Abstract
Background
The complement system plays a crucial role in acute xenogeneic reactions after cardiac transplantation. We used an ex vivo perfusion model to investigate the effect of Cp40, a compstatin analog and potent inhibitor of complement at the level of C3.
Methods
15 wild-type pig hearts were explanted, cardiopleged, and reperfused ex vivo after 150 min of cold ischemia. Hearts were challenged in a biventricular working heart mode to evaluate cardiac perfusion and function. In the treatment group (n=5), the complement cascade was blocked at the level of C3 using Cp40, using diluted human blood. Untreated human and porcine blood was used for controls.
Results
Throughout the perfusion, C3 activation was inhibited when Cp40 was used (mean of all time points: 1.11 ±0.34 % vs. 3.12 ±0.48 % control activation; p<0.01). Compared to xeno-perfused controls, the cardiac index improved significantly in the treated group (6.5 ±4.2 vs. 3.5 ±4.8 ml/min/g; p=0.03, 180 min perfusion), while the concentration of lactate dehydrogenase as a maker for cell degradation was reduced in the perfusate (583 ±187 U/ml vs. 2108 ±1145 U/ml, p=0.02). Histological examination revealed less hemorrhage and edema, and immunohistochemistry confirmed less complement fragment deposition than in untreated xeno-perfused controls.
Conclusions
Cp40 efficiently prevents C3 activation of the complement system, resulting in reduced cell damage and preserved function in wild-type porcine hearts xenoperfused ex vivo. We suggest that this compstatin analog, which blocks all main pathways of complement activation, could be a beneficial perioperative treatment in preclinical and in future clinical xenotransplantation.
Introduction
The demand for human organs and tissues far exceeds the supply, and this shortage has exacerbated over recent years. Discordant porcine xenotransplants could provide a much-needed alternative.
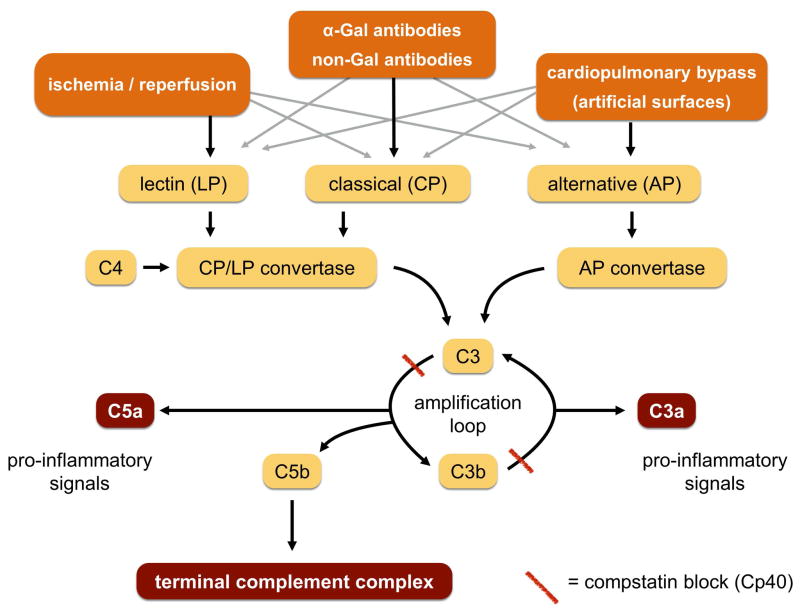
However, genetically unmodified porcine hearts transplanted into primates, including humans, encounter rapid and powerful xenogeneic reactions mediated by preformed antibodies, most notably those against αGal epitopes. In GGTA1-knockout organs that lack αGal this hyperacute response is almost completely prevented, but non-Gal-antibodies binding to other porcine antigens induce a delayed but strong humoral rejection. Antigen-antibody binding leads to rapid activation of the complement system. The classical (CP) and lectin pathways (LP) converge at the level of the CP/LP convertase C4b2b, whereas the alternative pathway (AP) culminates in the AP convertase C3bBb. Both convertases cleave C3, the central component of the complement system, to C3b and C3a. Amplification loops then follow, producing the anaphylatoxins C3a and C5a and resulting in the assembly of the cell-perforating terminal complement complex (TCC) (Fig. 1). The activated complement system damages transplanted hearts by direct cell lysis and via signals that cause leucocyte infiltration; microthrombi are another undesirable consequence [1, 2].
Fig. 1.
Extended survival of transplants has been achieved using transgenic porcine hearts that express membrane-bound complement regulator proteins such as human CD46, CD55, CD59, or GGTA1-knockout organs that lack αGal [3, 4]. Systemic fluid-phase complement inhibition is needed to further reduce complement activation. Compstatin is a peptide that selectively binds C3 and C3b and reversibly inhibits all downstream cell-surface complement components in humans and non-human primates (Fig. 1), but does not affect non-primates [5, 6, 7]. Structure-function refinement of compstatin has led to a series of analogs with improved inhibitory potency and pharmacokinetic profiles that show promise in many clinical applications and several primate models of disease [8]. The therapeutic C3 inhibitor Cp40 has been reported to inhibit complement-induced inflammation in cynomolgus monkeys triggered by hemodialysis filters [9]. Cp40 abrogates complement activation, conferring significant protection from inflammatory and procoagulation in septic animals [10]. Two further studies have described promising results with compstatin in the field of xenotransplantation, providing new insight into its therapeutic potential. Fiane et al. reported that compstatin prolongs survival of porcine kidneys perfused ex vivo with human blood [11]. Recently, Kourtzelis et al. found that Cp40 abrogates endothelial activation and leukocyte attachment in porcine endothelium exposed to human blood in vitro [12].
Cp40 is specific for human and non-human primate C3, so only ex vivo perfusion models using primate (human) blood may serve as an alternative to non-human primate xenotransplantation models. We tested the effect of complement inhibition by Cp40 on a working porcine heart in a customized xeno-perfusion system. These experiments enabled direct investigation of possible off-target toxic effects on the heart without confounding effects from off-target toxicity in other organs.
Material and methods
Animals and anesthesia
Fifteen juvenile pigs (German Landrace; weight 15.0 ±2.9 kg, blood group 0) were used as donors. Anesthesia was achieved with fentanyl (10–15 μg/kg) and propofol (0.15 μg/kg/min).
The study was conducted according to the European Law on Protection of Animals for Scientific Purposes and was approved by the Government of Upper Bavaria. Humans and animals were handled according to the guidelines of the Ludwig-Maximilian University, Munich.
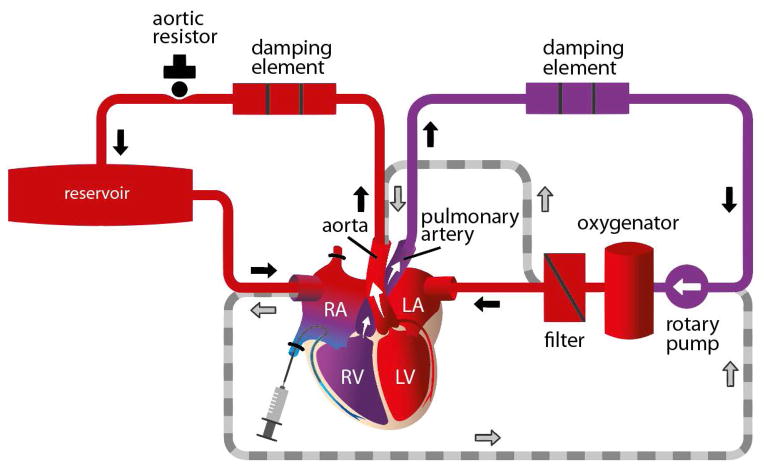
Surgical procedure and the biventricular heart perfusion system
A schematic representation of the ex vivo reperfusion system is shown in Figure 2. In brief, median sterno-/pericardiotomy was performed, followed by heparin administration, cross-clamping of the ascending aorta, and coronary infusion of histidine-tryptophan-ketoglutarate cardioplegia (HTK, 30 ml/kg body weight; Bretschneider solution, Custodial, Dr. F. Köhler, Bernsheim, Germany) at 4° C [13, 14]. The hearts were topically cooled with ice-cold saline solution.
Fig. 2.
The ascending aorta and pulmonary artery trunk were cut and cannulas inserted. After mass ligation of both lung hila, the corresponding structures were transected distally. Both atria were cannulated, and the superior and inferior venae cavae were ligated and cut distally. The hearts were then removed and stored in lactated Ringer’s solution (Fresenius, Kabi, Bad Homburg, Germany) at 4°C for 150 min to simulate an ischemic period similar to that occurring during orthotopic (xeno-) heart transplantation. The cannulated pig hearts were then attached to the perfusion system, which was filled with 500cc of freshly drawn human blood, 450cc of hydroxyl ethyl starch 6% (Volulyte, Freseniuns, Bad Homburg, Germany), 5cc of sodium bicarbonate, 8.4%, 0.25cc of 10% calcium gluconate, 1cc of 20% glucose, 1 IU insulin, and 5000 IU heparin (activated clotting times achieved were longer than 400 sec).
Five healthy male volunteers (blood groups 0, 0, A, A, B; no blood group mismatches, since all of the porcine organs were 0) donated blood twice, once for the control and a second time for a compstatin experiment.
In another control group of autologous ex vivo perfusion the system was filled with porcine blood of the donor animal. Blood was drawn before and during clamping of the aorta. Heparin, bicarbonate, calcium and hydroxyethyl starch were added.
The perfusate was first circulated in the absence of the donor organ (Fig 2). The hollow-fiber oxygenator (HILITE 1000, Medos, Stolberg, Germany) maintained partial pressures of 100–150 mmHg O2 and 35–40 mmHg CO2. A heater attached to the oxygenator maintained heart temperatures at 36–37°C; 400 mg/hr glucose and 0.2 IU/hr insulin were added, and resulting glucose levels were 120–140 mg/dl. Bicarbonate was used to keep the base excess between +2 and −2 mEq/l.
Pre- and afterloads were adjusted to maintain the following mean pressures (in mm Hg): ascending aorta, 65, pulmonary artery trunk, 15, both atria, 5–7. Ex vivo heart perfusion was then started with 15 min of reperfusion in a non-working Langendorff preparation (Fig. 2). Thereafter, the biventricular working mode commenced, lasting for another 165 min.
Five 3 F catheters (Arrow, Reading, USA) were inserted into the ascending aorta, pulmonary artery trunk, both atria, and the left ventricle (the catheter in the right atrium enabled measurement of key parameters in venous coronary blood, including lactate, blood gases, and pH). In-line sensors measured aortic and pulmonary artery flow (Ultra Sonic Flow Meter UF&B, Cynergy Components Limited, Dorset, England). The coronary blood flow index (CBFI) was calculated as: (pulmonary blood flow − aortic blood flow) / heart weight. Epicardial temperature (measured with an infrared thermometer, VC 110, Conrad Electronics SE, Hirschau, Germany), activated clotting time, PO2, pCO2, pH, base excess, serum lactate, serum glucose, and hematocrit were assessed and kept constant (Rapidlab, 1265, Siemens, Erlangen, Germany); the myocardial oxygen consumption index was calculated as (VO2) = CBFI x (CaO2 − CvO2). In five experiments, Cp40 (32 mg; 18 μM) was added to the perfusate when filling the perfusion system.
Hemodynamic data collection and blood sampling
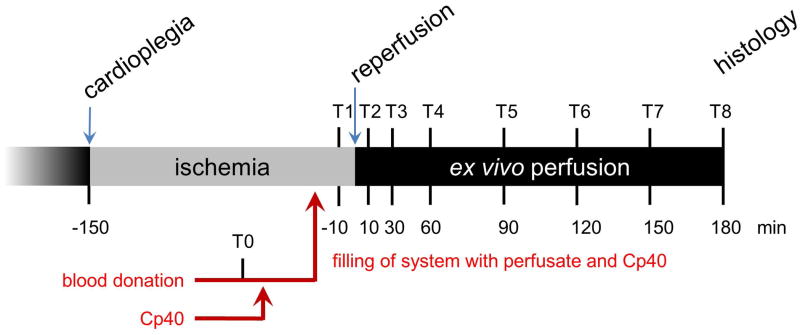
A time line of the experiments is displayed in Figure 3. Samples from the collected human blood were taken at time point T0. Prior to contact with the heart, but after equilibration (temperature, pO2, CO2, pH, Ca++), samples were taken from the circulating system (T1). Blood samples were taken from the coronary sinus after 10, 30, 60, 90, 120, 150, and 180 min perfusion (T2-T8). EDTA was added for later complement analysis. All samples were centrifuged at 2000 rpm for 15 min at 4°C to separate plasma from cells; samples were then divided into aliquots and stored at 80°C for further analysis. Lactate dehydrogenase activity (LDH, commercial assay, clinical laboratory) was measured at T1 and T8. Heart rate (HR), stroke volume index (SVI), cardiac index (CI) and oxygen consumption index (VO2) were measured at the beginning of ex vivo perfusion (T2) at the beginning of working heart mode (T3) and at the end of perfusion (T8).
Fig. 3.
Quantitation of Cp40 in plasma
The amount of Cp40 in plasma was determined using solid-phase extraction (SPE) and liquid chromatography–mass spectrometry as previously described [15, 7].
Measurement of activated C3 and the TCC in plasma
Concentrations of activated fragments of human C3 were measured in plasma by a sandwich ELISA as previously described [9]. In brief, microtiter plates were coated with 50 μl of a 2 μg/ml solution of capture antibody C3-28. This antibody specifically recognizes neo-epitopes exposed on molecules of activated C3 (C3b, iC3b, C3c). Wells were then blocked with 1% BSA in PBS for 1 h, followed by addition of diluted plasma samples and serial dilutions of plasma activated with cobra venom factor (equal to 100% of activated C3) for 1 h. Bound C3 fragments were detected using a peroxidase-conjugated polyclonal rabbit anti-human C3 IgG (MP Biomedicals). Plates were developed by adding hydrogen peroxide plus 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) according to the manufacturer’s instructions (Roche) and read at 405 nm. Optical density (O.D.) values were converted to percent total C3 activation using the following formula: x (%) = [O.D. curve/(O.D. sample × 100)] : (dilution curve/dilution sample) [16].
At T0, T1, T2, T4, T6, and T8, the TCC of the perfusate was measured using a commercially available ELISA (TCC, Human ELISA kit, HK328-02, Hycult Biotech, Netherlands).
Thromboelastography
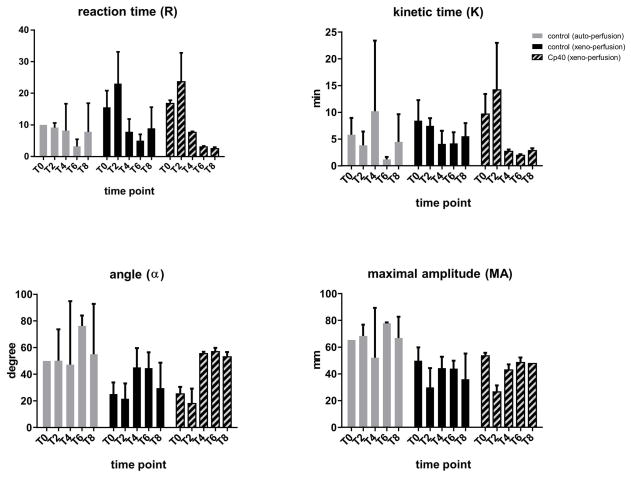
The process of thrombus formation was quantified using thrombelastography at time points T0, T2, T4 and T8. After sampling of 1ml perfusate from the coronary sinus, 1μl protamine was added immediately before thromboelastography (TEG5000, Haemonetics Corporation, MA, USA) was started. The reaction time (R) required to initiate clot formation, kinetic time (K) measuring the speed of clot development, α-angle characterizing the kinetics of clot growth and the maximum amplitude (MA) describing clot strength were all calculated.
Histology and immunohistochemistry
After 180 min perfusion, porcine hearts were weighed and compared to baseline values. Left ventricular biopsies were taken, fixed in formalin and embedded in paraffin. Four-micrometer sections were prepared and either processed immediately, or stored at room temperature until further analysis. Samples were hematoxylin-eosin (H&E) stained and evaluated in a blinded manner. The following histological changes were classified: perivascular edema, erythrocyte extravasation, and thrombus formation. Findings were scored as: 0 = absent, 1 = scarce, 2 = intermediate and 3 = major pathological changes. Additional immunohistochemical analyses were performed to detect deposition of IgM, C4d, fibrinogen, and C5b-9 (TCC). The following primary antibodies were used: polyclonal rabbit anti-human C4d (Emelca; 1:50), IgM (Dako; 1:50), fibrinogen (Biozol; 1:800), and monoclonal mouse anti-human C5b-9 (Abcam TCC; 1:200); secondary antibodies were either swine anti-rabbit biotin or peroxidase (for positive controls).
Staining intensities were scored semi-quantitatively in a blinded manner using a slide scanner microscope (Hamamatsu, Nanozoomer 2.0-HT, Japan) from 0 to 3 (0=no binding, 3= strong binding of positive control).
Statistics
Data were summarized as arithmetic means and standard deviations. Two-tailed values of p<0.05 were considered statistically significant. For each parameter, a paired Student’s t-test (Prism 6.0, GraphPad Software, La Jolla, CA, USA) was used to compare the two groups of matching blood donors at a single time point. Xeno- and autologous perfused groups were compared by paired Student’s t-test. Pearson product-moment correlation coefficients were calculated and linear regression models used to describe relationships between parameters.
Results
Hemodynamic parameters
The independent hemodynamic parameters, aortic pressure (62.7 ±5.2 mmHg), pulmonary artery pressure (10.8 ±1.2 mmHg), left atrial pressure (9.2 ± 0.9 mmHg) and right atrial pressure (5.4 ± 0.2) were kept stable throughout the Cp40 treated experiments and did not differ from xeno- or autologous perfused controls (Table 1).
Table 1.
Left and right ventricular pre- and afterload were kept stable throughout the experiments. Values are given as mean of T2-T8 (ex vivo perfusion) for the aortic pressure and mean of T3-T8 (working heart perfusion) pulmonary artery and atrial pressure.
| group | autologous perfusion (conrol) | auto. vs. cp40 | xeno-perfusion (control) | xeno-p. vs. cp40 | cp40 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| parameter | abbreviation | units | mean | SD | p | mean | SD | p | mean | SD |
| mean aortic pressure mmHg | MAP | mmHg | 68 | ±5 | 0,50 | 59 | ±7 | 0.32 | 63 | ±5 |
| mean pulmonary artery pressure mean | MPAP | mmHg | 15 | ±4 | 0.12 | 10 | ±1 | 0.19 | 11 | ±1.2 |
| mean left atrial pressure | LAP | mmHg | 7.5 | ±1.5 | 0.615 | 11 | ±2 | 0.10 | 9.2 | ±0.9 |
| mean right atrial pressure | RAP | mmHg | 5.2 | ±0.2 | 0.15 | 6 | ±1 | 0.22 | 5.4 | ±0.2 |
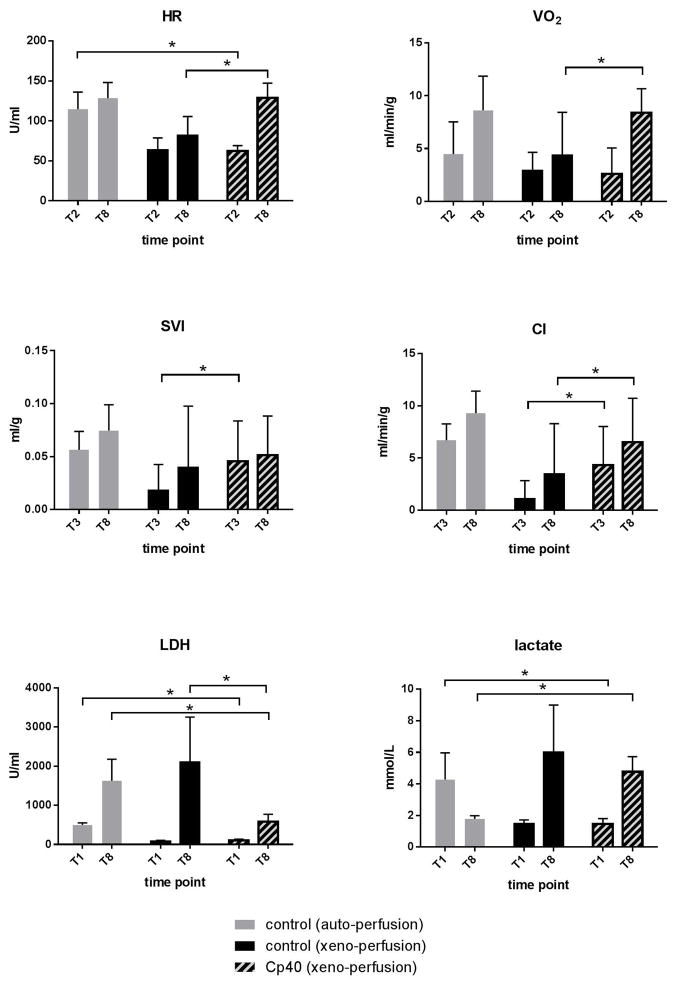
Figure 4 shows the variable hemodynamic parameters. At the beginning of reperfusion (T2) the heart rate in the treated group was lower than in autologous controls, but not different from xeno-perfused untreated hearts. At the end of perfusion (T8), the heart rate exceeded that of untreated controls.
Fig. 4.
Oxygen consumption was higher in the Cp40 treated group at the end of working heart perfusion (T8), but not during Langendorff perfusion (non-working) at time point T2.
Compared to xeno-perfused controls, treated hearts showed a higher stroke volume at the beginning (T3), and a better cardiac index at the beginning and end of working heart perfusion (T3 and T8).
LDH and lactate
During Cp40 treated perfusion, the LDH concentration in the perfusate remained lower than in xeno- and autologous perfused controls (Fig. 4). While lactate level decreased during autologous perfusion, an increase was seen in xeno-perfused experiments. The reduction by Cp40 treatment was not significant (Fig. 4).
Effect of Cp40 on complement activation
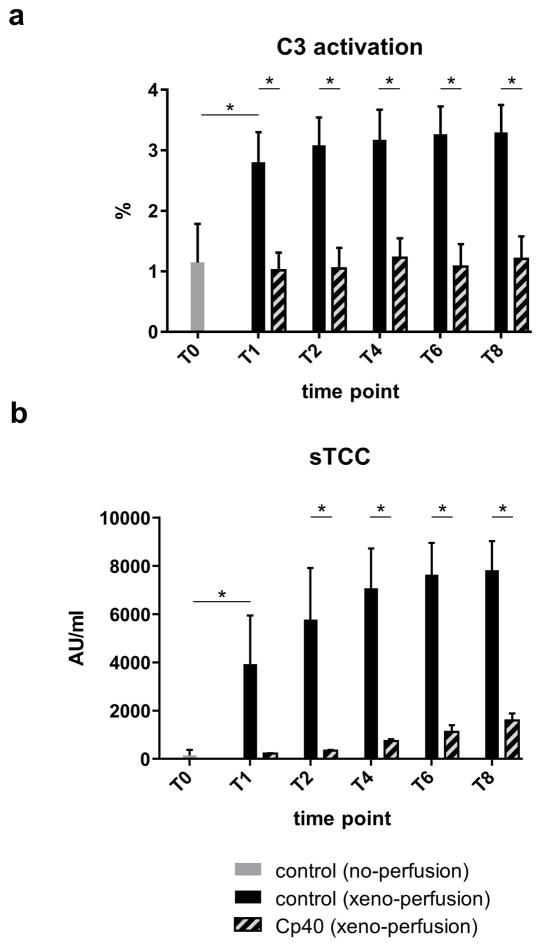
At the beginning of the treatment experiments, Cp40 levels averaged 19.7 ±3.6 μM and remained within that range throughout the observation time. Cp40 significantly inhibited C3 activation throughout the experiment compared to controls at the same time points (Fig. 5a).
Fig. 5.
Compared to samples taken immediately after blood donation, C3 activation only increased in the untreated experiments.
After connection to the perfusion system, sTCC increased in the xeno-perfused control group, but not in the treated group. During ex vivo perfusion, sTCC remained significantly lower in the treated group at all time points (Fig 5b).
Correlation and linear regression
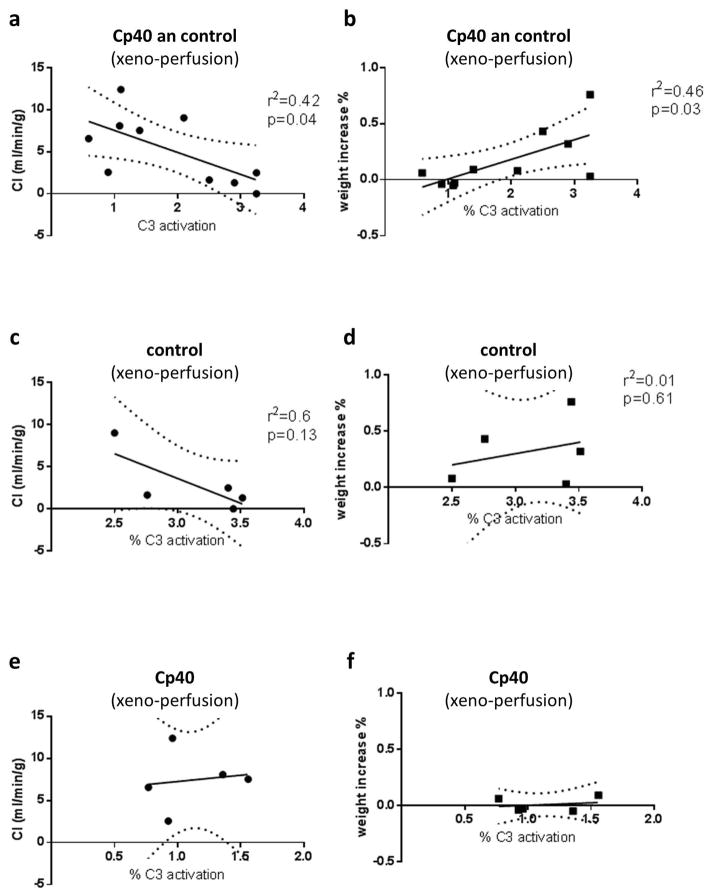
Figure 6 shows correlation and linear regression analysis between C3 activation at time point T1 and cardiac index (CI) as well as increase of heart weight during all ex vivo perfusion experiments. Correlation was significant but weak (CI: r2=0.42, p=0.04; weight increase: r2=0.46, p=0.03). When control or treatment experiments were analyzed separately, data did not reach statistical significance.
Fig. 6.
Thromboelastography
There were no differences between the treated and untreated xeno-perfused groups (Fig. 7). The thrombelastographic parameters of the autologous perfused hearts were more stable over time compared with those of the xeno-perfusion experiments.
Fig. 7.
Histology and immunohistochemistry
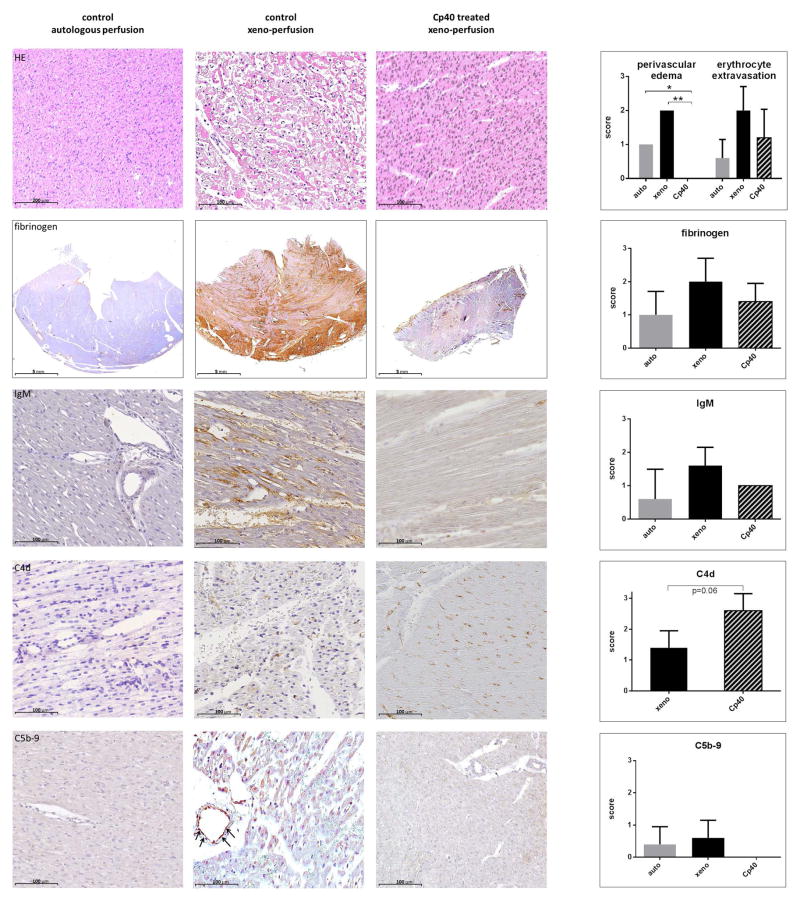
Compared to baseline, weights of control hearts had increased at the end of the perfusions by 32 ±26% (p=0.03), whereas weights of Cp40 treated (1 ±5%, p=0.95) and autologous perfused hearts (2 ±3%, p=0.81) showed no change. Consistent with this, histological analysis (Fig. 8) revealed no perivascular edema formation in the treated group. Erythrocyte extravasations were also less pronounced in the treated group. No thrombus formation was seen in either group. Immunohistochemical staining revealed a trend towards less fibrinogen and less endothelial IgM deposits in the Cp40 group. Staining of complement degradation products showed increased C4d deposits and no C5b-9 in the Cp40-treated group.
Fig. 8.
Discussion
The damaging role of complement after xenogeneic heart transplantation
A properly functioning complement system safeguards the integrity of the body’s structure by promptly detecting foreign entities and mediating their immediate lysis and removal [17, 2].
When a genetically unmodified foreign organ, such as a heart from a discordant species such as the pig, is perfused with human or other primate blood, it immediately experiences complement-driven hyperacute rejection [18]. Endothelial barrier function is lost, leading to thrombosis, erythrocyte extravasation, edema formation, myocardial damage, and finally irreversible organ dysfunction [reviewed in 3, 4].
All of these changes, except thrombosis, were seen in the control hearts perfused with our special biventricular working heart system using diluted but freshly drawn human blood. Previous experiments have indicated that the extent of these changes is highly dependent on the particular donor of the blood used for perfusion. We therefore stress that in our experiments all human volunteers donated blood twice, once for the control and once for the Cp40 experiment, and results were compared pairwise. This reduced variation due to immunological differences between blood donors, such as different levels of pre-existing anti-pig antibodies. Blood from donors with known weak xeno-reactivity was not used.
Besides the xeno-reaction itself, other features of life-supporting cardiac xenotransplantation can induce complement activation, particularly ischemia/reperfusion and contact of blood with plastics and gaseous surfaces within the heart lung machine [19, 20] (Fig. 1). In our ex vivo model these are represented by T1 (complement activation by the perfusion system with the heart excluded), and T2 to T8 the reperfusion of ischemic xenotransplants (control values increase mainly as a result of the non-abrogated xenoantigens). Interestingly, the amount of C3 activation triggered by the perfusion system was higher than subsequent activation by the xenograft (Fig. 5a). Accordingly, the formation of soluble terminal complement complex increased when the perfusate was circulated and further increased at the time of reperfusion (Fig. 5b). In this model, the effect of xeno-perfusion on the complement system cannot be investigated in isolation, however in clinical and preclinical xeno-transplantation both triggers also inevitably arise in combination.
Blockade of complement activation in a pre-clinical xenogeneic heart transplantation setting
Inhibition of damaging complement reactions is a major prerequisite for successful clinical cardiac xenotransplantation. Fundamental strategies are genetic ablation of major porcine xenoreactive antigens, principally α-Gal and Hanganutziu-Deicher antigen / Neu5GC, and prevention of coagulation disorders such as thrombotic microangiopathy [21]. These disorders can be prevented by overexpressing human thrombomodulin to supplement activation of protein C [22, 23]. Recently, Mohiuddin and co-workers had considerable success using triple genetically modified donors (GGTA1-KO/h-thrombomodulin/CD46 complement regulator) for five pig-to-baboon heart transplants. In that study, recombinant cobra venom factor was administered post-operatively for 3 days (day −1, 0, 1) for complement inhibition [24, 25]. Co-stimulation pathway blockade with anti-CD40 was used as the main immunosuppressive agent to control B- and T-cell activation. Encouragingly, two of the five porcine hearts continued beating for 616 and 945 days, only failing after anti-CD40 therapy was reduced and finally withdrawn completely. Long-term biopsies on postoperative days 378 and 640 revealed apparently normal myocardium. The other three hearts continued beating for 147, 159, and 298 days; a total mean survival time of 298 days [26, 27 and personal communication]. The importance of initial complement depletion in the therapeutic regimen used in this experiment is not clear. Its success however indicates that systemic perioperative complement inhibition may also be beneficial when using genetically modified hearts, e.g. overexpressing human complement regulator and human thrombomodulin.
However, these hearts were all implanted heterotopically in the abdomen and retrograde-perfused via the abdominal aorta, and so were beating but not ejecting. The Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation has set guidelines for experimental studies prerequisite for commencing clinical trials [28]. Recommendations are: graft survival for at least 3 post-operative months in 6 of 10 consecutive cases (a target that Mohiuddin’s group has more or less fulfilled), and a life-supporting orthotopic position of the transplanted hearts (29–33), or at least a heterotopic intrathoracic position [34]. For both procedures, the use of a heart-lung machine, also known to be a complement stimulus, is necessary. Effective blockade of the complement system is therefore vital, and we anticipate there will be roles for both transgenic expression of complement inhibitors such as human CD46, and administration of soluble complement blockers such as compstatin.
The peptide Cp40 has an advantage over other complement inhibitors such as the monoclonal anti-C5 antibody Eculizumab [35, 36], because it acts at an earlier stage of the complement cascade and should thus avoid stimulation of additional damaging pathways as a result of additional proinflammatory signals (Fig. 1) [6–9, 12]. Since Cp40 has no relevant adverse effects, as demonstrated by other preclinical and even clinical studies, it could theoretically be used over a longer period after xenotransplantation to overcome humoral rejection [37].
Cp40 effectively inhibited complement activation, and therefore the triggering of rapid and strong xenogeneic reactions, in all our perfused pig hearts (Fig. 5a). Consequently, sTCC were almost undetectable in the perfusate (Fig. 5b) and myocardial immunohistochemistry slides (Fig. 8). Complement inhibition at the pivotal C3 level led to agglomeration and increased endothelial deposition of the C4d located just upstream, but no downstream deposition of C5b-9 (see Fig. 1 and Fig. 8). In consequence, C4d deposition should not been used to evaluate complement activation in future preclinical or clinical xenotransplantation if Cp40 is used.
The decreased IgM deposition we observed in the treated group might be related to reduced endothelial activation and injury; IgM might bind unspecifically or to newly exposed antigens. At necropsy, hearts treated with Cp40 weighed significantly less than control hearts, since they had less edema and revealed less erythrocyte extravasation, as confirmed by histological examination (Fig. 8). Functional cardiac parameters such as cardiac index, heart rate, and stroke volume index were increased, however they did not reach the level of autologous perfused hearts, indicating that Cp40 does not block the non-complement xenoreactive responses. In the treated hearts oxygen consumption increased along with improved cardiac work and was compensated by more effective myocardial perfusion (Fig. 4). Lactate metabolism could not be reversed as in autologous perfused hearts but improved compared to untreated experiments.
Conclusion
Cp40 effectively attenuates complement mediated injury triggered by endothelial antibody binding and extracorporal circulation. Cp40 is a promising adjunct for preclinical and future clinical cardiac xenotransplantation.
Acknowledgments
This work was supported by the German Research Foundation within the Collaborative Research Center 127, the European Community’s Seventh Framework Programmes FP7/DIREKT (grant no. 602699; to J.D.L.), and grants by the National Institutes of Health (AI068730 to J.D.L.).
Footnotes
Conflict of interest
J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications.
References
- 1.COWAN PJ, D’APICE AJ. Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 2009;87:203–208. doi: 10.1038/icb.2008.107. [DOI] [PubMed] [Google Scholar]
- 2.RICKLIN D, HAJISHENGALLIS G, YANG K, LAMBRIS JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PIERSON RN, 3RD, DORLING A, AYARES D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EKSER B, EZZELARAB M, HARA H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 5.SAHU A, KAY BK, LAMBRIS JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 6.RICKLIN D, LAMBRIS JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–292. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RISITANO AM, RICKLIN D, HUANG Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MASTELLOS DC, YANCOPOULOU D, KOKKINOS P, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.REIS ES, DEANGELIS RA, CHEN H, et al. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2015;220:476–482. doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SILASI-MANSAT R, ZHU H, POPESCU NI, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FIANE AE, MOLLNES TE, VIDEM V, et al. Compstatin, a peptide inhibitor of C3, prolongs survival of ex vivo perfused pig xenografts. Xenotransplantation. 1999;6:52–65. doi: 10.1034/j.1399-3089.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 12.KOURTZELIS I, FERREIRA A, MITROULIS I, et al. Complement inhibition in a xenogeneic model of interactions between human whole blood and porcine endothelium. Horm Metab Res. 2015;47:36–42. doi: 10.1055/s-0034-1390452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BRETSCHNEIDER HJ, HUBNER G, KNOLL D, et al. Myocardial resistance and tolerance to ischemia: physiological and biochemical basis. J Cardiovasc Surg (Torino) 1975;16:241–260. [PubMed] [Google Scholar]
- 14.HOLSCHER M, GROENEWOUD AF. Current status of the HTK solution of Bretschneider in organ preservation. Transplant Proc. 1991;23:2334–2337. [PubMed] [Google Scholar]
- 15.QU H, RICKLIN D, BAI H, et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SOERMAN A, ZHANG L, DING Z, et al. How antibodies use complement to regulate antibody responses. Mol Immunol. 2014;61:79–88. doi: 10.1016/j.molimm.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 17.ATKINSON JP, FARRIES T. Separation of self from non-self in the complement system. Immunol Today. 1987;8:212–215. doi: 10.1016/0167-5699(87)90167-8. [DOI] [PubMed] [Google Scholar]
- 18.CALNE RY. Organ transplantation between widely disparate species. Transplant Proc. 1970;2:550–556. [PubMed] [Google Scholar]
- 19.NILSSON B, EKDAHL KN, MOLLNES TE, LAMBRIS JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.EKDAHL KN, TERAMURA Y, ASIF S, JONSSON N, MAGNUSSON PU, NILSSON B. Thromboinflammation in therapeutic medicine. In: LAMBRIS JD, EKDAHL KN, RICKLIN D, NILSSON B, editors. Immunresponses to biosurfaces. Springer International Switzerland Publishing; 2015. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- 21.BYRNE GW, MCGREGOR CG, BREIMER ME. Recent investigations into pig antigen and anti-pig antibody expression. Int J Surg. 2015 doi: 10.1016/j.ijsu.2015.07.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PETERSEN B, RAMACKERS W, TIEDE A, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009;16:486–495. doi: 10.1111/j.1399-3089.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 23.WUENSCH A, BAEHR A, BONGONI AK, et al. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplantation. 2014;97:138–147. doi: 10.1097/TP.0b013e3182a95cbc. [DOI] [PubMed] [Google Scholar]
- 24.MIYAGAWA S, HIROSE H, SHIRAKURA R, et al. The mechanism of discordant xenograft rejection. Transplantation. 1988;46:825–830. doi: 10.1097/00007890-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 25.VOGEL CW, FRITZINGER DC, HEW BE, THORNE M, BAMMERT H. Recombinant cobra venom factor. Mol Immunol. 2004;41:191–199. doi: 10.1016/j.molimm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 26.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. discussion 1113–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COOPER DK, KEOGH AM, BRINK J, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19:1125–1165. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 29.SCHMOECKEL M, BHATTI FN, ZAIDI A, et al. Orthotopic heart transplantation in a transgenic pig-to-primate model. Transplantation. 1998;65:1570–1577. doi: 10.1097/00007890-199806270-00006. [DOI] [PubMed] [Google Scholar]
- 30.VIAL CM, OSTLIE DJ, BHATTI FN, et al. Life supporting function for over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant. 2000;19:224–229. doi: 10.1016/s1053-2498(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 31.BRANDL U, MICHEL S, ERHARDT M, et al. Administration of GAS914 in an orthotopic pig-to-baboon heart transplantation model. Xenotransplantation. 2005;12:134–141. doi: 10.1111/j.1399-3089.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 32.BRENNER P, SCHMOECKEL M, WIMMER C, et al. Mean xenograft survival of 14.6 days in a small group of hDAF-transgenic pig hearts transplanted orthotopically into baboons. Transplant Proc. 2005;37:472–476. doi: 10.1016/j.transproceed.2004.12.241. [DOI] [PubMed] [Google Scholar]
- 33.BYRNE GW, MCGREGOR CG. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17:148–154. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ABICHT J, MAYR T, REICHART B, et al. Preclinical heterotopic intrathoracic heart xenotransplantation: a possibly useful clinical technique. Xenotransplantation. 20015 doi: 10.1111/xen.12213. [DOI] [PubMed] [Google Scholar]
- 35.THOMAS TC, ROLLINS SA, ROTHER RP, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389–1401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 36.KELLY RJ, HOCHSMANN B, SZER J, et al. Eculizumab in Pregnant Patients with Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med. 2015;373:1032–1039. doi: 10.1056/NEJMoa1502950. [DOI] [PubMed] [Google Scholar]
- 37.2016 Jan; http://www.sec.gov/Archives/edgar/data/1492422/000119312515342824/d23325ds1.htm.