase 3, randomized-controlled trial showing a clear survival benefit.27–29 The exact for reason for this is unknown but some have proposed that the current dosing strategies may favor cytotoxic effects over DNMT inhibitory effects,30 the ideal dose/schedule has not been determined,31,32 and the fact that decitabine has a different mechanism of action as it only incorporates into DNA, while azacitidine incorporates into both DNA and RNA.33 Overall, decitabine is thought to have similar rates of progression-free survival and hematologic responses compared with azacitidine in retrospective studies but may be associated with slightly more neutropenic/infectious complications especially in an older patient population.34
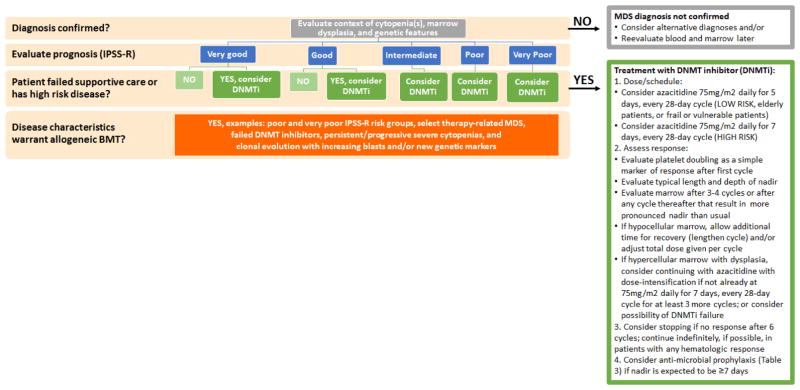
Figure 2. How I evaluate and treat myelodysplastic syndrome.
In this schematic, I summarize my general approach to patients with MDS. I provide additional details on how I choose the dose/schedule of azacitidine and monitor for response on a practical level. Many patients at the beginning of therapy (e.g., first and second cycles) will need close monitoring of their blood counts (e.g., two or three times a week) but this is usually adjusted during later cycles based on a patient’s typical length and depth of nadir. Table 1: Prediagnostic stages toward myelodysplastic syndrome.
In this clinical vignette, the patient had a deeper and longer nadir (period of lowest blood cell counts) during the 3rd cycle compared to the previous two cycles when she had an excellent hematologic response and marrow recovery between the 1st and 2nd cycles and the 2nd and 3rd cycles. However, she had not fully recovered counts on day one of the 4th cycle but was given this cycle at full dose, on time. During her 4th cycle, she end up having her deepest and longest nadir yet and presented with neutropenic fever. Work-up in the hospital revealed she had aspergillus pneumonia, an infection associated with high mortality rates in patients with hematologic malignancies.35 Her marrow evaluation revealed a hypocellular marrow (~10% cellularity) with trilineage hypoplasia, megaloblastoid erythropoiesis, megakaryocytic dysplasia, no increase in blasts (1%), and no evidence of acute leukemia. Thus, she was experiencing a cumulative azacitidine toxicity effect leading to severe pancytopenia and marrow hypoplasia. She had not been on prophylaxis antibiotics. I have indicated the antibiotic prophylaxis regimen I use in my clinical practice (Figure 2). In this case, I would have evaluated her marrow after the 3rd cycle and considered reducing her azacitidine dose (e.g., to a 5-day regimen) or lengthening her cycles to a 35- or 42-day cycle. In this case, the patient survived this critical illness and eventually recovered her counts approximately 8 weeks later. Interestingly, she had one additional year of transfusion independence but had to restart azacitidine when her anemia returned. She maintained some degree of response for another year before her disease progressed to a highly proliferative CMML-like disease.
In summary, there may be multifactorial reasons for patients who fail DNMT inhibitors. The reasons may not be strictly due to disease characteristics. The reasons for failing DNMT inhibitors should be looked at carefully in order to avoid a premature conclusion of azacitidine failure when actually the treatment had worked too well. Appropriate marrow evaluation, dose/schedule adjustment, and supportive care should be instituted. Patients who fail DNMT inhibitors due to disease characteristics have a poor prognosis,36,37 including those patients who were initially thought to have low risk MDS.38
Clinical vignette #3: Risk-adapted treatment and allogeneic BMT
A 70 year old man with history of chronic lymphocytic leukemia (normal karyotype) and diffuse large B-cell lymphoma transformation (DLBCL) presented with progressive neutropenia and a new clonal derivative chromosome 7, resulting in del7q and gain in 1q in approximately 10% of marrow cells. He had received five different multi-agent cytotoxic chemotherapy regimens over the course of six years, including the last regimen consisting of carmustine + etoposide + cytarabine + melphalan (BEAM) as high dose chemotherapy followed by autologous stem cell rescue. He had a DNMT3A p.R635L mutation. Dysplasia was prominent (>10% level) in the granulocytic series (cytoplasmic to nuclear asynchrony and abnormal granulation) and the megakaryocytes (separate nuclear lobes and hyperchromatic nuclei). Blasts were less than 5%. These findings are most compatible with therapy-related MDS. He is being referred for evaluation of treatment options.
Assessment of clinical vignette #3
This is a patient with therapy-related MDS. His cytogenetic findings suggest that of the two “pathways” toward therapy-related MDS (Table 4), the disease pathogenesis was more consistent with the alkylating agent-driven pathway. He had received both types of chemotherapy in the past (alkylating and topoisomerase II inhibitors). In Table 4, I compare and contrast the typical features of these two pathways.39–42 Recently, prognostic systems specific for therapy-related MDS were validated.43,44 High risk features include age ≥65 years, ECOG performance status 2 to 4, poor-risk cytogenetics (−7 and/or complex), WHO MDS subtype (RARS or RAEB-1/2), presence of cytopenias, and transfusion dependency. Cytogenetic abnormalities are present in approximately 80–90% of patients with therapy-related MDS39–41 versus approximately 50% of patients with de novo MDS (patients without prior chemotherapy or radiation). 5,6 MDS with complex cytogenetics (defined as 3 or more cytogenetic abnormalities) and monosomy karyotype (defined as 2 or more monosomies or a single monosomy with other structural abnormalities) carries a very poor prognosis and are characteristically associated with low responses to cytotoxic chemotherapy and poor survival outcomes after allogeneic BMT.45–53 Mutations in TP53 are more common in therapy-related MDS than in de novo MDS.54,55 Secondary MDS/AML, as discussed in this review, is a condition in which MDS/AML develops after having another type of hematologic malignancy such as primary myelofibrosis. Secondary MDS has similar poor-risk characteristics to therapy-related MDS, both having higher frequency of complex and monosomy karyotypes and higher rates of AML transformation compared to de novo MDS.56–58
Table 4.
Therapy-related myelodysplastic syndrome and their genetic and clinical pathways.
| Pathway | Clinical features | Genetic and biologic features |
|---|---|---|
|
Alkylating agents inter- and intrastrand DNA crosslinks |
|
|
|
Topoisomerase II inhibitors intercalate dsDNA |
|
|
Alkylating agents, examples include: melphalan, cyclophosphamide, busulfan, carmustine, cisplatin, carboplatin, dacarbazine, chlorambucil
Antitumor antibiotics and epipodophyllotoxins, examples include: etoposide, doxorubicin, daunorubicin, mitoxantrone
Due to prior cumulative chemotherapy toxicities, patients who have therapy-related MDS/AML may have increased risks for organ toxicities and infectious complications with intensive chemotherapy. The decision to treat therapy-related or secondary MDS/AML patients with multi-agent cytotoxic chemotherapy should be carefully reviewed as those with high-risk cytogenetic disease may not realize any survival benefit from multi-agent cytotoxic chemotherapy either as the only therapy or as cytoreductive therapy ahead of allogeneic BMT.59 Various calculators are available to estimate in-hospital and/or early mortality rates after multi-agent cytotoxic chemotherapy for more patient-specific risk/benefit discussions.60–62 Many of these calculators include prognostic factors such as age, functional status, kidney function, cytogenetics, blast counts, and de novo vs. therapy-related or secondary MDS/AML. Incorporation of genetic mutations into these risk calculators are likely forthcoming.
Thus, this patient should be considered for allogeneic BMT if he has a suitable donor option. In addition to therapy-related and secondary MDS, allogeneic BMT should also be considered early in disease course for de novo MDS patients in the IPSS intermediate-2 and high risk groups.63 A question that often comes up is whether azacitidine treatment should be used as a “bridge to transplant.”64,65 Treatment with azacitidine ahead of allogeneic BMT may serve several purposes including improving marrow function, reducing blast counts, and delaying disease transformation to AML. Generally, if the patient has disease characteristics that warrants allogeneic BMT, is an allogeneic BMT candidate, and has less than 10% marrow myeloblasts, proceeding with transplant in the short term as upfront therapy is recommended because there may be increased adverse outcomes with a prolonged pre-transplant period such as immunosuppression, infectious complications, transfusion dependence, transfusion-related alloimmunization, and iron overload that may delay or affect transplant success. The strongest predictors of relapse after allogeneic BMT are related to disease cytogenetics and marrow myeloblasts.66 While reducing marrow myeloblasts to less than 10% has been cited as an optimal goal before proceeding with transplant conditioning chemotherapy and is often included as an inclusion criterion for BMT clinical trials, there are no prospective, randomizedcontrolled studies based on disease characteristics to support that giving azacitidine or chemotherapy to achieve this goal provides overall survival benefit if the marrow myeloblasts are just above this value (e.g., 10–20%) or if the disease is associated with high-risk cytogenetics. High-risk cytogenetics and high percentage of marrow myeloblasts reflect disease biology; therefore, those patients with these characteristics have worse outcomes compared to those without these characteristics, perhaps regardless of the pre-allogeneic BMT treatment.59
Curing patients of their high risk hematologic malignancies requires an adequate and steady graft versus leukemia effect. While the donor and host are typically matched at key human leukocyte antigen (HLA) loci to minimize acute graft rejection by the host and acute graft versus host disease, the unmatched antigens (including tumor antigens) not considered in identifying a matched donor ideally induces donor immunogenic responses against host leukemia cells (graft versus leukemia effect) more so than against other host tissues (graft versus host disease). In this case, the patient had an HLA-identical sibling donor. The majority of transplant centers consider reduced intensity conditioning chemotherapy for patients over 60 years old. Conditioning chemotherapy serves several main purposes: 1) reduce disease burden as much as possible; 2) clear out the cellular components of the host bone marrow, and 3) suppress the host immune system to minimize graft rejection. Reduced intensity conditioning regimens are associated with lower rates of treatment-related mortality but higher rates of relapsed-related mortality compared to conventional myeloablative conditioning regimens such as busulfan-cyclophosphamide or cyclophosphamide-total body irradiation where the reverse is true.66 Retrospective studies have generally indicated similar overall survival rates when comparing reduced intensity versus myeloablative chemotherapy.67 However, the major caveats for applying this data to clinical practice include the inherent selection bias of choosing various treatment approaches based on patient and disease characteristics and the relatively short term follow-up data. The decision to use reduced intensity versus myeloablative conditioning regimens should be carefully considered based on age, comorbidities (e.g., hematopoietic cell transplantation comorbidity index), donor type, graft source, and disease biology.67,68 A randomized-controlled US study (BMT CTN 0901) was closed early in 2014 due to preliminary analysis indicating superior relapse reduction of myeloablative regimens compared to reduced intensity regimens.67 The study has not been published but was recently presented at the American Society of Hematology meeting in 2015 (Dr. Bart Scott, et al. late-breaking abstract) so the overall study design and quality of the data have not been fully evaluated. In the meantime, in appropriate patients, a conventional myeloablative regimen or the most intense reduced intensity regimen should be considered.67 In this case, the patient received one of the more intense reduced intensity regimens (fludarabine and melphalan).
Long-term survival in remission following HLA-matched related or unrelated donor sources for IPSS intermediate-2 and high risk groups are approximately 40 and 30%, respectively.69 For those with high risk cytogenetics (complex or monosomy karyotype), long-term survival is 10% or less.70 In this current era of nearly complete genetic characterization of hematologic malignancies, more sensitive, convenient markers of disease may be followed to monitor residual disease or early relapse. Studies are ongoing to define the use of disease-related genetic markers after allogeneic BMT. If a genetic marker persists, would a post-allogeneic BMT maneuver improve survival and prevent disease relapse? Such maneuvers might include early donor leukocyte infusions, tapering of immunosuppressive agents, small molecule inhibitors, and/or other targeted interventions. DNMT and FLT3 inhibitors are being investigated as maintenance therapy post-allogeneic BMT in cases of very high risk disease characteristics and minimal residual disease detection.71–78 In this case, the patient’s DNMT3A p.R635L mutation may be followed in his blood after transplant and DNMT inhibitors may be beneficial to reduce his risk for disease relapse.
Summary and future considerations
In summary, in the time span of approximately ten years, three FDA-approved drugs are available for MDS and several groups have genetically characterized MDS to a greater extent than previously. The genetic data will aid in diagnosis of MDS with equivocal pathologic data, and in time, enhance utilization of novel and existing drugs (singly or in combination) and treatment after post-allogeneic BMT. An important question is could there be a role for earlier treatment with DNMT inhibitors for select CCUS and low risk MDS phases if a convenient oral formulation allows for chronic, less toxic dosing? We should also reconsider the necessity to eradicate the disease versus stabilization of a disease so that it never transforms into something much worse such has high risk MDS and AML. We know that DNMT inhibitors fail to induce complete remission in the vast majorly of patients yet those with hematologic responses experience survival benefit and longer time to AML transformation. Novel, less toxic biologic and targeted therapies are needed in MDS, such as those that target mutations in IDH1/2 and spliceosome genes. Antibody-based therapies such as those that target CD33 and PD1 are also being actively investigated in clinical trials. Having better tools, such as more cost-effective massive parallel sequencing and newer strategies for gene editing (CRISPR/Cas9), will help advance MDS basic, translational, and clinical research. For example, many of the genetic mutations in MDS result in states of haploinsufficiency for the genes affected. Therefore, to better recapitulate the human disease in mouse models, one wildtype allele and one mutant allele should be maintained in mouse bone marrow cells. These models provide important preclinical tools for basic research and drug testing. In addition, first-in-human studies using CRISPR/Cas9 are now underway in other human diseases to correct specific gene defects. Research progress in MDS has occurred at an exciting pace. As I have illustrated here, there is still an art to taking care of MDS patients since no two cases are exactly the same and there are mimickers of MDS. Hopefully there will be substantial improvements in quality of life and survival of patients with MDS in the next ten years with recent advances in research.
Key points.
MDS is characterized by low blood cell counts, abnormal blood cell development, clonal genetic markers, and increased propensity towards AML
The recent genetic characterization of MDS has advanced various aspects of clinical management
Novel therapies are emerging to address common drivers of MDS development and disease progression
Patients with MDS should always be considered for clinical trial participation whenever possible to support scientific discoveries toward improving outcomes
Footnotes
Disclosure statement: I have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ma X. Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125(7 Suppl):S2–5. doi: 10.1016/j.amjmed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: Contemporary review and how we treat. Am J Hematol. 2016;91(1):76–89. doi: 10.1002/ajh.24253. [DOI] [PubMed] [Google Scholar]
- 3.Hartsock RJ, Smith EB, Petty CS. NORMAL VARIATIONS WITH AGING OF THE AMOUNT OF HEMATOPOIETIC TISSUE IN BONE MARROW FROM THE ANTERIOR ILIAC CREST. A STUDY MADE FROM 177 CASES OF SUDDEN DEATH EXAMINED BY NECROPSY. Am J Clin Pathol. 1965;43:326–331. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]
- 4.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4. World Health Organization; 2008. [Google Scholar]
- 5.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 6.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazzola M, Della Porta MG, Malcovati L. The genetic basis of myelodysplasia and its clinical relevance. Blood. 2013;122(25):4021–4034. doi: 10.1182/blood-2013-09-381665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejar R, Ebert BL. The genetic basis of myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24(2):295–315. doi: 10.1016/j.hoc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–3627. doi: 10.1182/blood-2013-08-518886. quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKerrell T, Park N, Moreno T, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10(8):1239–1245. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 15.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 17.Komrokji R, Ramadan H, Al Ali N, et al. Validation of the Lower-Risk MD Anderson Prognostic Scoring System for Patients With Myelodysplastic Syndrome. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S60–63. doi: 10.1016/j.clml.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Valcarcel D, Sanz G, Ortega M, et al. Use of newer prognostic indices for patients with myelodysplastic syndromes in the low and intermediate-1 risk categories: a population-based study. Lancet Haematol. 2015;2(6):e260–266. doi: 10.1016/S2352-3026(15)00067-8. [DOI] [PubMed] [Google Scholar]
- 19.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850–1856. doi: 10.1200/JCO.2008.17.1058. [DOI] [PubMed] [Google Scholar]
- 21.Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120(6):1037–1046. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111(2):574–582. doi: 10.1182/blood-2007-06-096370. [DOI] [PubMed] [Google Scholar]
- 23.Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–2712. doi: 10.1182/blood-2014-06-582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 25.Metzeler KH, Walker A, Geyer S, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26(5):1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traina F, Visconte V, Elson P, et al. Impact of molecular mutations on treatment response to DNMT inhibitors in myelodysplasia and related neoplasms. Leukemia. 2014;28(1):78–87. doi: 10.1038/leu.2013.269. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 28.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 29.Lubbert M, Suciu S, Hagemeijer A, et al. Decitabine improves progression-free survival in older high-risk MDS patients with multiple autosomal monosomies: results of a subgroup analysis of the randomized phase III study 06011 of the EORTC Leukemia Cooperative Group and German MDS Study Group. Ann Hematol. 2016;95(2):191–199. doi: 10.1007/s00277-015-2547-0. [DOI] [PubMed] [Google Scholar]
- 30.Saunthararajah Y, Sekeres M, Advani A, et al. Evaluation of noncytotoxic DNMT1- depleting therapy in patients with myelodysplastic syndromes. J Clin Invest. 2015;125(3):1043–1055. doi: 10.1172/JCI78789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantarjian HM, Issa JP. Decitabine dosing schedules. Semin Hematol. 2005;42(3 Suppl 2):S17–22. doi: 10.1053/j.seminhematol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Santos FP, Kantarjian H, Garcia-Manero G, Issa JP, Ravandi F. Decitabine in the treatment of myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10(1):9–22. doi: 10.1586/era.09.164. [DOI] [PubMed] [Google Scholar]
- 33.Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5(2):e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YG, Kim I, Yoon SS, et al. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol. 2013;161(3):339–347. doi: 10.1111/bjh.12256. [DOI] [PubMed] [Google Scholar]
- 35.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32(3):358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 36.Duong VH, Lin K, Reljic T, et al. Poor outcome of patients with myelodysplastic syndrome after azacitidine treatment failure. Clin Lymphoma Myeloma Leuk. 2013;13(6):711–715. doi: 10.1016/j.clml.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29(24):3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prebet T, Thepot S, Gore SD, Dreyfus F, Fenaux P, Vey N. Outcome of patients with low-risk myelodysplasia after azacitidine treatment failure. Haematologica. 2013;98(2):e18–19. doi: 10.3324/haematol.2012.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen-Bjergaard J, Andersen MT, Andersen MK. Genetic pathways in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2007:392–397. doi: 10.1182/asheducation-2007.1.392. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen-Bjergaard J, Pedersen M, Roulston D, Philip P. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. Blood. 1995;86(9):3542–3552. [PubMed] [Google Scholar]
- 41.Pedersen-Bjergaard J. Insights into leukemogenesis from therapy-related leukemia. N Engl J Med. 2005;352(15):1591–1594. doi: 10.1056/NEJMe048336. [DOI] [PubMed] [Google Scholar]
- 42.Qian Z, Joslin JM, Tennant TR, et al. Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chem Biol Interact. 2010;184(1–2):50–57. doi: 10.1016/j.cbi.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ok CY, Hasserjian RP, Fox PS, et al. Application of the international prognostic scoring system-revised in therapy-related myelodysplastic syndromes and oligoblastic acute myeloid leukemia. Leukemia. 2014;28(1):185–189. doi: 10.1038/leu.2013.191. [DOI] [PubMed] [Google Scholar]
- 44.Quintas-Cardama A, Daver N, Kim H, et al. A prognostic model of therapy-related myelodysplastic syndrome for predicting survival and transformation to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(5):401–410. doi: 10.1016/j.clml.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernasconi P, Klersy C, Boni M, et al. Incidence and prognostic significance of karyotype abnormalities in de novo primary myelodysplastic syndromes: a study on 331 patients from a single institution. Leukemia. 2005;19(8):1424–1431. doi: 10.1038/sj.leu.2403806. [DOI] [PubMed] [Google Scholar]
- 46.Patnaik MM, Hanson CA, Hodnefield JM, Knudson R, Van Dyke DL, Tefferi A. Monosomal karyotype in myelodysplastic syndromes, with or without monosomy 7 or 5, is prognostically worse than an otherwise complex karyotype. Leukemia. 2011;25(2):266–270. doi: 10.1038/leu.2010.258. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi H, Matsuyama T, Ueda M, et al. Predictive factors of response and survival following chemotherapy treatment in acute myeloid leukemia progression from myelodysplastic syndrome. Intern Med. 2009;48(18):1629–1633. doi: 10.2169/internalmedicine.48.2362. [DOI] [PubMed] [Google Scholar]
- 48.Kayser S, Zucknick M, Dohner K, et al. Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood. 2012;119(2):551–558. doi: 10.1182/blood-2011-07-367508. [DOI] [PubMed] [Google Scholar]
- 49.Valcarcel D, Adema V, Sole F, et al. Complex, not monosomal, karyotype is the cytogenetic marker of poorest prognosis in patients with primary myelodysplastic syndrome. J Clin Oncol. 2013;31(7):916–922. doi: 10.1200/JCO.2012.41.6073. [DOI] [PubMed] [Google Scholar]
- 50.van Gelder M, de Wreede LC, Schetelig J, et al. Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia. Leukemia. 2013;27(4):879–888. doi: 10.1038/leu.2012.297. [DOI] [PubMed] [Google Scholar]
- 51.Wudhikarn K, Van Rheeden R, Leopold C, Rattanaumpawan P, Gingrich R, de Magalhaes Silverman M. Outcome of allogeneic stem cell transplantation in myelodysplastic syndrome patients: prognostic implication of monosomal karyotype. Eur J Haematol. 2012;89(4):294–301. doi: 10.1111/j.1600-0609.2012.01830.x. [DOI] [PubMed] [Google Scholar]
- 52.Koenecke C, Gohring G, de Wreede LC, et al. Impact of the revised International Prognostic Scoring System, cytogenetics and monosomal karyotype on outcome after allogeneic stem cell transplantation for myelodysplastic syndromes and secondary acute myeloid leukemia evolving from myelodysplastic syndromes: a retrospective multicenter study of the European Society of Blood and Marrow Transplantation. Haematologica. 2015;100(3):400–408. doi: 10.3324/haematol.2014.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ustun C, Trottier BJ, Sachs Z, et al. Monosomal karyotype at the time of diagnosis or transplantation predicts outcomes of allogeneic hematopoietic cell transplantation in myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015;21(5):866–872. doi: 10.1016/j.bbmt.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shih AH, Chung SS, Dolezal EK, et al. Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Haematologica. 2013;98(6):908–912. doi: 10.3324/haematol.2012.076729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ok CY, Patel KP, Garcia-Manero G, et al. Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases. Leuk Res. 2015;39(3):348–354. doi: 10.1016/j.leukres.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015;5:e366. doi: 10.1038/bcj.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105(3):973–977. doi: 10.1182/blood-2004-07-2864. [DOI] [PubMed] [Google Scholar]
- 58.Bjorkholm M, Hultcrantz M, Derolf AR. Leukemic transformation in myeloproliferative neoplasms: therapy-related or unrelated? Best Pract Res Clin Haematol. 2014;27(2):141–153. doi: 10.1016/j.beha.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Yakoub-Agha I, Deeg J. Are hypomethylating agents replacing induction-type chemotherapy before allogeneic stem cell transplantation in patients with myelodysplastic syndrome? Biol Blood Marrow Transplant. 2014;20(12):1885–1890. doi: 10.1016/j.bbmt.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376(9757):2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 61.Pastore F, Dufour A, Benthaus T, et al. Combined molecular and clinical prognostic index for relapse and survival in cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2014;32(15):1586–1594. doi: 10.1200/JCO.2013.52.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–4423. doi: 10.1200/JCO.2011.35.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 64.Ahn JS, Kim YK, Min YH, et al. Azacitidine Pre-Treatment Followed by Reduced-Intensity Stem Cell Transplantation in Patients with Higher-Risk Myelodysplastic Syndrome. Acta Haematol. 2015;134(1):40–48. doi: 10.1159/000368711. [DOI] [PubMed] [Google Scholar]
- 65.Kim DY, Lee JH, Park YH, et al. Feasibility of hypomethylating agents followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome. Bone Marrow Transplant. 2012;47(3):374–379. doi: 10.1038/bmt.2011.86. [DOI] [PubMed] [Google Scholar]
- 66.Deeg HJ. Hematopoietic cell transplantation for myelodysplastic syndrome. Am Soc Clin Oncol Educ Book. 2015:e375–380. doi: 10.14694/EdBook_AM.2015.35.e375. [DOI] [PubMed] [Google Scholar]
- 67.Reshef R, Porter DL. Reduced-intensity conditioned allogeneic SCT in adults with AML. Bone Marrow Transplant. 2015;50(6):759–769. doi: 10.1038/bmt.2015.7. [DOI] [PubMed] [Google Scholar]
- 68.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation for myelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia. 1998;12(Suppl 1):S25–29. [PubMed] [Google Scholar]
- 70.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120(7):1398–1408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761–1769. doi: 10.1016/j.bbmt.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115(9):1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffin PT, Komrokji RS, De Castro CM, et al. A multicenter, phase II study of maintenance azacitidine in older patients with acute myeloid leukemia in complete remission after induction chemotherapy. Am J Hematol. 2015;90(9):796–799. doi: 10.1002/ajh.24087. [DOI] [PubMed] [Google Scholar]
- 74.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116(23):5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winkler J, Rech D, Kallert S, et al. Sorafenib induces sustained molecular remission in FLT3-ITD positive AML with relapse after second allogeneic stem cell transplantation without exacerbation of acute GVHD: a case report. Leuk Res. 2010;34(10):e270–272. doi: 10.1016/j.leukres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Tarlock K, Chang B, Cooper T, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. 2015;62(6):1048–1054. doi: 10.1002/pbc.25437. [DOI] [PubMed] [Google Scholar]
- 77.Sharma M, Ravandi F, Bayraktar UD, et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant. 2011;17(12):1874–1877. doi: 10.1016/j.bbmt.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schiller GJ, Tuttle P, Desai P. Allogeneic Hematopoietic Stem Cell Transplantation in FLT3-ITD-Positive Acute Myelogenous Leukemia: The Role for FLT3 Tyrosine Kinase Inhibitors Post-Transplantation. Biol Blood Marrow Transplant. 2016;22(6):982–990. doi: 10.1016/j.bbmt.2016.01.013. [DOI] [PubMed] [Google Scholar]