Abstract
In response to stress, plants accumulate Pro, requiring degradation after release from adverse conditions. Δ1-Pyrroline-5-carboxylate dehydrogenase (P5CDH), the second enzyme for Pro degradation, is encoded by a single gene expressed ubiquitously. To study the physiological function of P5CDH, T-DNA insertion mutants in AtP5CDH were isolated and characterized. Although Pro degradation was undetectable in p5cdh mutants, neither increased Pro levels nor an altered growth phenotype were observed under normal conditions. Thus AtP5CDH is essential for Pro degradation but not required for vegetative plant growth. External Pro application caused programmed cell death, with callose deposition, reactive oxygen species production, and DNA laddering, involving a salicylic acid signal transduction pathway. p5cdh mutants were hypersensitive toward Pro and other molecules producing P5C, such as Arg and Orn. Pro levels were the same in the wild type and mutants, but P5C was detectable only in p5cdh mutants, indicating that P5C accumulation may be the cause for Pro hypersensitivity. Accordingly, overexpression of AtP5CDH resulted in decreased sensitivity to externally supplied Pro. Thus, Pro and P5C/Glu semialdehyde may serve as a link between stress responses and cell death.
INTRODUCTION
Pro is required for protein biosynthesis and in many organisms also fulfills other important functions as protective agent for cells under osmotic stress, as a scavenger of radicals, for transient storage of nitrogen, as a source of reducing power, and in pH regulation. In Arabidopsis thaliana, Pro is synthesized in the cytosol from Glu via Glu semialdehyde (GSA), which spontaneously cyclizes to Δ1-pyrroline-5-carboxylate (P5C). Pro synthesis is catalyzed by two enzymes, P5C-synthetase (P5CS) and P5C-reductase, both using NADPH as a cofactor. Genes encoding the two enzymes for Pro anabolism have been identified in several plant species and reported to be upregulated in response to water deprivation and high salinity (Hare et al., 1999). P5CS appears to catalyze the rate-limiting step in Pro biosynthesis because overexpression led to increased Pro levels, whereas under stress conditions P5CS antisense repressed plants were unable to accumulate Pro to levels as in the wild type (Nanjo et al., 1999). The degradation of Pro is catalyzed by sequential action of two mitochondrial enzymes, Pro-dehydrogenase (ProDH) and P5C-dehydrogenase (P5CDH). ProDH is bound to the inner mitochondrial membrane with its active site facing the matrix, whereas P5CDH is located in the mitochondrial matrix (Elthon and Stewart, 1981). Arabidopsis ProDH and P5CDH have been characterized at the molecular level (Kiyosue et al., 1996; Peng et al., 1996; Verbruggen et al., 1996; Deuschle et al., 2001). AtProDH may be upregulated by Pro, hypoosmolarity, and dark adaptation (Hayashi et al., 2000). Cofactors of ProDH are unknown, whereas P5CDH uses NAD+ or NADP+ as electron acceptors, with a preference for NAD+ (Forlani et al., 1997). P5CDH is inducible by externally supplied Pro, but induction is retarded compared to ProDH (Deuschle et al., 2001). ProDH inhibition by knockout or antisense repression resulted in no or only marginal increases in Pro levels under normal conditions. However, during stress, Pro levels were several-fold higher as in the wild type (Nanjo et al., 1999, 2003; Mani et al., 2002). Both ProDH antisense plants and prodh knockouts were sensitive to treatment with Pro during early seedling development. No data have been presented so far to explain whether this is because of a specific requirement for Pro degradation during seedling development or because of accumulation of Pro or Pro metabolism intermediates.
Accumulation of Pro is observed under water limiting conditions (e.g. drought, salinity, or cold stress) and during developmental desiccation processes, such as pollen maturation (Chiang and Dandekar, 1995; Hare and Cress, 1997). During recovery from stress, accumulated Pro is rapidly oxidized to Glu, thus serving as source of nitrogen and energy. Furthermore, Pro is an important energy source during pollen tube elongation or in insect flight (Dashek and Harwood, 1974; Zhang et al., 1982; Gade and Auerswald, 2002).
Despite its protective role under a variety of stress conditions, external supply of Pro was found to be toxic to plant and animal cells (Hellmann et al., 2000; Maxwell and Davis, 2000; Deuschle et al., 2001; Donald et al., 2001). It has been proposed that Pro-induced damage is mediated by P5C/GSA, which, if not metabolized rapidly, might induce cell death (Hellmann et al., 2000). Consistently, yeast strains deficient in P5CDH were hypersensitive to Pro (Deuschle et al., 2001; Nomura and Takagi, 2004). In humans, defects in HsP5CDH result in type 2 hyperprolinemia, with patients experiencing seizures and variable degrees of mental retardation (Geraghty et al., 1998; Morita et al., 2002). Furthermore, both overexpression of ProDH or P5C treatment (400 μM) induced apoptosis in human tumor cell lines (Maxwell and Davis, 2000), and Donald et al. (2001) reported ProDH-dependent formation of reactive oxygen species (ROS) in human cells and induction of apoptosis. HsP5CDH seems to be a direct target of the mammalian tumor suppressor protein p53 and inhibition of expression enhanced p53-induced cell death (Yoon et al., 2004). Thus, in many organisms, synthesis, transport, and degradation of Pro and P5C/GSA must be tightly regulated to prevent cell death.
To study Pro degradation in plants, a yeast p5cdh mutant was used to clone the Arabidopsis P5CDH gene (AtP5CDH) by functional complementation (Deuschle et al., 2001). AtP5CDH is a single copy gene. Other dehydrogenases share <16% similarity on the amino acid level. Delayed induction of AtP5CDH relative to ProDH after external supply of Pro further strengthened the hypothesis that accumulation of P5C/GSA caused the symptoms of Pro-induced cell death. According to this model, P5CDH may have an important function in preventing damaging effects of P5C/GSA and subsequent cell death.
To analyze the physiological role of the ubiquitously expressed P5CDH in planta, two T-DNA insertion lines for AtP5CDH were isolated. Degradation of Pro was undetectable in p5cdh-1, indicating that P5CDH is the primary enzyme for P5C/GSA degradation. Surprisingly, the defect in P5CDH did not lead to visible alterations in phenotype under normal growth conditions, suggesting that Pro degradation is not essential for vegetative plant growth. p5cdh mutants were hypersensitive to external Pro supply and accumulated both Pro and P5C. Hypersensitivity of the p5cdh mutants to Pro, Orn, and Arg and increased tolerance of P5CDH overexpressors support the hypothesis that P5C/GSA is the causative agent of cell death induced by Pro supply.
RESULTS
Regulation of P5CDH Expression
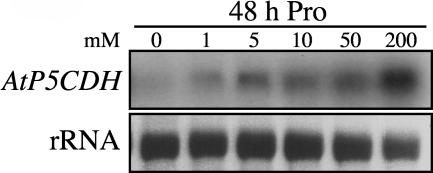
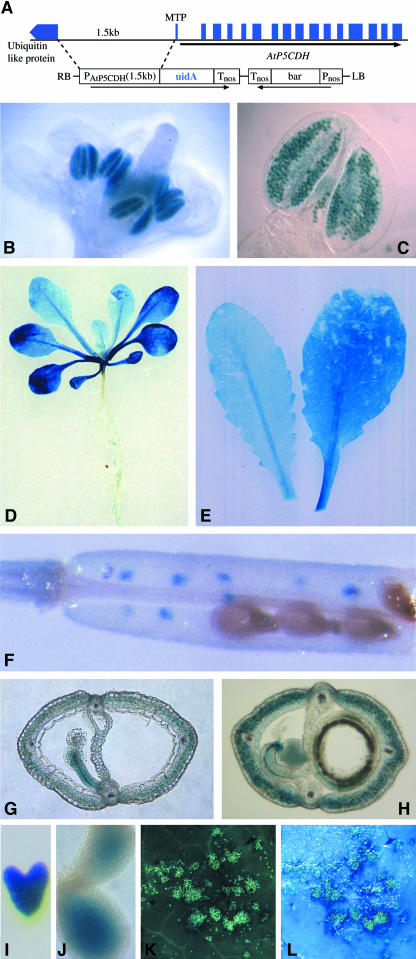
P5CDH expression increased slightly in the presence of a variety of amino acids, such as Pro, Arg, Glu, and Orn (data not shown). Within 48 h, induction by Pro was observed at external concentrations of 5 mM (Figure 1). The spatial expression pattern was analyzed in transgenic plants carrying an AtP5CDH promoter-β-glucuronidase (GUS) fusion construct, showing constitutive low levels of expression in vegetative tissue (Figure 2). Under normal growth conditions, high AtP5CDH promoter activity was only found in pollen (Figures 2B and 2C). Staining of leaves increased with leaf age, potentially indicating an elevated need for Pro degradation during senescence (Figures 2D and 2E). In siliques, aborted seeds showed strong GUS staining, as well as the silique walls, funiculi, and developing embryos (Figures 2F to 2J).
Figure 1.
Induction of AtP5CDH Expression by Pro.
Gel blot analysis of RNA extracted from 3-week-old Arabidopsis plants 48 h after transfer to liquid MS medium with Pro. Equal amounts of total RNA were loaded in each lane (13 μg).
Figure 2.
AtP5CDH Is Strongly Expressed in Pollen and Tissue Undergoing Cell Death.
(A) Construct used for AtP5CDH promoter analysis. Including the translation initiation codon, 1.5 kb of 5′-upstream sequence was fused to uidA, and GUS expression was monitored in transgenic Arabidopsis plants carrying the fusion construct. LB, left border; RB, right border.
(B) and (C) GUS expression in developing and mature pollen grains.
(D) Three-week-old seedling showing GUS expression in senescing cotyledons.
(E) Rosette leaves of soil-grown plants.
(F) Silique with aborted seeds.
(G) and (H) Silique cross sections showing staining in funiculi and silique coat.
(I) and (J) Stained embryos.
(K) and (L) Autofluorescent lesions caused by spraying with Pro.
(L) Overlay of (K) with induced GUS expression.
In flax (Linum usitatissimum), the P5CDH ortholog was found as an unknown gene induced around lesions induced by compatible rust infection (Roberts and Pryor, 1995; Ayliffe et al., 2002). Consistently, induction of AtP5CDH in Arabidopsis was detected around lesions induced by spraying with Pro (Figures 2K and 2L).
Isolation of P5CDH Insertion Mutants
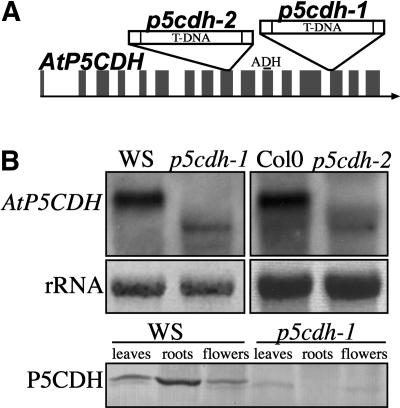
To study the role of P5CDH, insertion mutants were generated in which P5CDH expression was impaired. Screening for T-DNA insertions using the Arabidopsis Knockout Facility (University of Wisconsin) led to the identification of p5cdh-1 carrying a T-DNA in the 13th of 16 exons of AtP5CDH (Figure 3A). RNA gel blot analyses detected residual amounts of a truncated transcript, whereas protein gel blot analyses using polyclonal antibodies raised against purified potato (Solanum tuberosum) P5CDH did not show detectable amounts of P5CDH in various organs of p5cdh-1 (Figure 3B). A second insertion mutant (p5cdh-2) in the 9th exon lacking detectable transcript in the original size was identified in the Salk Insertion Sequence Database (Alonso et al., 2003) (Figures 3A and 3B). The insertion in the 9th exon is located upstream of the conserved aldehyde dehydrogenase domain, thus probably leading to a complete loss of function. In both p5cdh mutants, ProDH mRNA levels were not altered significantly as compared to the wild type (data not shown).
Figure 3.
T-DNA Insertions in AtP5CDH.
From the Wisconsin and Salk collections, two Arabidopsis lines carrying single T-DNA insertions in the 13th (p5cdh-1) and 9th (p5cdh-2) exons of AtP5CDH, respectively, were identified. The aldehyde dehydrogenase domain (ADH) of AtP5CDH is completely encoded by the 11th exon.
(A) Schematic illustration of T-DNA insertion sites in the mutant lines p5cdh-1 and p5cdh-2.
(B) RNA gel blot analysis of AtP5CDH transcript in both mutant lines shown on top. Bottom, polyclonal antibodies raised against potato P5CDH cross-reacted with AtP5CDH, but no protein could be detected in extracts of p5cdh-1. A second cross-reacting protein with slightly lower molecular mass was present in both the wild type and mutant, but absent in roots.
Absence of Alternative Pathways for Pro Degradation
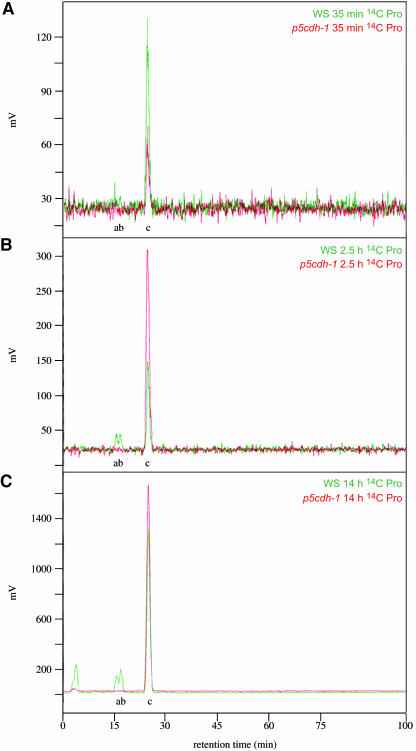
Despite the relevance of Pro and Pro-degradation for many physiological processes (e.g. energy supply during release from stress or in pollen tube growth), p5cdh mutants showed no visible defects. Also, photosynthesis seemed unaffected; however, the germination rate of the mutant lines was reduced (data not shown). To test whether the second step of Pro degradation is conducted primarily by P5CDH, leaves of Arabidopsis were fed with 14C-labeled Pro. After 35 min, 14C-labeled Gln and Glu were detected in the wild type but not in p5cdh-1. Even after 14 h the mutant did not accumulate Pro-derived amino acids (Figures 4A to 4C). Interestingly, under normal conditions, no significant increase of Pro levels was found in both p5cdh mutants as compared to the wild type. When Pro was supplied externally, internal Pro levels in p5cdh-1 and the wild type increased eightfold to 10-fold within 17 h (Figure 5A). However, p5cdh-1 was unable to degrade imported Pro over a period of at least 48 h, indicating that under normal conditions Pro degradation may not be essential for normal growth and development in the vegetative phase.
Figure 4.
No Labeled Pro Degradation Products Could Be Detected in p5cdh-1 after Feeding with 14C Pro.
HPLC analysis of radioactive peaks in p5cdh-1 and the wild type (Wassilewskija) after feeding with 14C-labeled Pro. a, Glu; b, Gln; c, Pro.
(A) HPLC chromatograms from leaves after 35 min incubation with 14C Pro.
(B) After 2.5 h.
(C) After 14 h incubation.
Figure 5.
Internal Pro Levels Were Not Increased in p5cdh-1.
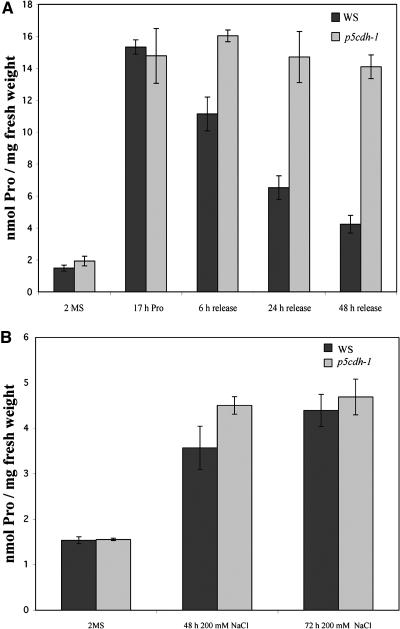
(A) p5cdh-1 and wild-type plants were grown on solid 2MS (MS supplemented with 60 mM sucrose) medium for 3 weeks, then they were incubated in MS medium supplemented with 100 mM Pro and 60 mM sucrose for 17 h. Pro treatment increased Pro levels in both the mutant and the wild type. Subsequently, plants were rinsed and transferred to MS medium without Pro for the indicated periods of time. Already 6 h after removal of Pro, the Pro levels in wild-type plants decreased, whereas even after 48 h release, Pro levels in the p5cdh-1 mutant were not significantly changed.
(B) Plants were precultured as in (A) and transferred to liquid MS supplemented with 200 mM NaCl and 20 mM sucrose. The 72-h NaCl treatment increased Pro levels in the wild type and p5cdh-1 mutant approximately threefold. All values are mean ± sd, n ≥ 3.
P5CDH Is Not Essential for Stress Tolerance
Reduced ProDH activity results in increased Pro accumulation under stress conditions (Nanjo et al., 1999; Mani et al., 2002). After salt treatment, both p5cdh-1 and the wild type accumulated Pro approximately threefold relative to pre-stress conditions. Thus, in contrast with ProDH inhibition, a block of degradation in P5CDH does not result in higher accumulation of Pro under stress conditions (Figure 5B). Consistent with the unchanged Pro levels, in a gauntlet with adverse conditions, such as salt stress, drought, temperature stress, light stress, and release of stress, no significant visible differences between the wild type and mutants were observed. These findings and the absence of growth defects of a prodh mutant (Nanjo et al., 2003) suggest that P5CDH and Pro degradation are not essential for survival under stress conditions or even during recovery after stress release. These findings prompted us to focus on the proposed function of Pro degradation in cell signaling (Deuschle et al., 2001).
Induction of Cell Death by Pro Supply
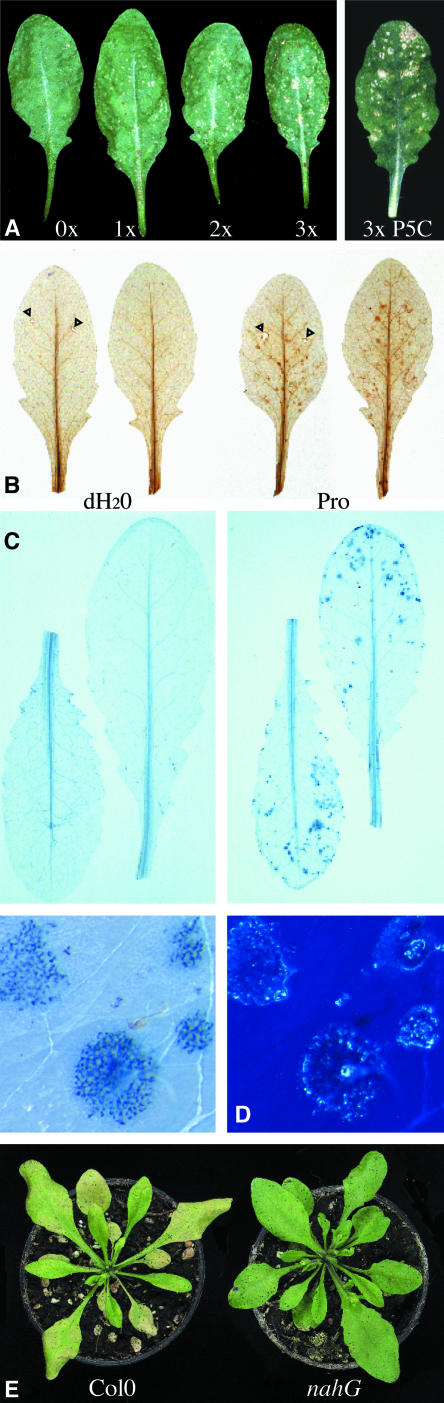
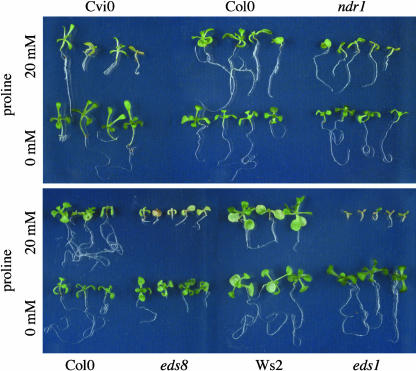
Spraying Arabidopsis leaves with Pro or its degradation product P5C led to cell death (i.e., foci of dead cells), a phenotype comparable to leaf spot disease or ozone induced lesions (Figure 6A) (Rao and Davis, 1999; Pilloff et al., 2002). The damaged tissue accumulated phenolics, callose, and H2O2 as indicated by autofluorescence, aniline blue, and 3,3-diaminobenzidine (DAB) staining, respectively. Trypan blue staining confirmed cell death in the center of the lesions (Figures 6B to 6D). Pro-dependent induction of cell death seems to be signal mediated because transgenic NahG plants, in which the stress hormone salicylic acid is degraded, were less sensitive to external Pro in the dark (Figure 6E) (Bowling et al., 1994). Mutants with altered induction of hypersensitive cell death in response to pathogens (e.g., eds1 and eds8 [enhanced disease susceptibility]) were hypersensitive to Pro (Figure 7). Eds1 was found to encode a protein with similarity to eukaryotic lipases, whereas eds8 has not been characterized at the molecular level yet (Falk et al., 1999). Also, ndr1 (non-race specific disease resistance) and ecotype Cvi-0 (Cape Verde Islands) displayed hypersensitivity to Pro. Cvi-0 plants were reported to accumulate higher levels of salicylic acid as compared to other ecotypes in response to ozone treatment, whereas Ndr1 encodes a presumptive membrane-associated protein required for salicylic acid–dependent, resistance gene–mediated pathogen defense (Century et al., 1997; Rao et al., 2000).
Figure 6.
Pro Induces HR-Like Lesions in Arabidopsis.
(A) Mature rosette leaves from Arabidopsis were sprayed one to three times with 20 mM Pro or three times with 750 μM P5C. Pictures were taken at the fifth day after the initial spraying.
(B) to (E) Leaves were sprayed at three consecutive days with 20 mM Pro.
(B) H2O2 detection by DAB staining; arrowheads indicate staining in wounding sites as control.
(C) and (D) Staining of dead cells with trypan blue.
(D) Formation of callose at sites of Pro-induced cell death is shown as fluorescence after staining with aniline blue.
(E) Transgenic plants expressing a bacterial salicylate hydroxylase (NahG) and control plants were sprayed with water (data not shown) or with 20 mM Pro and kept in the dark for 4 d.
Figure 7.
Pro Hypersensitivity of Ecotype Cvi-0 and Pathogen Response Mutants eds1, eds8, and ndr1.
Shown are 15-d-old seedlings of Cvi-0, ndr1, eds1, and eds8 and the corresponding wild type grown in the presence or absence of 20 mM Pro.
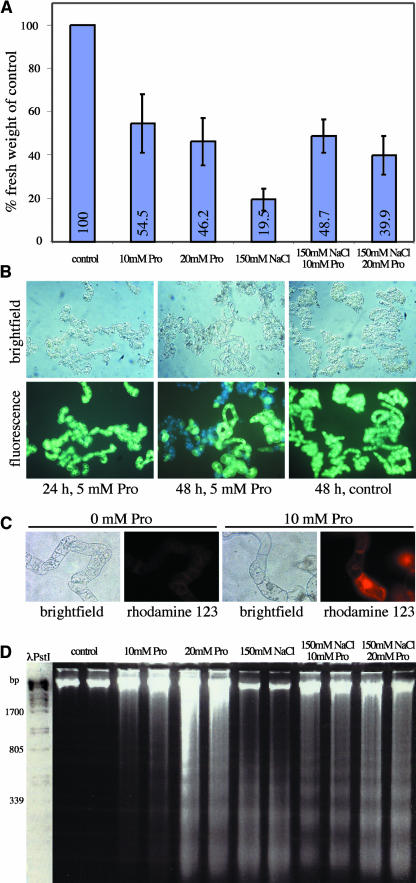
To further substantiate that Pro supply can induce programmed cell death (PCD), tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) suspension cultures were treated with Pro (10 and 20 mM). Pro addition led to reduced growth, cell death as indicated by fluorescein diacetate staining, production of ROS indicated by dihydrorhodamine 123 staining, and DNA laddering (Figures 8A to 8D). Similar symptoms were observed after addition of salt (data not shown) (Katsuhara, 1997). Addition of Pro in the presence of salt ameliorated toxicity shown by improved growth (Figure 8A).
Figure 8.
Pro Addition to Tobacco BY-2 Suspension Cultures Causes Symptoms of PCD.
(A) Accumulation of biomass in tobacco BY-2 cells 48 h after addition of Pro or NaCl to the growth medium.
(B) Viability of BY-2 cells 24 and 48 h after addition of 5 mM Pro. After addition of membrane-permeable fluorescein-diacetate, living cells accumulate green fluorescent fluorescein, and dead cells appear light blue because of autofluorescence. Light microscopy revealed cytoplasmic condensation in BY-2 cells 48 h after addition of Pro.
(C) H2O2 production in BY-2 cells treated with 10 mM Pro for 48 h was detected by staining with dihydrorhodamine 123.
(D) Nuclear DNA of BY-2 cells showed fragmentation into oligonucleosome fragments after treatment with Pro and/or NaCl for 48 h.
Effect of Pro on Gene Expression Profiles
To study the effect of Pro treatment on gene expression profiles, two custom GST arrays (one representing 3292 genes known or predicted to code for chloroplast proteins, the other representing membrane transport proteins plus 222 selected other proteins) were hybridized with probes derived from 3-week-old Arabidopsis plants supplied for 48 h with liquid medium supplemented with 100 mM Pro. After 48 h Pro treatment, 520 genes on the chloroplast array were significantly induced, whereas 507 genes were significantly downregulated (see Supplemental Table 1A online). Among the genes induced by Pro, many have known or predicted functions in stress defense and signal transduction. Highest changes in transcript abundance after 48 h Pro treatment were observed for genes encoding a putative ABC transporter (At1g66950, 11-fold), followed by two proteins from the protease inhibitor/seed storage/lipid transfer family, similar to pEARLI1 (AT4g12490, 11-fold; AT4g12470, 10-fold), a putative glutathione S-transferase (AT4g02520, 10-fold) and a putative Leu-rich repeat protein (At2g30100, 10-fold). The observed increase in expression of stress-related genes supports the notion that external Pro supply induces oxidative stress and release of ROS. When the same RNA preparations were used to probe the transporter array, several ABC transporters potentially involved in Pro efflux were significantly induced (see Supplemental Table 1B online). Furthermore, many genes related to amino acid metabolism were upregulated (i.e., Glu family).
p5cdh Mutants Are Pro Hypersensitive
The p5cdh mutant lines were used to assess the involvement of P5CDH in Pro-induced cell death. Previous studies suggested that accumulation of the Pro degradation intermediate P5C/GSA may be the causing agent of the observed stress responses. Thus, p5cdh mutants are expected to accumulate P5C and therefore be hypersensitive toward externally supplied Pro.
A method for P5C determination was developed for plant extracts. P5C was below the detection limit (<0.05 μmol/g fresh weight) in untreated Columbia-0 (Col-0), Wassilewskija (Ws), and p5cdh-1 and -2 mutant plants, as well as in Pro-treated Col-0 and Ws wild-type plants (Table 1). P5C was detected only in the p5cdh mutants when exposed to Pro. A 24-h exposure of p5cdh-2 to 10 mM Pro was sufficient to obtain P5C levels that were more than threefold above the detection limit. When exposed to 60 mM Pro, P5C was more than threefold higher as compared to treatments with 10 mM Pro. Longer treatments did not further affect P5C levels (data not shown).
Table 1.
P5C Contents in p5cdh Mutants and Wild-Type Plants
| Genotype | Untreated | 24 h 10 mM Pro | 24 h 60 mM Pro | 48 h 60 mM Pro |
|---|---|---|---|---|
| Ws | n.d. | – | n.d. | – |
| p5cdh-1 | n.d. | – | 0.56 ± 0.06 | 0.59 ± 0.12 |
| Col-0 | n.d. | – | n.d. | – |
| p5cdh-2 | n.d. | 0.17 ± 0.01 | 0.60 ± 0.09 | – |
p5cdh-1 and -2 seedlings were treated with either 10 or 60 mM Pro; 24 or 48 h after the treatment, P5C content was evaluated. Values are expressed as μmol (g fresh weight)−1 and are mean ± se over three independent replicates. n.d., not detectable, <0.05 μmol (g fresh weight)−1; –, not analyzed.
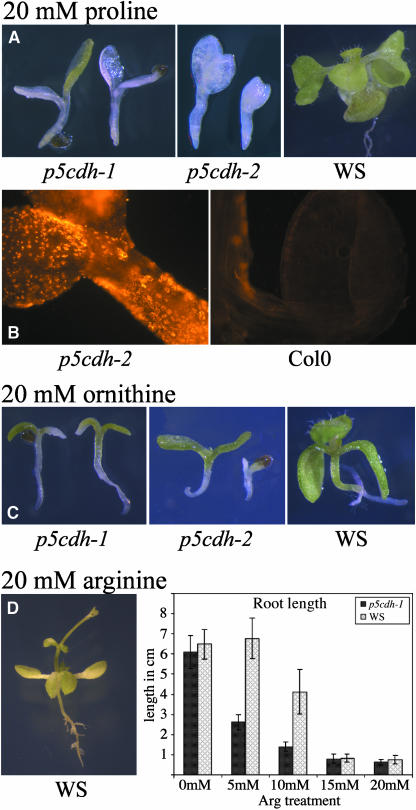
When grown in the presence of 20 mM Pro, p5cdh mutants bleached rapidly after germination and were not able to develop roots. p5cdh-1 seedlings (background Ws) died in the presence of 20 mM external Pro before the formation of true leaves, whereas in p5cdh-2 seedlings (Col-0 background), development was arrested even earlier and cotyledons hardly ever developed chlorophyll (Figure 9A). Pro-treated mutants also accumulated ROS as indicated by dihydrorhodamine 123 staining (Figure 9B). Hypersensitivity toward exogenous Pro supply was observed also in P5CDH antisense lines (see Supplemental Figure 1 online).
Figure 9.
Cell Death Caused by Exogenously Supplied Pro and Other P5C-Producing Substrates in p5cdh Mutants.
(A) Both p5cdh mutant lines were arrested in growth in the presence of 20 mM Pro. The mutants did not form roots or true leaves and bleached rapidly, whereas wild-type plants were only mildly affected.
(B) Production of ROS in p5cdh-2 seedlings grown on 20 mM Pro, shown by staining with dihydrorhodamine 123.
(C) p5cdh mutants are hypersensitive to growth on 20 mM Orn.
(D) Growth on 20 mM Arg causes decreased root growth in the wild type and mutants.
Mutation of P5CDH Mimics the Effect of Pro Treatment on Global Gene Expression Profiles
To test for more subtle changes in p5cdh mutants, transcript profiling was applied. The mutation in p5cdh-1 led to altered transcript levels for 1420 out of 2573 genes on a GST array with mainly chloroplast-targeted genes (see Supplemental Table 1C online). Although p5cdh-1 neither contained elevated Pro levels nor detectable amounts of P5C under normal conditions (Figure 5, Table 1), the expression profile of p5cdh-1 in the control treatment resembled that of Pro-treated wild type, which showed up to 10-fold increased Pro levels. A total of 111 genes were induced in untreated p5cdh-1 as well as in Pro-treated wild type, and only four genes that were induced in p5cdh-1 were downregulated in Pro-treated wild type. A total of 309 genes were downregulated both in p5cdh-1 and in Pro-treated wild type, and only 67 were downregulated in p5cdh-1 and upregulated in Pro-treated wild type.
Treatment of 3-week-old p5cdh-1 plants with 100 mM Pro significantly enhanced expression of 1320 genes, many of which have a proposed function in signal transduction, secondary metabolism, or stress defense. A total of 317 genes were significantly downregulated, 64 of them are predicted photosynthesis-related genes, which is in agreement with the observed bleaching of the p5cdh mutant lines grown on Pro (see Supplemental Table 1D online).
Alternative Sources of P5C Cause Symptoms Similar to Pro
The effect of Orn and Arg on p5cdh-1 and -2 was tested to analyze whether other compounds producing P5C have similar effects as Pro. Orn also induced toxicity in Arabidopsis wild-type plants, and p5cdh mutants were hypersensitive to Orn. Symptoms resembled those of Pro treatment, but were less severe (Figure 9C). Supply of Arg as the sole nitrogen source in the medium led to a dwarf phenotype, shortened roots, and premature bolting and flowering in axenic culture (Figure 9D). Inhibition of root elongation by Arg was more severe in p5cdh-1 than in the wild type. Hypersensitivity of p5cdh mutants to Orn and Arg further supports the hypothesis that cell death induced by Pro supply is linked to P5C accumulation.
P5CDH Overexpression Leads to Reduced Pro Sensitivity
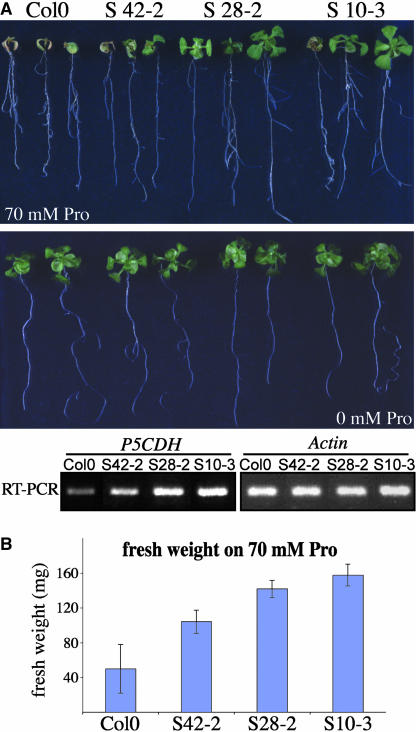
Hypersensitivity of p5cdh mutants to Pro and other agents degraded via P5C as well as the direct demonstration of P5C accumulation in p5cdh mutants after Pro supply indicated that P5C/GSA accumulation may induce cell death. Thus, increased P5CDH activity should lead to amelioration of Pro-induced cell death. P5CDH was overexpressed under control of the 35S promoter of Cauliflower mosaic virus. Under normal growth conditions, plants overexpressing P5CDH were indistinguishable from the wild type, but consistent with a function of P5CDH in protection from P5C effects, the overexpressing lines tolerated higher concentrations of external Pro than the wild type (Figure 10).
Figure 10.
Overexpression of P5CDH Reduces Pro Sensitivity.
(A) Arabidopsis lines overexpressing AtP5CDH and corresponding wild-type Col-0 grown on MS medium containing 30 mM sucrose and 0 or 70 mM Pro.
(B) Plants were grown on medium containing 30 mM sucrose and 70 mM Pro for 18 d. Fresh weight shown is mean ± SD, n ≥ 3.
DISCUSSION
Pro accumulation is a common response of plants to stress. Pro is supposed to act as a compatible osmolyte, as a protective agent for membranes and enzymes, as a scavenger of radicals, and as a transient storage form of organic nitrogen (Hare and Cress, 1997). Surprisingly, external supply of Pro, or the degradation intermediate P5C/GSA, were found to be toxic (Bonner et al., 1996; Hellmann et al., 2000; Deuschle et al., 2001; Hare et al., 2002). Although synthesis has been studied extensively, little is known about the events after stress relief, when Pro is degraded. Here, we show that the second enzyme of Pro catabolism, P5CDH, is essential for Pro degradation. Furthermore, we provide evidence that the induction of cell death by Pro is caused by P5C/GSA, which triggers a salicylic acid–mediated signaling cascade leading to cell death. An increase in the capacity to metabolize P5C/GSA consistently leads to a protection from cell death induced by Pro supply.
Role of P5CDH in Metabolism under Normal Growth Conditions
Promoter analysis revealed expression of P5CDH in all tissues, especially in pollen, where ProDH is also expressed to high levels (Nakashima et al., 1998). Pollen grains are known to have a high content of free Pro, supposedly functioning as an osmolyte during the desiccation process and as an energy source during pollen germination (Schwacke et al., 1999). To obtain more information about Pro degradation and P5CDH function, two insertion mutants for P5CDH were isolated. Both mutant lines were visibly indistinguishable from the wild type under normal growth conditions, and also the level of free Pro was not significantly elevated in the p5cdh-1 mutant. However, no degradation of externally supplied Pro was detected, indicating that alternative Pro degradation pathways not using P5CDH do not exist. Pollen germination and pollen tube growth in vitro were unaffected in p5cdh-1, suggesting that Pro is not the only/major energy source of pollen tube growth (data not shown). Another situation where Pro might play an important role is during the early phases of development. Seed imbibition was recently reported to induce a fourfold increase in free Pro levels, and seedling development was inhibited by externally supplied Pro in ProDH-antisense lines (Mani et al., 2002; Hare et al., 2003). Because the germination rate of p5cdh mutants was reduced, Pro degradation seems to be important for seed maturation or germination.
P5C/GSA as a Feedback Regulator for Pro Biosynthesis and Degradation
Because Pro levels in p5cdh-1 were not significantly higher as compared with the wild type both under stress or nonstress conditions, Pro synthesis must be subject to allosteric regulation. Feedback inhibition of Pro synthesis by Pro had previously been described (Hu et al., 1992; Zhang et al., 1995; Hong et al., 2000). Inhibition of Pro degradation by knockout or antisense repression of ProDH resulted in no or only marginal increases in Pro levels under normal conditions. However, during stress, Pro levels increased severalfold higher than in the wild type (Nanjo et al., 1999, 2003; Mani et al., 2002). Enhanced accumulation of Pro in prodh but not p5cdh mutants was unexpected and indicates the existence of a feedback mechanism that determines the upper limit of Pro accumulation and is dependent on ProDH but not P5CDH activity. It is conceivable that the degradation and synthesis intermediate P5C/GSA may be the signal limiting Pro biosynthesis under stress. Because synthesis and degradation occur in the cytosol and mitochondrial matrix, respectively, the P5C pools must communicate, potentially involving a P5C transporter in the inner mitochondrial membrane (proposed in Phang, 1985), or the P5C-generated signal must be perceived and converted within the mitochondria.
As in yeast, elevated amounts of P5C were observed only in p5cdh mutants after supply with exogenous Pro (Nomura and Takagi, 2004). In Arabidopsis, Pro levels were the same as in the wild type, and P5C levels were 30 times lower, indicating that ProDH activity was also impaired. It remains to be determined if ProDH activity is inhibited already by low concentrations of P5C or if accumulating P5C is exported into the cytosol and converted to Pro by P5C-reductase.
Role of P5CDH in Metabolism under Stress Conditions and in Pathogen Infection
During salt stress, Pro levels increased threefold both in p5cdh-1 and in the wild type. In all experiments performed, stress tolerance of p5cdh mutants was similar as in the wild type, supporting the hypothesis that accumulation of Pro is important for stress resistance, whereas redox shuttling between the mitochondria and the cytosol by means of Pro synthesis and degradation probably does not contribute to stress tolerance, as postulated for animal cells (Phang, 1985). The presence of high Pro levels in p5cdh-1 for extended periods after stress release did not seem to have obvious consequences.
The spectrum of stress symptoms induced by Pro application is very similar to the well-characterized hypersensitive response (HR) of plants to incompatible pathogens. Strikingly, Fabro et al. (2004) recently reported that plants undergoing HR accumulated Pro, probably because of upregulation of the key enzyme in Pro biosynthesis, P5CS, at and around the sites of HR. Further evidence for a function of Pro degradation or more specifically P5CDH in PCD derived from the regulation of the flax ortholog of AtP5CDH, LuFIS1 (Roberts and Pryor, 1995). LuFIS1 was upregulated around rust infection sites only during susceptible infection with flax rust but not when the plants were resistant because of a hypersensitive reaction (Ayliffe et al., 2002). The pathogen may induce P5CDH to prevent accumulation of P5C and induction of PCD by the plant. Expression of P5CDH around HR-like lesions might contribute to restrict HR to the area of infection and prevent uncontrolled spreading of cell death. Reduced sensitivity of salicylic acid–deficient NahG plants to exogenous Pro application in the dark but not in plants exposed to the normal dark–light cycles further supports the concept of a P5C-mediated signal because darkness was reported to induce ProDH expression (Hayashi et al., 2000). Damages occurring in the light seem to depend on salicylic acid–independent mechanisms, potentially mediated by ROS formation. Consistently, Cvi-0, an Arabidopsis ecotype overproducing salicylic acid in response to ozone stress, is hypersensitive to Pro (Rao et al., 2000). The link between P5C and pathogen-induced cell death is further substantiated by the finding that cpr5, eds1, eds8, and ndr1 are hypersensitive to Pro. Thus, what had been termed Pro toxicity is most probably not a direct toxicity of P5C/GSA but cell death induced via a signaling cascade.
P5C/GSA as a Downstream Effector after Pro Treatment
Symptoms of cell death observed after external Pro application included local browning, accumulation of fluorescent compounds, callose deposition, DNA laddering, and local cell death, and Arabidopsis plants died after long-term incubation, suggesting a link between P5C accumulation and PCD. Hare et al. (2002) showed disruption of chloroplast and mitochondria ultrastructure among the early symptoms of Pro application, which might be connected to the mitochondrial permeability transition frequently observed during the onset of apoptosis (Tiwari et al., 2002).
Interestingly, PCD was also observed when plants were exposed to salt stress (Katsuhara, 1997; Huh et al., 2002). It is therefore conceivable that Pro accumulation under salt stress or during HR to pathogens provides the link to cell death. This hypothesis may be tested by analyzing the effect of salt stress on PCD in P5CS mutants. By contrast, growth amelioration of NaCl-treated BY-2 suspension cultures by addition of Pro shows that Pro supply can have positive effects on plant growth, at least when supplied under stress conditions.
P5C application in animals and plants led to cell death–like symptoms similar to those observed as a consequence of external Pro supply, the simplest interpretation being that the effects of Pro treatment are mediated by P5C (Hellmann et al., 2000; Maxwell and Davis, 2000). P5C accumulation may be because of delayed induction of P5CDH relative to ProDH during external Pro supply (Deuschle et al., 2001). Consistently, P5C was detectable in p5cdh mutants after external Pro supply but not in the wild type. Hypersensitivity of p5cdh-1 mutants to other P5C-producing substances, such as Arg and Orn, may support the hypothesis that the cellular damage caused by Pro application is induced by P5C or P5C-derived signals.
A similar conclusion may be drawn from studies in human cell lines, where induction of ProDH expression by p53 or ProDH overexpression induced apoptosis (Polyak et al., 1997; Maxwell and Davis, 2000). Accordingly, HsP5CDH was also found to be a direct target of p53, and overexpression was able to reduce ROS accumulation caused by H2O2 or UV treatment (Yoon et al., 2004). Alternative to the hypothesis that an increase in P5C as a consequence of Pro treatment leads to cell death via a signaling cascade, it has been suggested that ROS triggering cell death are produced by a hypothetical Pro cycle (Yoon et al., 2004). In this model, both Pro and P5C cycle across the mitochondrial membrane. The oxidation of Pro to P5C by ProDH can contribute to the energy supply of the cell and enhance generation of ROS. P5CDH would normally remove P5C irreversibly from this cycle by converting it to Glu, reducing the production of ROS. The existence of a mitochondrial P5C/Pro shuttle remains to be demonstrated.
Unexpectedly, plants impaired in ProDH expression were also developmentally arrested by the supply of exogenous Pro (Mani et al., 2002; Nanjo et al., 2003). To differentiate between the possibilities whether P5C also accumulates in these mutants or whether other factors play a role in Pro-induced developmental arrest, it will be important to determine P5C and ROS levels in prodh mutants.
However, strong support for the role of P5C/GSA in Pro-induced cell death derives from constitutive overexpression of AtP5CDH in Arabidopsis, which resulted in decreased sensitivity to externally supplied Pro.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana Heynh. ecotypes Col-0 and Ws-2 were grown in axenic culture or in the greenhouse both with 16 h of light and 8 h of dark at 21.5°C. For axenic culture, seeds were surface sterilized for 10 min in 70% ethanol and 20 min in 1.2% sodium hypochlorite and 0.01% Triton X-100, rinsed three times with sterile water, and vernalized for 24 h at 4°C. Plants were grown on MS medium (MS salts from Duchefa, Haarlem, The Netherlands) with 60 mM sucrose solidified with 7.5 g/L of BiTek agar in parafilm-sealed Petri dishes or Weck round rim jars (Weck, Wehr-Öflingen, Germany) in a climate chamber. For foliar application, greenhouse plants were sprayed with a solution of 0.0025% silwet-L-77 in distilled water plus the desired substances.
BY-2 tobacco (Nicotiana tabacum) suspension culture was grown in constant darkness in liquid culture. Fresh weight is an average of five independent treatments and measurements.
DNA Extraction from Tobacco BY-2 Cells
Tobacco BY-2 cells, a kind gift from T. Merkle (University of Freiburg, Germany), were maintained on an orbital shaker at 25°C in the dark in 125-mL Erlenmeyer flasks containing MS medium. Cells were subcultured once a week. At 48 h after subculturing, different concentrations of Pro and NaCl were added to the culture medium. At 48 h after treatment, tobacco BY-2 cells were collected by filtration and weighed, and genomic DNA was isolated according to Young and Gallie (2000). For DNA fragmentation analysis, 20 μg of each sample was resolved on a 1.8% TBE agarose gel.
Stress Treatment of Arabidopsis Plants
Cold stress was imposed by growth at 4°C or incubation at −20°C for different periods of time. Heat treatment was performed at 37°C. Salt stress was applied by either growth on MS medium supplemented with different concentrations of NaCl, by watering plants with water containing different concentrations of NaCl, or by transfer of plants grown on solid 2MS medium to liquid medium containing 200 mM NaCl. Light stress was applied by shifts from low light (50 μmol/s/m2) to high light (400 μmol/s/m2) and back. Drought stress was achieved by lack of watering of plants in soil for different periods of time.
Mutant Identification
The Wisconsin mutant population (Krysan et al., 1999) (www.biotech.wisc.edu/Arabidopsis/default.htm) was screened using the gene-specific primer 5′-TTATTAGTGGACACACCACTTCTAAGTAG-3′. Out of 94 plants from pool 4005, four plants carrying the p5cdh-1 insertion were recovered. For further analyses, the mutant was backcrossed twice to the Ws wild type.
Insertion mutant information about p5cdh-2 (Salk_018453) was obtained from the SIGnAL Web site at http://signal.salk.edu. The insertion was verified by PCR with primers designed by the SIGnAL iSect Web tool and sequencing of the obtained PCR product from the T-DNA left boarder.
Construction of Overexpressers, Antisense Lines, and GUS Plants
For stable transformation, Agrobacterium tumefaciens strain pGV2260 (Deblaere et al., 1985) was transformed with the mini binary vector pCB302.3 containing an AtP5CDH cDNA in sense or antisense orientation and used for transformation of Arabidopsis by floral dip (Clough and Bent, 1998; Xiang et al., 1999). The AtP5CDH coding sequence was amplified by PCR from a vector containing the cDNA from ecotype Landsberg erecta and inserted into pCB302.3 using the unique BamHI site. T0 seeds were sown in the greenhouse, and transformants were selected by spraying the plants with 0.1% BASTA (Aventis CropScience, Gent, Belgium) at the four leaf stage. AtP5CDH-GUS plants are described by Deuschle et al. (2001).
Array Analysis and RNA Gel Blot Analyses
Plants were cultured on solid MS medium containing 60 mM sucrose. Three-week-old plants were transferred to chambers (Weck round-rim jar 100) in which roots had contact with 10 mL of liquid MS medium supplemented with salt, sugars, or different amino acids. After 48 h of incubation, the roots were washed in distilled water, and the plants were rapidly frozen in liquid N2. Analysis of the expression profile at earlier time points (0.5 to 6 h) showed that transfer of plants led to high variability in both Pro-treated and untreated samples, thus, data were not considered. RNA extraction, gel electrophoresis, and blotting were performed according to Lehrach et al. (1977) and Logemann et al. (1987). Hybridizations were conducted according to Martin et al. (1992) using the cDNA of AtP5CDH labeled with [α32P]dCTP by random priming. For the array analysis, RNA from 3-week-old plants grown on solid 2MS and transferred to liquid MS medium supplemented with either 100 mM Pro or 100 mM glucose for 48 h was used. The array containing 3292 Arabidopsis plastidic protein GSTs, probing, and data analysis are described by Pesaresi et al. (2003) and Richly et al. (2003). The custom-made Arabidopsis transporter array carries gene-specific cDNA fragments (cGSTs) for 1274 (putative) membrane proteins with four or more transmembrane spans and 222 soluble proteins spotted on Hybond N+ filters. The filters were hybridized overnight with 33P-labeled cDNA in DIG EasyHyb solution (Roche, Indianapolis, IN) and washed two times for 5 min at 65°C in 2× SSC/0.1% SDS (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) and once for 20 min at 65°C in 0.2× SSC/0.1% SDS before exposure and evaluation.
Expression levels of AtP5CDH were determined by RT-PCR using primers specific for the coding sequence of AtP5CDH (forward, 5′-GCTTTTTGTTCATGAGAACTGGTC-3′; reverse 5′-CAGCCCGGGAGTAGATGGAGGAAGTTCCC-3′; 23 cycles), specific for endogenous AtP5DCH mRNA (forward, 5′-GCTTTTTGTTCATGAGAACTGGTC-3′; reverse, 5′-TTTATTTGATGCAACAGCACACTAAG-3′; 25 cycles), and for AtActin2 (forward, 5′-TCCAAGCTGTTCTCTCCTTG-3′; reverse, 5′-GAGGGCTGGAACAAGACTTC-3′; 20 cycles).
Staining Procedures and Detection
Histochemical GUS assays were performed according to Martin et al. (1992). Seedlings were stained for 24 h, flowers for 12 to 14 h.
For detection of dead cells, leaves were boiled for 3 min in trypan blue staining solution (10 mL of lactic acid, 10 g of phenol, 10 mL of glycerol, 10 mL of deionized water, 10 mg of trypan blue; freshly diluted 1:1 in 100% ethanol). Destaining was conducted overnight in chloral hydrate (2.5 g/mL).
H2O2 was visually detected in the leaves of plants using DAB. Briefly, leaves or whole plants were excised with a razor blade and supplied through the cut petioles or stems with a 1-mg/mL solution of DAB, pH4, for 10 h in the dark at 21°C. As positive control, leaves were wounded with a needle in the upper half of the leaf right before the DAB treatment. The experiments were terminated by immersion of the plants or leaves in warm ethanol (80%) until they were decolorized except for the deep brown polymerization product produced from DAB in the presence of H2O2.
H2O2 was also detected using dihydrorhodamine 123 (Sigma, St. Louis, MO), which was added from a 2.5-mg/mL stock solution in ethanol to give a final concentration of 5 μg/mL in MS medium. Seedlings were incubated for 30 min at room temperature and viewed under a Zeiss Axioplan epifluorescence microscope (Carl Zeiss, Oberkochen, Germany) through a rhodamine optical filter. For the detection of autofluorescence, plants were viewed directly under an epifluorescence stereomicroscope (Leica, Wetzlar, Germany) with a green fluorescent protein filter set. For callose visualization, plants were infiltrated with 4% paraformaldehyde in PBS and fixed overnight at 4°C. Plants were rinsed once in PBS before staining in 0.5% aniline blue in PBS, pH 8.5. Stained plants were mounted onto glass slides and viewed with a 4′,6-diamidino-2-phenylindole filter set.
For viability staining, 0.5 μL 0.5% fluorescein diacetate solution was added to each 10-μL aliquot of BY-2 culture. Living cells accumulate green fluorescent fluorescein, and dead cells appear light blue because of autofluorescence.
Pro and P5C Determination
Pro content of 18-d-old Arabidopsis seedlings was determined according to Bates et al. (1973). The standard isolation protocol for amino acids proved to be unsuitable for P5C detection, thus a special assay had to be developed and was applied. Plant material was resuspended in 10 mL g-1 50 mM HCl and extracted in a Teflon-in-glass Potter homogenizer by 2 × 12 strokes. After centrifugation for 10 min at 18,000g, 10-mL aliquots of the supernatant were immediately loaded onto Dowex AG50 (200 to 400 mesh) columns (5 mL bed-volume) equilibrated with water. After extensive washing with 50 mM HCl, P5C was eluted with 1 M HCl, collecting 1.5-mL fractions. P5C and Pro content in the eluate were quantified with the o-aminobenzaldehyde and the ninhydrin assay method, respectively, as previously described (Williams, 1975).
14C Pro Feeding Studies
Rosette leaves from 4-week-old Arabidopsis plants were cut under 2.5 mM EDTA, pH 6, and fed through the petiole with 160 μM Pro solution (86 μM labeled Pro [U-14C Pro; Amersham Biosciences, Piscataway, NJ], 74 μM unlabeled Pro, and 60 mM sucrose in deionized water) for different periods of time. After incubation, the leaves were rinsed with distilled water, and the amino acids were analyzed using HPLC.
Determination of Amino Acids
One hundred milligrams of ground Arabidopsis material was extracted once in 0.5 mL of methanol/water (80% methanol [v/v], 3 min at 95°C) and the sediment once again in 0.5 mL of methanol/water (20% methanol [v/v], 3 min at 95°C) after centrifugation (3 min at 20000g). The supernatants were combined, and the liquid was evaporated to dryness in a speed vac. The sediment was redissolved in 150 μL of lithium diluent Li220 (Pickering Lab, Mountain View, CA), and 10 μL were separated by HPLC on a cation-exchange column on a Kontron HPLC system (Neufahrn, Germany). Single amino acids were detected and quantified photometrically at 440 and 570 nm after post-column derivatization with ninhydrin in a derivatization oven (Pickering).
Analysis of Organic Acids and Sugars
After sample preparation as for amino acid analysis, 10 μL of the sample were separated by HPLC on an Aminex HPX 87-H column (Bio-Rad, Hercules, CA) using 2 mM H2SO4 as eluant. The retention times of 14C-labeled compounds were monitored with an LB 507B radiodetector (Berthold, Wildbad, Germany).
Supplementary Material
Acknowledgments
We would like to thank Yvonne Sauermann for excellent technical assistance. We thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed Arabidopsis T-DNA insertion mutants, D. Weigel for providing immediate access to seed material, and Jane Parker, Volker Lipka, and P. Schulze-Lefert for providing eds1 and ndr1 and for technical advice on staining methods. The NahG mutant was a generous gift from Xinnian Dong, and the eds8 mutant was obtained from the Arabidopsis Stock Center. We are deeply indebted to Bettina Stadelhofer for assistance and advice for radiotracer and metabolite analyses. This work was supported by grants to W.B.F. from Deutsche Forschungsgemeinschaft (Fr989/4-3), the Gottfried Wilhelm Leibniz award to W.B.F., and by Bundesministerium für Bildung und Forschung in the framework of Genomanalyse im biologischen System Pflanze–GABI.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Wolf B. Frommer (wfrommer@stanford.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.023622.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. [DOI] [PubMed] [Google Scholar]
- Ayliffe, M.A., Roberts, J.K., Mitchell, H.J., Zhang, R., Lawrence, G.J., Ellis, J.G., and Pryor, T.J. (2002). A plant gene up-regulated at rust infection sites. Plant Physiol. 129, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, L.S., Waldren, R.P., and Teare, I.D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. [Google Scholar]
- Bonner, C.A., Williams, D.S., Aldrich, H.C., and Jensen, R.A. (1996). Antagonism by L-glutamine of toxicity and growth inhibition caused by other amino acids in suspension cultures of Nicotiana silvestris. Plant Sci. 113, 43–58. [Google Scholar]
- Bowling, S.A., Guo, A., Cao, H., Gordon, A.S., Klessig, D.F., and Dong, X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century, K., Shapiro, A., Repetti, P., Dahlbeck, D., Holub, E., and Staskawicz, B. (1997). NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science 278, 1963–1965. [DOI] [PubMed] [Google Scholar]
- Chiang, H.-H., and Dandekar, A. (1995). Regulation of proline accumulation in Arabidopsis thaliana (L.) Heynh during development and in response to desiccation. Plant Cell Environ. 18, 1280–1290. [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dashek, W., and Harwood, H. (1974). Proline, hydroxyproline and lily pollen tube elongation. Ann. Bot. 38, 947–959. [Google Scholar]
- Deblaere, R., Bytebier, B., De Greve, H., Deboeck, F., Schell, J., Van Montagu, M., and Leemans, J. (1985). Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 13, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle, K., Funck, D., Hellmann, H., Daeschner, K., Binder, S., and Frommer, W.B. (2001). A nuclear gene encoding mitochondrial Δ1-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 27, 345–356. [DOI] [PubMed] [Google Scholar]
- Donald, S.P., Sun, X.Y., Hu, C.A., Yu, J., Mei, J.M., Valle, D., and Phang, J.M. (2001). Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61, 1810–1815. [PubMed] [Google Scholar]
- Elthon, T.E., and Stewart, C.R. (1981). Submitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol. 67, 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabro, G., Kovacs, I., Pavet, V., Szabados, L., and Alvarez, M.E. (2004). Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact. 17, 343–350. [DOI] [PubMed] [Google Scholar]
- Falk, A., Feys, B., Frost, L., Jones, J., Daniels, M., and Parker, J. (1999). EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad Sci. USA 96, 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani, G., Scainelli, D., and Nielsen, E. (1997). Δ1-Pyrroline-5-carboxylate dehydrogenase from cultured cells of potato: Purification and properties. Plant Physiol. 113, 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade, G., and Auerswald, L. (2002). Beetles' choice–proline for energy output: Control by AKHs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 132, 117–129. [DOI] [PubMed] [Google Scholar]
- Geraghty, M.T., Vaughn, D., Nicholson, A.J., Lin, W.W., Jimenez-Sanchez, G., Obie, C., Flynn, M.P., Valle, D., and Hu C.A. (1998). Mutations in the Δ1-pyrroline 5-carboxylate dehydrogenase gene cause type II hyperprolinemia. Hum. Mol. Genet. 7, 1411–1415. [DOI] [PubMed] [Google Scholar]
- Hare, P.D., and Cress, W.A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21, 79–102. [Google Scholar]
- Hare, P.D., Cress, W.A., and van Staden, J. (1999). Proline synthesis and degradation: A model system for elucidating stress-realetd signal transduction. J. Exp. Bot. 50, 413–434. [Google Scholar]
- Hare, P.D., Cress, W.A., and van Staden, J. (2002). Disruptive effects of exogenous proline on chloroplast and mitochondrial ultrastructure in Arabidopsis leaves. S. Afr. J. Bot. 68, 393–396. [Google Scholar]
- Hare, P.D., Cress, W.A., and van Staden, J. (2003). A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul. 39, 41–50. [Google Scholar]
- Hayashi, F., Ichino, T., Osanai, M., and Wada, K. (2000). Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol. 41, 1096–1101. [DOI] [PubMed] [Google Scholar]
- Hellmann, H., Funck, D., Rentsch, D., and Frommer, W.B. (2000). Hypersensitivity of an arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol. 123, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Z., Lakkineni, K., Zhang, Z., and Verma, D.P. (2000). Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 122, 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C.A., Delauney, A.J., and Verma, D.P. (1992). A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 89, 9354–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, G.H., Damsz, B., Matsumoto, T.K., Reddy, M.P., Rus, A.M., Ibeas, J.I., Narasimhan, M.L., Bressan, R.A., and Hasegawa, P.M. (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 29, 649–659. [DOI] [PubMed] [Google Scholar]
- Katsuhara, M. (1997). Apoptosis-like cell death in barley roots under salt stress. Plant Cell Physiol. 38, 1091–1093. [Google Scholar]
- Kiyosue, T., Yoshiba, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1996). A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan, P.J., Young, J.C., and Sussman, M.R. (1999). T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach, H., Diamond, D., Wozney, J.M., and Boedtker, H. (1977). RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16, 4743–4751. [DOI] [PubMed] [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Mani, S., Van De Cotte, B., Van Montagu, M., and Verbruggen, N. (2002). Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol. 128, 73–83. [PMC free article] [PubMed] [Google Scholar]
- Martin, T., Wöhner, R.V., Hummel, S., Willmitzer, L., and Frommer, W.B. (1992). The GUS reporter system as a tool to study plant gene expression. In GUS Protocols, S. Gallagher, ed (Orlando, FL: Academic Press), pp. 23–43.
- Maxwell, S.A., and Davis, G.E. (2000). Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc. Natl. Acad. Sci. USA 97, 13009–13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, Y., Nakamori, S., and Takagi, H. (2002). Effect of proline and arginine metabolism on freezing stress of Saccharomyces cerevisiae. J. Biosci. Bioeng. 94, 390–394. [DOI] [PubMed] [Google Scholar]
- Nakashima, K., Satoh, R., Kiyosue, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1998). A gene encoding proline dehydrogenase is not only induced by proline and hypoosmolarity, but is also developmentally regulated in the reproductive organs of Arabidopsis. Plant Physiol. 118, 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjo, T., Fujita, M., Seki, M., Kato, T., Tabata, S., and Shinozaki, K. (2003). Toxicity of free proline revealed in an arabidopsis T-DNA-tagged mutant deficient in proline dehydrogenase. Plant Cell Physiol. 44, 541–548. [DOI] [PubMed] [Google Scholar]
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Sanada, Y., Wada, K., Tsukaya, H., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 18, 185–193. [DOI] [PubMed] [Google Scholar]
- Nomura, M., and Takagi, H. (2004). Role of the yeast acetyltransferase Mpr1 in oxidative stress: Regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc. Natl. Acad. Sci. USA 101, 12616–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z., Lu, Q., and Verma, D.P. (1996). Reciprocal regulation of Δ1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol. Gen. Genet. 253, 334–341. [DOI] [PubMed] [Google Scholar]
- Pesaresi, P., Gardner, N.A., Masiero, S., Dietzmann, A., Eichacker, L., Wickner, R., Salamini, F., and Leister, D. (2003). Cytoplasmic N-terminal protein acetylation is required for efficient photosynthesis in Arabidopsis. Plant Cell 15, 1817–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang, J.M. (1985). The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Cell. Regul. 25, 91–132. [DOI] [PubMed] [Google Scholar]
- Pilloff, R.K., Devadas, S.K., Enyedi, A., and Raina, R. (2002). The Arabidopsis gain-of-function mutant dll1 spontaneously develops lesions mimicking cell death associated with disease. Plant J. 30, 61–70. [DOI] [PubMed] [Google Scholar]
- Polyak, K., Xia, Y., Zweier, J.L., Kinzler, K.W., and Vogelstein, B. (1997). A model for p53-induced apoptosis. Nature 389, 300–305. [DOI] [PubMed] [Google Scholar]
- Rao, M.V., and Davis, K.R. (1999). Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 17, 603–614. [DOI] [PubMed] [Google Scholar]
- Rao, M.V., Lee, H., Creelman, R., Mullet, J., and Davis, K. (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12, 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richly, E., Dietzmann, A., Biehl, A., Kurth, J., Laloi, C., Apel, K., Salamini, F., and Leister, D. (2003). Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO Rep. 4, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, J.K., and Pryor, A. (1995). Isolation of a flax (Linum usitatissimum) gene induced during susceptible infection by flax rust (Melampsora lini). Plant J. 8, 1–8. [DOI] [PubMed] [Google Scholar]
- Schwacke, R., Grallath, S., Breitkreuz, K.E., Stransky, E., Stransky, H., Frommer, W.B., and Rentsch, D. (1999). LeProT1, a transporter for proline, glycine betaine, and gamma-amino butyric acid in tomato pollen. Plant Cell 11, 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, B.S., Belenghi, B., and Levine, A. (2002). Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 128, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen, N., Hua, X.-J., May, M., and Van Montagu, M. (1996). Environmental and development signals modulate proline homeostasis: Evidence for a negative transcriptional regulator. Proc. Natl. Acad. Sci. USA 93, 8787–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, I., and Frank, L. (1975). Improved chemical synthesis and enzymatic assay of Δ1-pyrroline-5-carboxylic acid. Anal. Biochem. 64, 85–97. [DOI] [PubMed] [Google Scholar]
- Xiang, C., Han, P., Lutziger, I., Wang, K., and Oliver, D.J. (1999). A mini binary vector series for plant transformation. Plant Mol. Biol. 40, 711–717. [DOI] [PubMed] [Google Scholar]
- Yoon, K.A., Nakamura, Y., and Arakawa, H. (2004). Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J. Hum. Genet. 49, 134–140. [DOI] [PubMed] [Google Scholar]
- Young, T.E., and Gallie, D.R. (2000). Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol. Biol. 42, 397–414. [DOI] [PubMed] [Google Scholar]
- Zhang, C.S., Lu, Q., and Verma, D.P. (1995). Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J. Biol. Chem. 270, 20491–20496. [DOI] [PubMed] [Google Scholar]
- Zhang, H.Q., Croes, A., and Linskens, H. (1982). Protein synthesis in germinating pollen of Petunia: Role of proline. Planta 154, 199–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.