Abstract
Binge drinking and the onset of alcohol use disorders usually peak during the transition between late adolescence and early adulthood, and early adolescent onset of alcohol consumption has been demonstrated to increase the risk for alcohol dependence in adulthood. In the present study we describe an animal model of early adolescent alcohol consumption where animals drink unsweetened and unflavored ethanol in high concentrations (20%). Using this model we investigated the influence of drinking on alcohol-related appetitive behavior and alcohol consumption levels in early adulthood. Further, we also sought to investigate whether differences in alcohol-related drinking behaviors were specific to exposure in adolescence versus exposure in adulthood. Male Wistar rats were given a 2-bottle choice between 20% ethanol and water in one group and between two water bottles in another group during their adolescence (Postnatal Day (PD) PD26-59) to model voluntary drinking in adolescent humans. As young adults (PD85), rats were trained in a paradigm that provided free access to 20% alcohol for 25 min after completing up to a fixed ratio (FR) 16-lever press response. A set of young adult male Wistar rats was exposed to the same paradigm using the same time course beginning at PD92. The results indicate that adolescent exposure to alcohol increased consumption of alcohol in adulthood. Furthermore, when investigating differences between adolescent high and low adolescent drinkers in adulthood, high consumers continued to drink more alcohol, had fewer FR failures, and had faster completion of FR schedules in adulthood whereas the low consumers were no different than controls. Rats exposed to ethanol in young adulthood also increased future intake but there were no differences in any other components of drinking behavior. Both adolescent- and adult-exposed rats did not exhibit an increase in lever pressing during the appetitive challenge session. These data indicate that adolescent and early adult alcohol exposure can increase consumptive aspects of drinking but that adolescent exposure may preferentially influence the motivation to drink.
Keywords: Adolescence, Alcohol drinking, Appetitive responses, Consumptive responses, Wistar rats
Introduction
Adolescence is a critical time period for brain development when emotional, cognitive, and social maturation occur (see Dahl & Spear, 2004). The 2007 National Survey on Drug Abuse and Health has reported that approximately 16% of adolescents between the age of 12 and 17 were current users of ethanol, with 10% of these teens classified as binge drinkers (U.S. Department of Health and Human Services, 2008). Data from the Monitoring the Future study shows that 30-day prevalence and heavy drinking in men peaks at ages 21–22 and then declines linearly through adulthood (Bachman et al., 1997). In agreement with these findings, Grant et al. (2004) reported that individuals within the ages 18–29 exhibit the highest rates of past-year ethanol abuse and dependence.
Ethanol use during early adolescence has been clearly demonstrated to be a risk factor for the later development of alcohol dependence in a number of epidemiological studies (Ehlers et al., 2006, 2010; Grant, 1998; Grant & Dawson, 1997; Hicks, Iacono & McGue, 2010; Hingson, Heeren & Edwards, 2008). The mechanism by which early adolescent drinking leads to an increased risk for alcohol dependence in high-risk individuals is not known. One hypothesis suggests that early heavy drinking can interrupt the normal course of social and cognitive development leading to an increased risk for a number of pathologies including drug addictions (DeWit et al., 2000; York, 1999). An alternate hypothesis is that teens that initiate drinking during early adolescence may have an underlying predisposition to disinhibitory behaviors that drives their early drinking as well as other risky actions (Iacono et al., 2002; Jessor & Jessor, 1977). These hypotheses are difficult to distinguish in human studies; however, the development of an animal model to study the effects of adolescent ethanol exposure on drinking behaviors in adulthood could ultimately prove useful in the understanding of the brain mechanisms underlying the effects of early adolescent drinking.
The adolescent period in rodents has a number of similarities with the human condition making it a reasonable model to study the consequences of adolescent drinking (see Spear, 2000b, 2000c; Spear & Varlinskaya, 2005). Variables that have been used to investigate adolescent drinking patterns in animal models include: strain, sex, age, sweetened and/or flavored solutions, isolate-housing, investigator administered alcohol, and different operant or free consumption paradigms with variable lengths of access to ethanol during the light or dark part of the circadian cycle (Bell et al., 2006; Brunell & Spear, 2005; Criado & Ehlers, 2013; Doremus et al., 2005; Ehlers et al., 2007a; Fullgrabe, Vengeliene & Spanagel, 2007; Lancaster et al., 1996; Siciliano & Smith, 2001; Walker, Walker & Ehlers, 2008). Studies investigating developmental differences in drinking patterns indicate that adolescent rats show greater levels of ethanol intake when compared to adult rats (Brunell & Spear, 2005; Doremus et al., 2005; Fabio et al., 2014; Sarviharju et al., 2001; Spear, 2004, 2007; Vetter, Doremus-Fitzwater & Spear, 2007; Vetter-O’Hagen, Varlinskaya & Spear, 2009).
Studies using animal models have also indicated that voluntary ethanol drinking during adolescence can, in some models, be shown to facilitate the acquisition of alcohol self-administration, increase craving behavior, and/or increase the probability of relapse in those animals in adulthood (see Alaux-Cantin et al., 2013; Gilpin, Karanikas & Richardson, 2012; Jeanblanc et al., 2015; McBride et al., 2005; Serlin & Torregrossa, 2015; Spear, 2000a; Toalston et al., 2015). However, other studies have shown that ethanol exposure during adolescence has no effect on subsequent ethanol consumption in adulthood (Slawecki & Betancourt, 2002). For instance, Vetter, Doremus-Fitzwater & Spear (2007) found that adult rats trained to drink ethanol during adolescence showed no differences in ethanol drinking when compared to a control group not exposed to ethanol during adolescence. Additionally, Siegmund et al. (2005) have shown that Wistar rats that initiated alcohol consumption during adolescence, when not exposed to stress, actually consumed less alcohol and showed lower preference than rats that were initiated into drinking as adults. The reason that disparate findings have been obtained between studies is at this point not entirely clear. However, one reason may be the inclusion of sweeteners or flavorings into the ethanol solutions. In two recent studies, adolescent drinking of sucrose or sucrose/saccharin solutions with or without ethanol was found to increase the consumption of those sweetened solutions in adulthood but not the consumption of ethanol alone (Broadwater, Varlinskaya & Spear, 2013; Pian et al., 2009). This finding has been interpreted as suggesting that solution–specific increases in adulthood intake after adolescent exposure are most likely associated with solution “acceptance” due to familiarity (Broadwater, Varlinskaya & Spear, 2013). Thus, the drinking of ethanol in flavored or sweetened solutions during adolescence may confound the interpretation of potential increases in ethanol consumption in adulthood if the ethanol is presented in a sweet or flavored solution that the rat is familiar with.
The purpose of this present study was to develop a model of adolescent drinking of high concentrations of unsweetened and unflavored ethanol (20% ethanol in water) to test the three specific aims: (1) to determine if adolescent ethanol drinking affects future drinking in adulthood; (2) to test whether the amount of drinking during adolescence influences drinking in adulthood; and (3) to investigate whether adolescent alcohol drinking enhances appetitive (alcohol seeking or craving, as measured by increasing number of lever presses to drink) and/or consumptive (amount of alcohol drank) components of alcohol drinking. Further, we sought to determine whether these behavioral differences were specific to adolescent exposure or were also seen in young adults exposed to the same drinking paradigm. Voluntary ethanol drinking using a two-bottle choice paradigm was used to assess the effects of ethanol drinking in adolescence and young adulthood. In later adulthood in both groups of rats, the “sipper tube” model, developed by Samson et al. (1998), was used to independently measure the number of lever presses, (to evaluate appetitive behaviors), and the amount of ethanol consumed, (to measure consummatory behaviors).
Materials and Methods
Animals
Fifty-three adolescent and 16 young adult male Wistar rats were obtained from Charles River (USA). Adolescent rats were received and weaned on postnatal day (PD) 23, whereas adult rats were received at PD75. All animals were pair-housed in standard plastic cages and kept in a room with a light dark cycle (12-h of light/12-h of dark, lights on at 8:00 am) that was temperature controlled. Food and water were available ad libitum throughout the duration of the experiment. All experimental protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals.
Intermittent Ethanol 2-Bottle Choice Paradigm
To assess the effects of early ethanol drinking, adolescent rats and a comparison group of young adults were given the option to drink from two bottles. Experimental subjects (33 adolescents, 8 young adults) were given one bottle with 20% ethanol and one bottle with tap water. Control subjects (20 adolescents, 8 young adults) were provided with two bottles of tap water. At the start of each 2-bottle choice session animals were divided from their cage mate by a Plexiglas divider that separated the cage into two equal-sized compartments. Solutions were presented in 100 ml graduated cylinders that were fitted with ball-point sipper tubes. The position of each bottle was alternated daily to avoid position preference. Animals were given 24 h access to the solutions and food was available ad libitum. Two “leak” bottles, one for water and one for ethanol, were used to control for accidental sipper leakage during the experiment and were placed in an identical plastic cage with a wire top. Before each session, the amount of solution in each bottle was measured before presentation to the animal. During the session, each bottle was weighed after 30 min and 24 h of drinking. The amount consumed from each bottle was calculated by subtracting the amount removed from the corresponding leak bottle from the volume removed from each bottle by the animal’s drinking. After each 24-h session the pair-housed animals were placed back together in their home cage. Each week contained 3 sessions with a total of 15 sessions lasting 33 days (PD26-59 or PD92-125). Each animal underwent a tail bleed during their 13th or 15th session to measure their blood alcohol level (BAL). The BALs were mildly detectable (Adolescent = 21.51±2.50 mg/dL, Young Adult = 35.81±5.59 mg/dL) since alcohol drinking was relatively low and consumed across a 24-h period.
Operant Drinking
After a 4-week abstinence period, animals were trained in operant chambers for two days starting on PD85 for 2-bottle choice adolescence or PD152 for 2-bottle choice adults. Operant boxes were equipped with a house-light, a retractable lever, and a retractable sipper tube. This operant “sipper tube” model, developed by Samson et al. (1998), was used to independently measure the number of lever presses, (to evaluate appetitive behaviors), and the amount of ethanol consumed, (to measure consummatory behaviors). Each animal was water deprived for 23 h and then placed in an operant box with access to water with each lever press. Each training session was 25 min long and each lever press provided the animal with 30 sec of water access. To ensure that animals were properly hydrated following the training sessions, if an animal did not lever press during the 25 min training session they were provided with 60 min of free water access. If the animal did lever press they were given at least 35 more minutes of free water access. Operant sessions were performed 5 days per week around 9am. The paradigm then shifted to a fixed ratio (FR) schedule using ethanol to determine each rat’s motivation to consume ethanol. After two water training sessions, each rat was given 10 min to press the lever once to receive 20% ethanol for 25 min. The required number of presses then subsequently increased to FR 4, 6, 8, 10, 12, 14, then 16. If the animal succeeded at pressing the lever the number of times required, the animal was given 20% ethanol access for 25 min and advanced to the next level the following day. Animals that failed to complete the number of required lever presses did not receive access to ethanol and had to repeat that ratio level. For each session, the amount of ethanol consumed and the number of lever presses was recorded for each animal. Between each animal, a vinegar and water solution was used to clean each operant box and the bedding was changed. If an animal failed to reach criterion 6 times, or succeeded FR16 five times, it underwent an appetitive “challenge” session the next day. During the challenge session there was no fixed ratio, so that each animal could press the lever any number of times without receiving ethanol until a fixed time of 10 min. After the 10 min each animal received 25 min of 20% ethanol access and a tail bleed was done to measure the animal’s blood alcohol level (BAL).
Statistics
To determine if ethanol exposure in adolescence significantly influenced drinking in adulthood, adolescent rats were given a choice to consume alcohol for 15 intermittent days and then later tested on an operant FR drinking paradigm. During operant performance, two-way ANOVAs (group x session) were used to analyze the average amount of ethanol consumed during the first three easier schedule sessions (FR1, FR4, FR6) and the average amount of ethanol consumed during the last five more challenging FR schedule sessions (FR8, FR10, FR12, FR14, FR16) to further delineate whether there were differences between groups. If the result of the ANOVA was statistically significant (p < 0.05), Tukey’s post hoc analyses were conducted. Rats that failed before reaching an FR schedule were given a blank score for consumption (g/kg) for all the subsequent sessions. One adolescent rat (ethanol group) did not complete the early FR ratios and nine more (4 control, 5 ethanol) failed to reach FR16. One-way ANOVAs were utilized to determine differences between groups (ethanol vs. control) on the average ethanol consumption (g/kg) during the appetitive challenge session, the average number of times each animal failed to reach criterion, and the overall average time to reach criterion. To test if adolescent alcohol exposure also had an effect on the appetitive component of adult drinking; the average number of challenge lever presses for each group was analyzed using a one-way ANOVA.
To determine if individual differences in alcohol consumption during adolescence influenced ethanol consumption during adulthood, the adolescent ethanol exposed animals were split into two groups; one group which consumed an amount of alcohol, during the first 30 min of the 2-bottle choice, above the median (High EtOH) and another group which consumed below the median (Low EtOH). In adulthood, two-way ANOVAs (group x session) were used to determine if there were any significant differences in the average amount of alcohol consumed during the first three FR schedule sessions, and during the last five FR schedule sessions. A one-way ANOVA was used to determine differences in ethanol consumption (g/kg) during the appetitive challenge session between the high and low drinking groups as compared to the controls in adulthood. One-way ANOVAs were used to analyze the average number of times each animal failed to reach criterion, overall average time to reach criterion, and average number of lever presses during the challenge session. Follow-up Tukey’s post hoc analyses were conducted for statistically significant (p<0.05) ANOVAs.
To determine if ethanol exposure during early adulthood influenced drinking in later adulthood, adult rats were given an identical alcohol access period and were tested 4 weeks later on the same operant FR drinking paradigm as the adolescent-exposed rats. To determine if there were initial differences between young adult- and adolescent-exposure, a one-way ANOVA was conducted for the first 30 min of drinking and for 24 h of drinking over sessions 2–15. Three adult rats (2 ethanol, 1 control) did not complete the first 3 ratios and one additional rat (control group) did not reach FR16. A one-way ANOVA with Tukey post hoc analyses was used to determine differences in ethanol consumption (g/kg) during the challenge session, the average number of times each animal failed, average time to reach criterion, and average number of lever presses during the challenge session.
All study analyses were carried out using the statistical program SPSS (version 15.0, Chicago, IL: SPSS Inc.). All averages are reported as mean ± SEM (standard error of mean).
Results
Behavior in Adolescent Drinkers vs. Controls
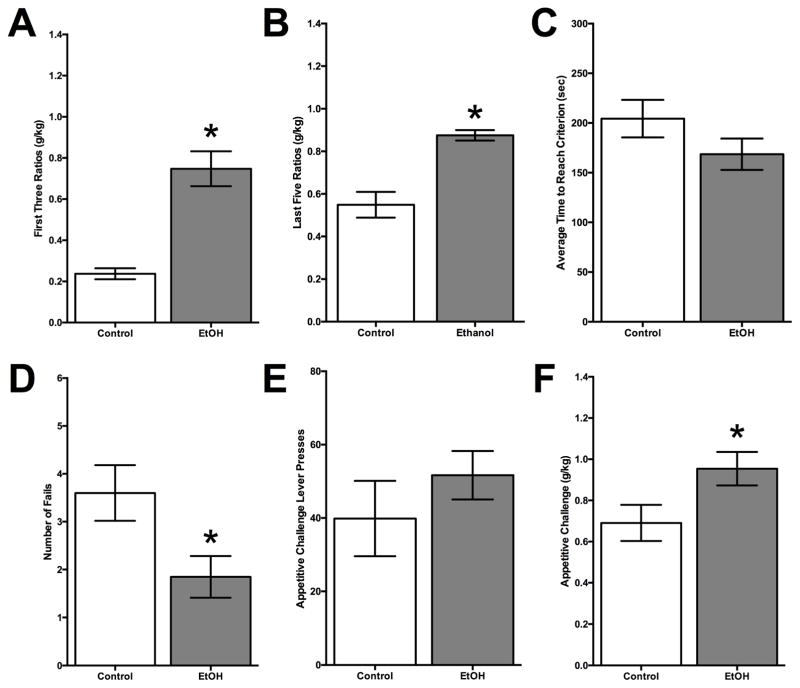
The mean ± SEM amount of alcohol consumed over the fixed ratio sessions by the adult rats that were exposed to ethanol or water during adolescence was compared using a two-way ANOVA (alcohol or water group X three FR sessions). Two-way ANOVA (group x session) revealed that there was a significant main effect of group (adolescent alcohol exposure vs. control) over the first three FR sessions of the operant training (F = 45.47, df = 1,152, p < 0.0001) but no effect of session (F = 1.903, df = 2,152, p = 0.15). Rats previously exposed to ethanol in adolescence consumed a significantly higher amount of ethanol (mean = 0.75±0.08 g/kg), than control rats (mean = 0.24±0.03 g/kg) during the first three FR ratio sessions (see Figure 1A). Two-way ANOVA also revealed that there was a significant effect of group over the last five FR sessions (F = 18.80, df = 1,220, p < 0.0001) but again no effect of session (F = 0.714, df = 4,220, p = 0.58). Adolescent-exposed rats continued to consume a significantly higher amount of ethanol (mean = 0.87±0.08 g/kg), than control rats (mean = 0.51±0.07 g/kg) during the last five FR ratio sessions (see Figure 1B). These results indicate that adolescent consumption of 20% ethanol over 15 days is sufficient to produce an increase in future drinking in adulthood compared to controls.
Figure 1. Ethanol Drinking in Adolescence on Behaviors in Adulthood.
(A) The mean (± SEM) amount of ethanol consumed during the first three FR sessions of the self-administration paradigm. Adult rats which were given the opportunity to freely consume ethanol during their adolescence. Adolescent-exposed rats consumed a significantly higher amount of ethanol compared to controls. (B) The mean (± SEM) amount of ethanol consumed during the last five FR sessions of the self-administration paradigm. Rats which were exposed to ethanol during adolescence again drank more ethanol during adulthood than control rats. (C) The overall mean (± SEM) time to reach any given FR criterion was similar for both groups. (D) The mean (± SEM) number of failed criterions was less for adolescent-exposed rats compared to controls (E) The mean (± SEM) number of lever presses during the appetitive challenge was not significantly different between the control and alcohol-exposed animals. (F) The mean (±SEM) ethanol consumed after the challenge lever pressing session. Ethanol-exposed rats consumed significantly more alcohol during this period compared to controls. (* p<0.05 vs. control group)
There was no significant difference in average time it took to lever press over any given FR criterion between adolescent alcohol drinking and control rats (F = 2.042, df = 1,51, p = 0.16; see Figure 1C). However, adolescent-exposed rats were more likely to reach criterion (failed less) over all incrementing ratios (mean = 1.85±0.44) compared to controls (mean = 3.60±0.58; F = 5.919, df = 1,51, p < 0.05; see Figure 1D). These results indicate that, overall, adolescent ethanol rats were more motivated to consume alcohol compared to controls.
The number of lever presses during the appetitive challenge session of the operant paradigm was compared to see if there was an enhancement in the appetitive component of alcohol in animals that drank alcohol in adolescence. Adolescent-exposed rats pressed the lever more (mean = 51.67±6.61) than controls (mean = 39.85±10.28; F = 1.026, df = 1,51, p = 0.32) but the results were not significant (see Figure 1E). However, adolescent ethanol drinking rats (mean = 0.95±0.08 g/kg) did consume more than controls (mean = 0.69±0.09 g/kg) after 10 minutes of challenge session lever pressing (F = 4.456, df = 1,51, p < 0.05; see Figure 1F). The BALs were taken immediately after the conclusion of the challenge session in a subset of animals. Ethanol consumption (g/kg) was not significantly correlated with BAL levels (mean = 32.09±3.23 mg/dL) taken immediately after the session [F = 3.083, df = 1,22, p = 0.09, R2 = 0.12]. These results indicate that while adolescent-exposed rats demonstrate a robust increase in alcohol consumption in adulthood, it does not appear to impact the appetitive components of alcohol related behaviors.
Behavior in Low and High Adolescent Drinker vs. Controls
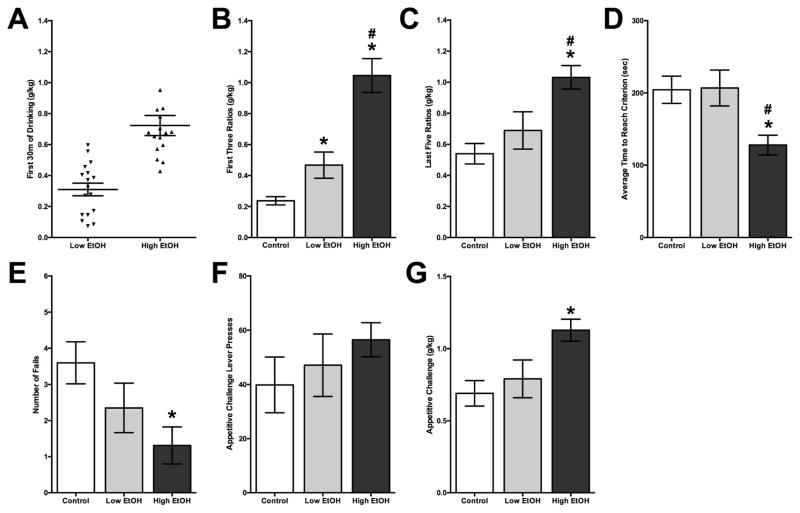
The mean ± SEM amount of ethanol consumed during the first 30 min of the ethanol 2-bottle choice paradigm was used to evaluate the effects of the amount of alcohol drank in rats exposed to alcohol during adolescence. The adolescent drinkers were split into two groups; a low drinking subgroup (Low EtOH, n = 17), which consumed ethanol at or above the median (0.45 g/kg/30m), and a high drinking subgroup (High EtOH, n = 16), that consumed ethanol above the median were formed. A significant difference was found in the amount of ethanol consumed in the first 30 min of sessions 2–15 during adolescence between the low drinking group (mean = 0.29±0.03 g/kg) and the high drinking group (mean = 0.65±0.06 g/kg; F = 41.345, df = 1,31, p < 0.0001; see Figure 2A). High adolescent drinkers (mean = 3.36±0.46g/kg) also consumed more ethanol over the 24-h period for sessions 2–15 compared to low drinkers (mean = 2.06±0.35g/kg; F = 5.085, df = 1,31, p<0.05).
Figure 2. High and Low Adolescent Ethanol Drinkers on Behaviors in Adulthood.
(A) The mean (±SEM) amount of ethanol consumed during the first 30 min of the 2-bottle choice paradigm. Adolescent high drinking animals (High EtOH) had significantly higher consumption of alcohol than low drinking animals (Low EtOH). (B) The mean (±SEM) amount of alcohol consumed during adulthood in the first three FR sessions was compared between high drinkers, low drinkers and controls to determine if an initial preference to alcohol had a long-term effect on alcohol consumption during adulthood. High drinking animals consumed significantly more ethanol than both low drinking animals and control animals. Low drinkers consumed significantly more alcohol than controls. (C) The mean (±SEM) amount of alcohol consumed during all sessions was compared between high drinkers, low drinkers and controls. High drinking animals consumed significantly more ethanol than both low drinking animals and control animals, but no difference between low drinkers and controls. (D) The overall mean (± SEM) time to reach FR criterion was significantly faster for the high drinkers compared to low drinkers and controls. (E) The mean (± SEM) number of failed criterions was less for the high drinkers compared to controls but no difference between controls and low drinkers. (F) The mean (±SEM) number of lever presses during the appetitive challenge session was compared between the high drinkers, low drinkers and control animals and no significant difference was found. (G) The mean (±SEM) amount of alcohol consumed during the challenge session was significantly different, with high consuming animals drinking significantly more than low drinkers and control. (* p<0.05 vs. control group, # p<0.05 vs. Low EtOH group)
The ethanol consumption during adulthood of the two adolescent-ethanol exposed subgroups was then compared to determine if the amount of ethanol drank during adolescence had an effect on drinking during adulthood. Using a two-way ANOVA (group x session), there was a significant effect of group (F = 50.47, df = 2,149, p < 0.0001) as measured by the mean amount of alcohol consumed between control animals (mean = 0.24±0.03 g/kg), low drinkers (mean = 0.47±0.08 g/kg), and high drinkers (mean = 1.05±0.11 g/kg) during the first three FR sessions (see Figure 2B). Further post-hoc analysis showed that the Low and High EtOH subgroups drank more than controls and the Low EtOH drinkers consumed significantly less than the High EtOH group. There was also a significant effect over the first three FR ratio session (F = 3.680, df = 2,149, p < 0.05), with more consumption occurring after the FR6 (mean = 0.68±0.08 g/kg) schedule compared to FR1 (mean = 0.47±0.06 g/kg). There was also a significant difference in the mean alcohol consumption during the last five operant sessions (F = 16.97, df = 2,215, p < 0.001; see Figure 2C) with high drinkers (mean = 1.03±0.08 g/kg) consuming more than control animals (mean = 0.54±0.07 g/kg) and low drinkers (mean = 0.69±0.12 g/kg). However, there was no difference between control and low drinking animals. There was also no significant effect of session (F = 0.494, df = 4,215, p = 0.74).
Differences in the behaviors associated with drinking were also compared in adolescent-exposed rats and controls in the high and low adolescent drinking subgroups. Figure 2D shows a significant difference in the mean time to lever press to reach criterion (F = 4.850, df = 2,50, p<0.05). Rats who drank higher levels of alcohol in adolescence were significantly faster at reaching criterion (mean = 127.9±13.61 sec) compared to control (204.4±18.88 sec, p < 0.05) and Low EtOH drinkers (206.8±24.78 sec, p < 0.05). There was a significant difference in number of failed sessions (F = 3.680, df = 2,50, p < 0.05; see Figure 2E). This measure indicates the number of times an animal did not meet the criterion within 10 min on any given session (i.e. did not reach 8 press within 10 min on an FR8 session). This effect was significant in a comparison of High EtOH drinkers (mean = 1.31±0.51) and controls (mean = 3.60±0.58; p < 0.05). No differences were observed between Low EtOH drinkers (mean = 2.35±0.69) and controls.
There was no difference in the number of lever presses during the appetitive challenge of operant training between the groups (F = 0.720, df = 2,50, p = 0.49; Figure 2F). However, there was a difference in alcohol consumption after 10min of challenge lever pressing (F = 5.023, df = 2,50, p < 0.05; Figure 2G). High EtOH rats (mean = 1.13±0.08 g/kg) consumed more than controls (mean 0.69±0.09 g/kg) on this last day of operant drinking. However, no difference in the amounts consumed was seen between Low EtOH drinkers (mean = 0.79±0.13 g/kg) and controls or high drinkers.
Behavior in Adult Drinkers vs. Controls
The mean ± SEM amount of ethanol consumed during the first 30 min of the ethanol 2-bottle choice paradigm was again used to determine initial differences in ethanol drinking (g/kg) between adolescent- and young adult rats. There was no significant difference in the amount of ethanol consumed in the first 30 min of sessions 2–15 between the adolescent-exposed (mean = 0.47±0.04g/kg) and the young adult-exposed groups (mean = 0.39±0.11 g/kg; F = 0.649, df = 1,39, p = 0.35). However, adolescent (mean = 3.50±0.27g/kg) rats did consumed more ethanol over a 24-h period compared to adults (mean = 2.20±0.53 g/kg; F = 5.915, df = 1,26, p < 0.05, not shown).
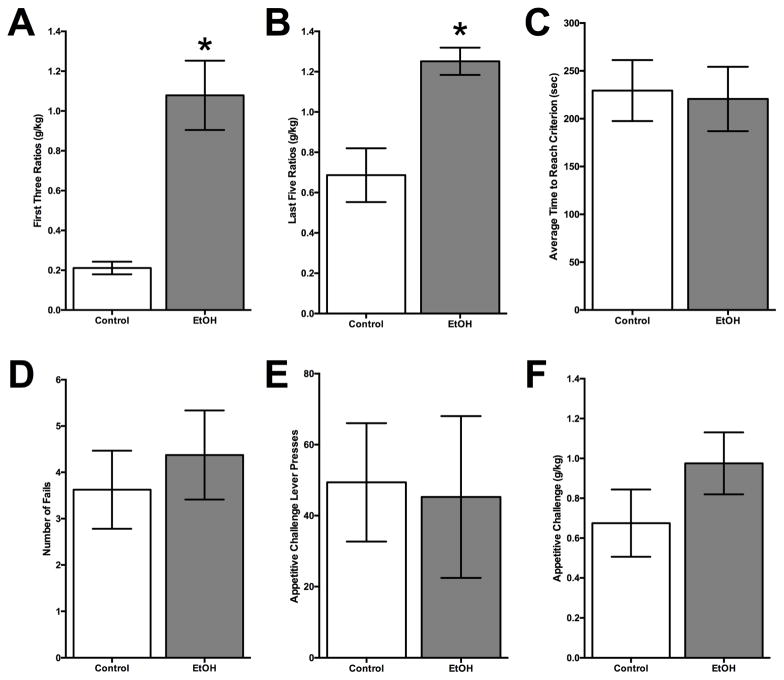
During operant FR drinking paradigm, the mean ± SEM amount of alcohol consumed by adult rats which were allowed access to alcohol in young adulthood and then performed the operant drinking later in life, were compared to water only controls using a two-way ANOVA (group X session). Young adult-exposed rats drank significantly more alcohol later in adulthood during the first three FR sessions (mean = 1.08±0.17 g/kg) as compared to controls (mean= 0.21±0.03 g/kg; F = 33.85, df = 1,37, p < 0.001; Figure 3A). There was also a significant effect between groups over the last five schedules (F = 33.69, df = 1,49, p < 0.0001). Ethanol adult rats also showed an increase in consumption in the last five schedules (mean = 1.25±0.07 g/kg) compared to controls (mean = 0.69±0.13 g/kg; Figure 3B). However, there were no differences in consumption across FR schedules for the first three fixed ratio sessions (F = 2.163, df = 2,37, p = 0.13) or last five fixed ratio sessions (F = 1.845, df = 4,49, p = 0.14) for adult rats. Anecdotally, young adult exposed rats seemed to consume more ethanol during this task compared to adolescent exposed. These results indicate that this model of intermittent access to unsweetened alcohol can increase future consumption, irrespective of when rats initially self-administered. There was also no difference in time to reach criterion for rats pre-exposed to ethanol in adulthood compared to controls (F = 0.036, df = 1,14, p = 0.85; Figure 3C). While adolescent-exposed rats were more likely to reach criterion (failed less) over all of the incrementing ratios, this difference in motivation to complete ratios was not demonstrated in the adults (Control = 3.63±0.84, EtOH = 4.38±0.96; F = 0.343, df = 1,14, p = 0.57; Figure 3D).
Figure 3. Ethanol Drinking in Young Adulthood on Behaviors Later in Adulthood.
(A) The mean (± SEM) amount of ethanol consumed during the first three FR sessions of the self-administration paradigm. Adult rats which were given the opportunity to freely consume ethanol during earlier in adulthood consumed significantly more ethanol compared to controls. (B) The mean (± SEM) amount of ethanol consumed during the last five FR schedules of the self-administration paradigm. Rats which were exposed to ethanol during adulthood again drank more ethanol later in adulthood than control rats. (C) The overall mean (± SEM) time to reach FR criterion was similar for both groups. (D) The mean (± SEM) number of failed criterions was not significantly different between ethanol-exposed and control group. (E) The mean (± SEM) number of lever presses during the appetitive challenge was not significantly different between the control and ethanol-exposed animals. (F) The mean (±SEM) amount of ethanol consumed during the challenge session was not significantly different between groups. (* p<0.05 vs. control group)
While rats pre-exposed to alcohol in young adulthood demonstrated increased consumption, we sought to determine if there was an age-dependent enhancement in the appetitive component of alcohol. Young adult ethanol drinking rats (mean = 45.25±22.80) pressed the lever less during appetitive challenge session compared to controls (mean = 49.38±16.68), but the finding was not significant (F = 0.021, df = 1,14, p = 0.89; Figure 3E). Similar to adolescent exposed rats, ethanol rats (mean = 0.98±0.16 g/kg) consumed more than controls (mean = 0.68 ± 0.17 g/kg) after the 10 min of challenge lever pressing, however this difference was not significant (F = 1.708, df = 1,14, p = 0.21; see Figure 3F). Ethanol consumption (g/kg) after the challenge session was significantly correlated with BAL levels (mean = 37.07±7.32 mg/dL) taken immediately after the session [F = 11.51, df = 1,14, p < 0.01, R2 = 0.45]. These results indicate that young adult rats presented alcohol during this intermittent 2-bottle choice paradigm do not increase their appetitive alcohol-related behaviors in later adulthood.
Discussion
The present study used a limited-access two-bottle consumption model that used unsweetened and unflavored ethanol (20%) during adolescence followed by a self-administration FR paradigm during adulthood to: (1) determine if the exposure to ethanol during adolescence has an effect on adult consumptive behavior; (2) to test if the amount of ethanol consumed during adolescence has an effect on adult drinking; and (3) to assess the effect of ethanol drinking during adolescence on appetitive and consumptive behaviors associated with ethanol in adulthood. These results indicate that adolescent alcohol exposure, using this intermittent drinking paradigm, can increase consumption in adulthood. Furthermore, when investigating differences between adolescent high and low adolescent drinkers, differences seemed to emerge in the adult rats that consumed higher amounts of alcohol in adolescence. These high consumers continued to drink more alcohol in adulthood across all FR ratios compared to controls whereas the low consumers drank more only during the first three ratios compared to controls. High consumers showed less FR failures and faster completion of FR schedules. However, there were still no differences in appetitive challenge lever pressing. We also investigated whether these behavioral differences were specific to adolescent exposure or were also seen in young adults exposed to the same drinking paradigm. Our results indicate that pre-exposure to alcohol increased alcohol intake irrespective of initial age of exposure. However, young adult-exposed rats did not show increased motivation to reach criterion (less fails) as seen with the adolescent exposure group.
Using this simple model of intermittent unsweetened 20% ethanol self-administration, rats previously exposed to ethanol during adolescence were found to consume significantly higher amounts in adulthood compared to control rats. Further, rats that consumed high amounts of ethanol during adolescence also drank significantly more ethanol during adulthood compared to low drinkers and control animals. This indicates that moderate exposure to ethanol during adolescence may be followed by an increase in alcohol consumption during adulthood. Low ethanol exposure also increased adulthood consumption but only after the first three FR schedules. This is consistent with previous studies which showed that early exposure to ethanol increased ethanol consumption in adulthood (Ho, Chin & Dole, 1989; Yoshimoto et al., 2002), and more specifically the studies of Alaux-Cantin et al. (2013), who demonstrated that intermittent free-choice ethanol consumption increased adult daily ethanol intake and the motivation to drink when the animal was provided with ethanol during early adolescence (PD30–43). However, there are some inconsistences in the literature with a few studies finding that ethanol exposure during adolescence has no effect on adult drinking (Fullgrabe, Vengelience & Spear, 2007; Siegmund et al., 2005; Slawecki & Betancourt, 2002; Vendruscoloa et al., 2010; Vetter, Doremus-Fitzwater & Spear, 2007). This result may be due to differences in methodology, for instance, how the ethanol was given to adolescent animals (voluntary or involuntary), whether the ethanol was administered with sucrose or another sweet substance, or the amount, length or time or timing of exposure. Further, voluntary administration used in this study may be considerably less stressful compared to intragastric infusions or injections, which may compound with the effects of alcohol during this critical period in development to impact future behavior (Lupien et al., 2009).
This effect of adolescent drinking on future consummatory behavior is not age specific. We found that adult rats, allowed to self-administer alcohol for 15 days would also increase consumption later during the operant FR task. While it would be interesting to determine whether differences emerged between high and low young adulthood drinkers, there were no clear changes in appetitive or motivational behaviors between treatment groups to suggest that further subdivision would provide a similar profile to adolescent drinkers. None the less, future studies are needed to further investigating the appetitive and motivational impact of adult alcohol exposure. Previous studies have shown that pre-exposure to ethanol in adulthood increased future consumption (de la Torre, Escarabajal & Agüero, 2015; Carrara-Nascimento, Olive & Camarini, 2014). While pre-exposure increased consumption in both adolescent and young adult rats, it modestly increased the motivation to consume alcohol in the adolescent-exposed rats compared to controls. This developmental effect, that drinking during adolescence leads to a different profile of behavior than drinking during adulthood, has also been shown in mice. Carrara-Nascimento, Olive & Camarini (2014) found that adolescent and adult alcohol-exposed mice consumed the same amount of alcohol after a 15-day treatment (2 g/kg injection) but only the adolescent-exposed mice showed a robust preference during the condition-place preference paradigm. These results indicate that administration of ethanol during adolescence can alter future behaviors in a different manor than when administered in adulthood. These findings may also support the hypothesis that different neurobiological systems facilitate consummatory and appetitive behaviors (Samson, Czachowski & Slawecki, 2000). However, it is unclear whether the neurobiological changes in adolescence causes altered alcohol related behaviors, or if it is caused by a direct effect of alcohol exposure. Further research on this causal relationship is important for understanding the impact of alcohol exposure during this sensitive period of development.
This intermittent access paradigm, using high concentrations of unsweetened and unflavored ethanol (20% ethanol in water), demonstrates that alcohol drinking in adolescence can increase drinking in adulthood. It is important to note that after 30 minutes of alcohol exposure, rodent BALs were around 0.03%, equivalent to 1–2 drinks in humans. This further suggests that even minimal alcohol consumption can have lasting changes on future drinking. Further studies using this model may be suitable for investigating the neurobiological mechanisms underlying the effects of voluntary ethanol exposure during adolescence on ethanol drinking during adulthood.
HIGHLIGHTS.
Rats that drank 20% ethanol as adolescents or young adults drank more as adults.
Adolescent or adult drinking did not increase future appetitive lever pressing.
Adolescents drank more and displayed more motivation to drink than adult drinkers.
Acknowledgments
Role of Funding Source
This study was supported in part by the National Institutes of Health (NIH), National Institute on Alcoholism and Alcohol Abuse grants, AA006059 and AA019969 awarded to CLE. NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Anita Desikan and Phil Lau for their assistance in data collection and analyses, and Shirley Sanchez for assistance in proofreading and editing.
Footnotes
Conflicts of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Bachman JG, Wadsworth KN, O’Malley PM, Johnston LD, Schulenberg JE. Smoking, drinking, and drug use in young adulthood: the impacts of new freedoms and new responsibilities. Mahwah, N.J: L. Erlbaum Associates; 1997. [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacology, Biochemistry and Behavior. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcoholism: Clinical and Experimental Research. 2013;37:1048–1055. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Olive MF, Camarini R. Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Developmental Psychobiology. 2014;56:36–48. doi: 10.1002/dev.21089. [DOI] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of Dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcoholism: Clinical and Experimental Research. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behavioural Brain Research. 2010;210:164–170. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacology, Biochemistry and Behavior. 2013;103:622–630. doi: 10.1016/j.pbb.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. New York, N.Y: The New York Academy of Sciences; 2004. [DOI] [PubMed] [Google Scholar]
- de la Torre ML, Escarabajal MD, Agüero Á. Sex differences in adult Wistar rats in the voluntary consumption of ethanol after pre-exposure to ethanol-induced flavor avoidance learning. Pharmacology, Biochemistry and Behavior. 2015;137:7–15. doi: 10.1016/j.pbb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- De Wit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. American Journal of Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Desikan A, Wills DN, Ehlers CL. Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacology, Biochemistry and Behavior. 2014;122:279–285. doi: 10.1016/j.pbb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clinical and Experimental Research. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clinical and Experimental Research. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicology and Teratology. 2007b;29:153–163. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behavioral Neuroscience. 2007a;121:111–119. doi: 10.1037/0735-7044.121.1.111. http://dx.doi.org/10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder A, Gilder DA, Stouffer GM, et al. Age at regular drinking, clinical course, and heritability of alcohol dependence in the San Francisco family study: a gender analysis. American Journal on Addiction. 2010;19:101–110. doi: 10.1111/j.1521-0391.2009.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT. Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcoholism: Clinical and Experimental Research. 2013a;37:1466–1475. doi: 10.1111/acer.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013b;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN. Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcoholism: Clinical and Experimental Research. 2014a;38:749–759. doi: 10.1111/acer.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Desikan A, Phillips E, Havstad J. Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Developmental Neuroscience. 2014b;36:175–195. doi: 10.1159/000358484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Stouffer GM, Gilder DA. Associations between a history of binge drinking during adolescence and self-reported responses to alcohol in young adult Native and Mexican Americans. Alcoholism: Clinical and Experimental Research. 2014c;38:2039–2047. doi: 10.1111/acer.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, Nizhnikov ME, Spear NE, Pautassi RM. Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Developmental Psychobiology. 2014;56:574–583. doi: 10.1002/dev.21101. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacology, Biochemistry and Behavior. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS ONE. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health and Research World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: adolescent risk factors and adult outcomes. Alcoholism: Clinical and Experimental Research. 2010;34:819–833. doi: 10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. Journal of Studies on Alcohol and Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. http://dx.doi.org/10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Ho A, Chin AJ, Dole VP. Early experience and the consumption of alcohol by adult C57BL/6J mice. Alcohol. 1989;6:511–515. doi: 10.1016/0741-8329(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Balguerie K, Coune F, Legastelois R, Jeanblanc V, Naassila M. Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addiction Biology. 2015;20:490–499. doi: 10.1111/adb.12146. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: a longitudinal study of youth. New York: Academic Press; 1977. [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcoholism: Clinical and Experimental Research. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Developments in Alcoholism. 2005;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcoholism: Clinical and Experimental Research. 2008a;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Research. 2008b;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Milk consumption during adolescence decreases alcohol drinking in adulthood. Pharmacology, Biochemistry and Behavior. 2009;94:179–185. doi: 10.1016/j.pbb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-d-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism: Clinical and Experimental Research. 1998;22:1783–1787. doi: 10.1111/j.1530-0277.1998.tb03980.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcoholism: Clinical and Experimental Research. 2000;24:766–773. doi: 10.1111/j.1530-0277.2000.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Sarviharju M, Jaatinen P, Hyytia P, Hervonen A, Kiianmaa K. Effects of lifelong ethanol consumption on drinking behavior and motor impairment of alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol. 2001;23:157–166. doi: 10.1016/S0741-8329(01)00132-X. [DOI] [PubMed] [Google Scholar]
- Serlin H, Torregrossa MM. Adolescent rats are resistant to forming ethanol seeking habits. Developmental Cognitive Neuroscience. 2015;16:183–190. doi: 10.1016/j.dcn.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiology and Behavior. 2001;74:637–643. doi: 10.1016/S0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 2005;29:1139–1145. doi: 10.1097/01.ALC.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Research: Developmental Brain Research. 2001;128:63–72. doi: 10.1016/S0165-3806(01)00150-X. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/S0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000a;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current Directions in Psychological Science. 2000b;9:111–114. doi: 10.1111/1467-8721.00072. [DOI] [Google Scholar]
- Spear LP. Adolescent period: Biological basis of vulnerability to develop alcoholism and other ethanol-mediated behaviors. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000c. pp. 315–333. NIAAA Research Monograph 34, NIH Pub. No. 00-4520 ed. [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Annals of the New York Academy of Sciences. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism. 2005;17:143–159. doi: 10.1007/0-306-48626-1_7. [DOI] [PubMed] [Google Scholar]
- Spear LP. Annenberg Public Policy Center, & Annenberg Foundation Trust at Sunnylands, editor. The developing brain and adolescent-typical behavior patterns: an evolutionary approach. In: Romer D, Walker RF, editors. Adolescent psychopathology and the developing brain: integrating brain and prevention science. Oxford: Oxford University Press; 2007. pp. 9–30. [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in wistar rats with a history of ethanol exposure. Alcoholism: Clinical and Experimental Research. 2005;29:584–590. doi: 10.1097/01.ALC.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Toalston JE, Deehan GA, Jr, Hauser SR, Engleman EA, Bell RL, Murphy JM, et al. The reinforcing properties of ethanol are quantitatively enhanced in adulthood by peri-adolescent ethanol, but not saccharin, consumption in female alcohol-preferring (P) rats. Alcohol. 2015;49:513–518. doi: 10.1016/j.alcohol.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National findings. Rockville, MD: U.S. Department of Health and Human Services, Office of Applied Studies; 2008. (DHHS Publication No. SMA 08-4343, NSDUH Series H-34) [Google Scholar]
- Vendruscolo LF, Gueye AB, Vendruscolo JC, Clemens KJ, Mormede P, Darnaudery M, et al. Reduced alcohol drinking in adult rats exposed to sucrose during adolescence. Neuropharmacology. 2010;59:388–394. doi: 10.1016/j.neuropharm.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clinical and Experimental Research. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behavioral Neuroscience. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JL. Clinical significance of alcohol intake parameters at initiation of drinking. Alcohol. 1999;19:97–99. doi: 10.1016/S0741-8329(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hori M, Sorimachi Y, Watanabe T, Yano T, Yasuhara M. Increase of rat alcohol drinking behavior depends on the age of drinking onset. Alcoholism: Clinical and Experimental Research. 2002;26:63S–65S. doi: 10.1111/j.1530-0277.2002.tb02704.x. [DOI] [PubMed] [Google Scholar]