Abstract
Objective
The purpose of the current study was to compare the donor age variation of chondrocytes from non-OA (osteoarthritic) trauma joints in patients of young to middle age (20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years) embedded in matrix-associated autologous chondrocyte transplantation (MACT) grafts (CaReS). The chondrocyte-specific gene expression of CaReS grafts were then compared to chondrocytes from OA joints (in patients aged 63.8 ± 10 years) embedded in a collagen type I hydrogel.
Design
OA chondrocytes and articular chondrocyte-laden grafts were cultured over 14 days in chondrogenic growth medium. We performed reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) to evaluate the mRNA expression levels of chondrocyte-specific and hypertrophic markers.
Results
Gene expression analysis with RT-qPCR revealed no significant difference in chondrocyte-specific genes (COL2A1, ACAN, SOX9, SOX5, SOX6) among 3 different age group of patients with CaReS grafts. In a comparative analysis of OA chondrocytes to articular chondrocytes, chondrogenic markers (COL2A1, SOX6) exhibited higher expression in OA chondrocytes (P < 0.05). Hypertrophic or OA cartilage pathogenesis marker (MMP3, MMP13) expression was higher and COL1A1 had significantly lower expression (P < 0.05) in OA chondrocytes than articular chondrocytes when cultivated in collagen type I hydrogels.
Conclusion
In summary, we identify that donor age variation does not influence the chondrogenic gene expression of the CaReS system. We also identified that freshly isolated OA chondrocytes embedded in collagen type I hydrogels can exhibit chondrogenic gene expression as observed in articular chondrocytes on the CaReS grafts. Transforming OA chondrocytes to articular chondrocytes can be regarded as an alternative option in the MACT technique.
Keywords: cartilage, chondrocytes, osteoarthritis, hydrogel, gene expression
Introduction
Articular cartilage is an avascular tissue that is limited by its poor healing capacity to restore tissue damage after sports injuries or trauma, ultimately ensuring an instability and loss of mobility in the joint tissue leading to osteoarthritis. Recent advances made in addressing the surgical intervention for repair of cartilage defects emphasize on healing the diseased fibrocartilaginous tissue to a healthy hyaline cartilaginous tissue. Collective strategies developed are either implantation of autologous cells that are cultivated in vitro or embedding cells into a chondro-inductive biomaterial and implanted directly at the site of defect.1-3 The prerequisite for the treatment of articular cartilage defects with chondrocyte implantation requires an excision biopsy from a healthy site of cartilaginous tissue from the same patient. The most challenging part when expanding the autologous articular chondrocytes is to overcome the state of dedifferentiation that occurs during in vitro expansion onto cell culture substrates. Chondrocyte dedifferentiation is primarily observed by the morphological transition of the cell from spherical to fibroblast-like structure, loss of collagen II and proteoglycans, and the transition to produce more collagen I and fibronectin.4-6 The dedifferentiation has to be reversed either by selection of appropriate substrates or culturing them on fibrous hydrogels or intact polymer scaffolds,7,8 supporting the cells to maintain their native spherical morphology.
Matrix-associated autologous chondrocyte transplantation (MACT) is a technique that employs a 2-step surgical intervention, with autologous chondrocytes being isolated from a biopsy at the non–load-bearing site of the cartilaginous tissue. The cells are expanded in a monolayer culture in vitro for up to 4 weeks and embedded onto the MACT scaffold and implanted at the site of cartilage defect. Commonly used MACT scaffolds for clinical implantation involve hyaluronic acid (Hyalograft C), type I collagen (Neocart, CaReS), collagen-chondroitin sulfate (Novocart), fibrin (Chondron), and agarose/alginate (Cartipatch).9-14 During the MACT procedure, combining the preliminary chondrocyte density with defined biomaterials such as collagen type I has been shown to improve the consistency of the initial cell density and eventually resulting in a composite cartilage matrix formation.15 Distinctive from other MACT scaffolds, CaReS (Arthro Kinetics Biotechnology GmbH, Krems, Austria) involves the strategy of embedding patients cells directly after isolation in the collagen gel graft system without further expansion on 2D substrates, which is imperative to impede the dedifferentiation process.
In this study, we compared the chondrogenic markers in terms of matrix production (COL2A1, COL1A1, ACAN), transcription factors (SOX9, SOX5, SOX6), and matrix metalloproteinases (MMP3, MMP13) among 3 different age groups of patients undergoing the MACT (CaReS) implantation procedure. We also report the differences in chondrogenic-specific gene expression of chondrocytes from osteoarthritic (OA) patients having collagen type I scaffolds, to articular chondrocytes currently used for the MACT procedure (CaReS).
Materials and Methods
Ethics Statement
Human articular chondrocytes were obtained from 25 patients (5 females, 20 males; 20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years) undergoing MACT (CaReS) treatment. Osteoarthritic human articular cartilage was obtained from knee joints of 9 patients (4 females, 5 males; 63.8 ± 10 years) undergoing total knee arthroplasty. All patients provided informed consent for the use of chondrocytes. The local ethical committee approved all experiments in this study (Approval No. GS4-EK-4/130-2011).
MACT Graft Specimens: CaReS
MACT grafts (CaReS) were prepared and provided by the cooperation partner organization (Arthro Kinetics Biotechnology GmbH) as a 2-step surgical procedure. The articular cartilage biopsy was acquired arthroscopically from the healthy site of the knee joint without any further expansion in a monolayer culture. On average 6 × 104 cells from 25 patient biopsies were embedded directly into the collagen type I hydrogel and cultivated for 14 days in autologous serum. Post 14 days of culture, the cell yield from the MACT grafts yielded on average about 2.3 × 106 cells/graft.
Human Osteoarthritic Chondrocyte Isolation
Human OA chondrocytes were isolated from articular cartilage of OA knee joints. The cartilage was minced into 2 mm3 pieces prior to enzymatic digestion with Liberase TM (0.2 WU/mL, Roche Diagnostics GmbH, Mannheim, Germany) in medium (GIBCO DMEM/F12 GlutaMAX-I; Invitrogen, LifeTech Austria, Vienna, Austria) containing antibiotics (penicillin 200 U/mL; streptomycin 0.2 mg/mL) and amphotericin B (2.5 µg/mL) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) under continuous agitation for 18 to 22 hours at 37°C. The resulting chondrocyte cell suspension was passed through a cell strainer with 40 µm pore size (BD, Franklin Lakes, NJ) to remove undigested debris. Cells were washed with phosphate-buffered saline (PBS), centrifuged (10 minutes, 500g, room temperature [RT]) and resuspended in growth medium (i.e., medium supplemented with antibiotics [see above], 10% FCS [GIBCO, Invitrogen, LifeTech Austria] and 0.05 mg/mL ascorbic acid (Sigma-Aldrich Chemie GmbH]). Viability was determined via trypan blue (Sigma-Aldrich Chemie GmbH) dye exclusion and cells were counted using a hemocytometer.
Preparation of Collagen Type I Hydrogels with OA Chondrocytes
Cells were embedded directly in rat-tail collagen type I hydrogel (6 mg/mL; Arthro Kinetics) as used in the MACT grafts (CaReS) at a concentration of 3 mg/mL with 1 × 105 cells/graft (6.2 cm3). The cell seeding densities were in equal proportion to the MACT (CaReS) transplants. Collagen type I hydrogel was casted similar to the MACT (CaReS) transplants. Briefly, FCS was diluted in a 1:4 dilution with 2× gel-neutralization buffer and encapsulated with OA chondrocytes as a mixture. In the next step, collagen type I hydrogel was added to the mixture in a 1:1 ratio and resuspended carefully. Cell-hydrogel constructs were cast at 4°C to prevent instantaneous polymerization. The cell-hydrogel constructs were then let to polymerize at 37°C, 5% CO2 for 30 minutes. The resulting grafts were cultivated in growth medium with 10% FCS and 0.05 mg/mL of ascorbic acid for 14 days. The medium was changed every 3 days.
RNA Isolation and Reverse Transcription
The experimental design was divided into 2 categories. In category 1 a division of 3 different age groups was compared (20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years). In category 2, chondrocytes from 9 MACT patients (41.9 ± 4.1 years) was compared to chondrocytes from 9 OA patients (63.8 ± 10 years). After 14 days of cultivation, MACT (CaReS) transplants and OA chondrocytes in collagen type I hydrogels were digested with collagenase II (120 U/mL) (Sigma-Aldrich Chemie GmbH) at 37°C for 1 to 2 hours. The cell suspension was centrifuged (5 minutes, 500g, RT) and total RNA was extracted from 1 × 106 cells per sample using high pure RNA Isolation Kit (Roche Diagnostics GmbH) according to manufacturer’s instructions. Complementary DNA (cDNA) was synthesized with the first strand cDNA synthesis kit for reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) (Roche Diagnostics GmbH) and Random Primer p(dN)6 according to the manufacturer’s instruction. The resulting cDNA was stored at −20°C until further usage.
Real-Time Reverse Transcriptase Quantitative PCR
To determine the chondrocyte-specific gene expression, the magnitude of mRNA was analyzed with RT-qPCR for collagen type II (COL2A1), collagen type I (COL1A1), aggrecan (ACAN), SOX9, SOX5, SOX6, matrix metalloproteinase 3 (MMP3), matrix metalloproteinase 13 (MMP13), from chondrocytes cultivated in MACT (CaReS) grafts and OA chondrocytes in collagen type I hydrogels. Probes and primers were selected with the Universal Probe Library System (Roche) and by performing in silico PCR. Optimum conditions and annealing temperature were determined experimentally. cDNA was amplified with the FastStart TaqMan Probe Master (Roche Diagnostics GmbH) with gene-specific primers (Eurofins MWG Synthesis GmbH, Ebersberg, Germany) in triplicates using the iCycler iQ (Bio-Rad Laboratories, Hercules, CA). Target cycle threshold (CT) values were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and compared to the corresponding sample using the comparative CT (ΔΔCT) method without efficiency correction (see Table 1 ).
Table 1.
Sequences of Primers and Conditions Used in Reverse Transcriptase Quantitative Polymerase Chain Reaction.
| Target Gene | Primer Forward | Primer Reverse |
|---|---|---|
| GAPDH | CTCTGCTCCTCCTGTTCGAC | ACGACCAAATCCGTTGACTC |
| ACAN | CCTCCCCTTCACGTGTAAAA | GCTCCGCTTCTGTAGTCTGC |
| COL2A1 | GTGTCAGGGCCAGGATGT | TCCCAGTGTCACAGACACAGAT |
| COL1A1 | GGGATTCCCTGGACCTAAAG | GGAACACCTCGCTCTCCAG |
| SOX9 | TACCCGCACTTGCACAAC | TCTCGCTCTCGTTCAGAAGTC |
| SOX5 | TTTACCTCAGGAGTTTGAAAGGA | GCTTGTCACCATGGCTACCT |
| SOX6 | TCAAAGGCAATTTACCAGTGATT | GGCTTGCTTGGAAGACATTC |
| MMP3 | CAAAACATATTTCTTTGTAGAGGACAA | TTCAGETATTCGCTTGGGAAA |
| MMP13 | TTTCCTCCTGGGCCAAAT | GCAACAAGAAACAAGTTGTAGCC |
Statistical Analysis
Statistical analysis was performed using the GraphPad PRISM (GraphPad Software, Inc., La Jolla, CA). All data are expressed as mean ± standard deviation. Significance between different age group subjects of MACT chondrocytes was analyzed with a nonparametric Kruskal-Wallis test and Dunn’s multiple comparison test. Significance between MACT and OA chondrocytes was compared using an unpaired nonparametric Mann-Whitney U test. P values <0.05 was considered significant.
Results
Analysis of Gene Expression between Age Groups of MACT (CaReS) Chondrocytes
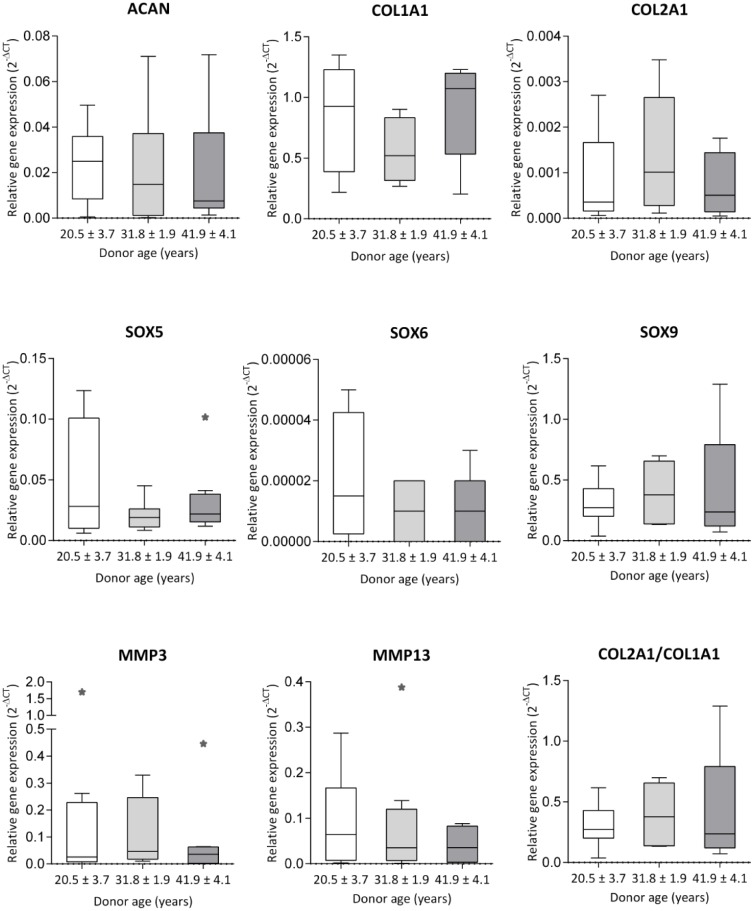
Gene expression evaluated in MACT (CaReS) chondrocytes among different age groups of patients (20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years) revealed a high variance of COL1A1 expression with no significant differences among the groups. Considerably lower expression of ACAN and COL2A1 expression was observed with no significant differences ( Fig. 1 ). Similarly, chondrogenic redifferentiation specific transcription factors SOX9, SOX5, SOX6 expressed lower mRNA among all the groups with no difference. MMP3 and MMP13 were observed in all age group subjects with lower expression with no significant difference.
Figure 1.
RT-qPCR analysis of chondrogenic gene expression in chondrocytes from MACT (CaReS) grafts. Chondrocytes were isolated from the healthy site of the articular cartilage in patients for 3 different age group subjects (20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years) (n = 8-9 per group) and cultivated in MACT (CaReS) grafts for 14 days. Gene expression of MACT cells at different ages were normalized to their respective GAPDH expression levels. Data expressed as median and range with Tukey box and whisker plot: lower box = 75 percentile, upper box = 25 percentile, whisker = non-outlier range, dot = outlier, asterisk = extreme outlier (>1.5-fold above/below the box).
Analysis of Gene Expression in Chondrocytes From MACT (CaReS) and OA Grafts
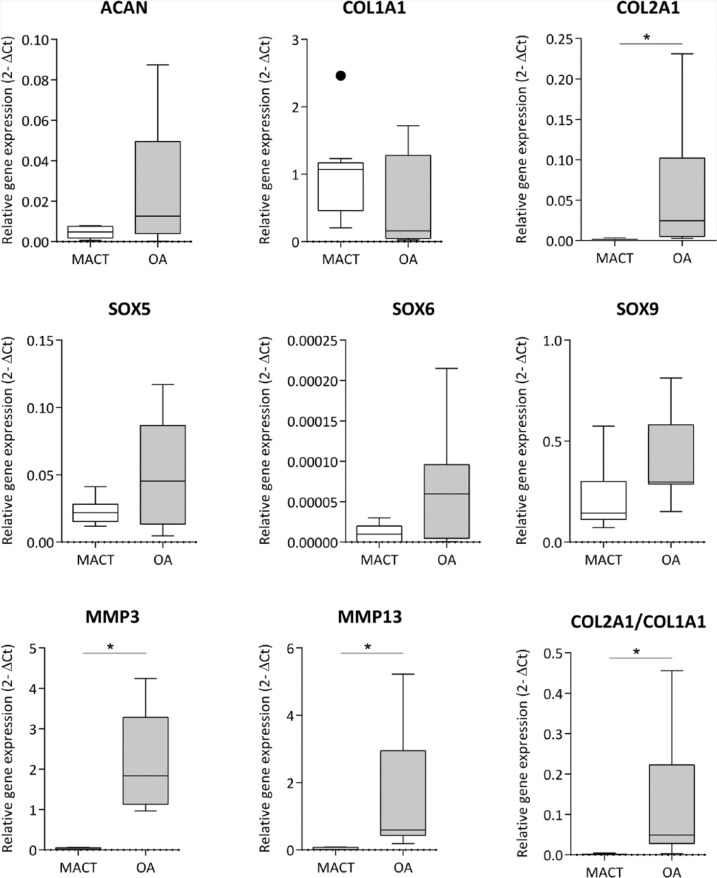
Remarkably, higher expression of COL2A1 was observed in OA chondrocytes with a significant difference (P < 0.001) than in the MACT chondrocytes, correlating to the lower expression of COL1A in OA chondrocytes (P = 0.01658). Similar results were observed in the differentiation index of COL2A1/COL1A1 ratio with a significant difference (P < 0.001). Comparably, expression of ACAN mRNA levels had a high variance in OA chondrocytes in comparison to MACT chondrocytes with no significant differences. Chondrogenic redifferentiation specific transcription factors SOX9, SOX5 had an equal expression in OA chondrocytes as observed in the MACT chondrocytes. SOX6 expression was higher in OA chondrocytes than MACT chondrocytes with a significant difference (P = 0.0397). MMP3 and MMP13 exhibited a higher expression in OA chondrocytes with significant difference (P < 0.001) than in the MACT chondrocytes ( Fig. 2 ).
Figure 2.
RT-qPCR analysis of chondrogenic gene expression in both MACT (CaReS) and OA grafts. Gene expression levels of MACT and OA cells at the different ages were normalized to their respective GAPDH expression levels. MACT represents articular chondrocytes isolated from MACT grafts (CaReS) of age group subjects (41.9 ± 4.1) (n = 9) at day 14. OA represents osteoarthritic chondrocytes isolated from collagen type I hydrogels age group subjects (63.8 ± 10) (n = 9) at day 14. Data expressed as median and range with Tukey box and whisker plot: lower box = 75 percentile, upper box = 25 percentile, whisker = non-outlier range, dot = outlier, asterisk = extreme outlier (>1.5-fold above/below the box).
Discussion
Patient age regularly has been reported to be a major factor determining the changes in the molecular organization, mechanical properties, cell yield, and redifferentiation potential of the articular cartilage tissue structure.16-18 To determine the possible influence of aging in isolated articular chondrocytes when embedded onto MACT (CaReS) implants, we compared the chondrogenic specific gene expression among 3 different subjects of age groups (20.5 ± 3.7, 31.8 ± 1.9, 41.9 ± 4.1 years). We clearly noted no significant differences in variation in the mRNA expression levels of redifferentiation markers COL2A1, ACAN; the transcription factors SOX5, SOX6, SOX9; and dedifferentiation marker COL1A1, MMP3, MMP13 in all the age groups. A recent study demonstrated that donor age was not an influencing factor when OA chondrocytes from a 78-year-old patient exhibited better tissue formation with a low cell seeding density than younger donors aged 50 to 68 years when used in the autologous chondrocyte transplantation technique.19 Our results indicate that the patient age by itself does not influence the overall chondrogenic gene expression during preimplantation of the articular chondrocytes in MACT (CaReS) grafts. However, the outcomes from the post follow-up study of these implants received in diverse age groups of patients could further support the observed data notably. It should be taken into account that the chondrogenic gene expression would differ in a different hydrogel-based scaffold.
During developmental chondrogenesis, SOX9 (Sry-type high mobility group [HMG] box 9) concomitantly binds to the sequence along the COL2A1 enhancer, the gene encoding type II collagen, an important structural component of cartilage.20 We observed that COL2A1 expression was higher in OA chondrocytes from collagen type I hydrogels than the articular chondrocytes with a significant difference analogous to expression levels of SOX6 mRNA in OA chondrocytes. SOX9 exhibited similar expression in both the cell types and no direct correlation to COL2A1 could be indicated. SOX5 and SOX6 cooperatively combine with SOX9 activating COL2A1, leading to a higher expression in OA chondrocytes.21 In another study, it has been identified that low expression of SOX9 does not correlate with the higher expression of COL2A1 in OA chondrocytes.22 Matrix metalloproteinase 3 (stromelysin) has been reported to activate the collagenase-I MMP1, which in turn cleaves a broad range of proteins inside the cartilage tissue matrix.23 Similarly, the collagenase-III MMP13 is commonly observed during the developmental phase of chondrogenesis and in the OA tissue environment.24,25 This phenomenon was observed in our study when OA chondrocytes were embedded in collagen type I scaffolds. MMP3 and MMP13 exhibited a higher mRNA expression with a significant difference in comparison to the healthy articular chondrocytes in the MACT (CaReS) scaffolds. Several studies have reported that this phenomenon of higher expression in MMPs under pathological conditions like osteoarthritis leads to cartilage degradation26 and a lower expression of MMPs in a cartilaginous healthy tissue supporting tissue remodeling.27 The marker for a dedifferentiated phenotype in chondrocytes, COL1A1, exhibited a higher expression in articular chondrocytes from the MACT than in the OA chondrocytes significantly. We assume that the redifferentiation potential of the articular chondrocytes in MACT (CaReS) will be enhanced once the transplant receiving patients, post recovery, initiate to perform under normal physical conditions. This can be further supported by our recent study, where chondrocytes from OA patients embedded in collagen type I hydrogels under compressive mechanical loading stimulation regimes redifferentiated from catabolic to anabolic phenotype.28 Our current study limits to the assessment of the chondrogenic redifferentiation markers and a few hypertrophic markers. Hypertrophic markers like RUNX2, ALP, and COL10A1 could have further supported the arguments to the redifferentiation potential of the OA chondrocytes in a collagen type I hydrogel. Similarly, the significance of no differences in gene expression levels at different age group subjects of the MACT grafts (CaReS) could have been correlated with the protein levels, to strengthen further this observation, and it has to be taken into consideration that it might influence the results based only on mRNA transcript levels without correlating to the levels of functional protein.
OA chondrocytes have been shown to express increased levels of COL2A1 while retaining the expression throughout the in vitro culture period starting from 7 to 21 days in comparison to the chondrogenic differentiation of bone marrow derived MSCs.29 Similarly, when OA chondrocytes were long cultured over 6 months the proliferation of cells decreased over time but without reaching the senescence phase of growth and no alterations were found in the DNA levels indicating the genetic stability and a positive correlation was observed among several mismatch repair (MMR) genes.30 Tallheden et al. performed a similar study where OA chondrocytes exhibited an increase in redifferentiation that occurred simultaneously with increase in proliferation in a 3-dimensional pellet culture model. The accumulation of proteoglycans and type II collagen matrix was comparable to ACT donors, whereas total collagen was significantly lower in redifferentiated OA chondrocytes.31 The OA chondrocytes further being loaded into a hyaluronic acid based scaffold produced cartilage-specific genes as well. This indicates that autologous chondrocytes from OA patients are a potential source for treatment but the limited collagen synthesis needs to be further addressed. Especially the poor collagen production is of importance to note, as collagen is the major factor for the stability of the construct.
In conclusion, our present study identifies that donor age is not an influence of chondrogenic gene expression for healthy articular chondrocytes embedded in CaReS cell seeded grafts. Freshly isolated chondrocytes from OA patients could be a source of a replacement for articular chondrocytes being commonly used in MACT (CaReS) and have positive implications for patients suffering from an early onset of osteoarthritis.
Footnotes
Acknowledgments and Funding: The authors acknowledge Arthrokinetics Biotechnology GmbH, Austria, for providing the MACT (CaReS) grafts. This work was funded by the Life Science Call (LS-09.009), and the project was administered by the Lower Austria Research & Education Agency (NFB).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Danube University Krems ethical committee approved all experiments in this study (Approval No. GS4-EK-4/130-2011).
Informed Consent: All patients provided written informed consent for the use of chondrocytes.
References
- 1. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14), 889-95. [DOI] [PubMed] [Google Scholar]
- 2. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640-5. [DOI] [PubMed] [Google Scholar]
- 3. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13(3):194-202. [DOI] [PubMed] [Google Scholar]
- 4. von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267(5611):531-2. [DOI] [PubMed] [Google Scholar]
- 5. Marlovits S, Hombauer M, Truppe M, Vècsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Joint Surg Br. 2004;86(2):286-95. [DOI] [PubMed] [Google Scholar]
- 6. Dessau W, Sasse J, Timpl R, Jilek F, von der Mark K. Synthesis and extracellular deposition of fibronectin in chondrocyte cultures. Response to the removal of extracellular cartilage matrix. J Cell Biol. 1978;79(2 pt 1):342-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12(7):1775-85. [DOI] [PubMed] [Google Scholar]
- 8. Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67(4):1105-14. [DOI] [PubMed] [Google Scholar]
- 9. Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57(1):3-8. [DOI] [PubMed] [Google Scholar]
- 10. Crawford DC, Heveran CM, Cannon WD, Jr, Foo LF, Potter HG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009;37(7):1334-43. [DOI] [PubMed] [Google Scholar]
- 11. Schneider U, Rackwitz L, Andereya S, Siebenlist S, Fensky F, Reichert J, et al. A prospective multicenter study on the outcome of type i collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med. 2011;39(12):2558-65. [DOI] [PubMed] [Google Scholar]
- 12. Panagopoulos A, van Niekerk L, Triantafillopoulos I. Autologous chondrocyte implantation for knee cartilage injuries: moderate functional outcome and performance in patients with high-impact activities. Orthopedics. 2012;35(1):e6-14. [DOI] [PubMed] [Google Scholar]
- 13. Kim MK, Choi SW, Kim SR, Oh IS, Won MH. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):528-34. [DOI] [PubMed] [Google Scholar]
- 14. Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90(5):597-604. [DOI] [PubMed] [Google Scholar]
- 15. Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668-75. [DOI] [PubMed] [Google Scholar]
- 16. Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through. Arthritis Rheum. 2002;46(1):114-23. [DOI] [PubMed] [Google Scholar]
- 17. Verbruggen G, Cornelissen M, Almqvist KF, Wang L, Elewaut D, Broddelez C, et al. Influence of aging on the synthesis and morphology of the aggrecans synthesized by differentiated human articular chondrocytes. Osteoarthritis Cartilage. 2000;8(3):170-9. [DOI] [PubMed] [Google Scholar]
- 18. Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: molecular and cellular aspects. Matrix Biol. 2002;21(5):449-59. [DOI] [PubMed] [Google Scholar]
- 19. Stoop R, Albrecht D, Gaissmaier C, Fritz J, Felka T, Rudert M, et al. Comparison of marker gene expression in chondrocytes from patients receiving autologous chondrocyte transplantation versus osteoarthritis patients. Arthritis Res Ther. 2007;9(3):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16(2):174-8. [DOI] [PubMed] [Google Scholar]
- 21. Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561-73. [DOI] [PubMed] [Google Scholar]
- 22. Aigner T, Gebhard PM, Schmid E, Bau B, Harley V, Pöschl E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003;22(4):363-72. [DOI] [PubMed] [Google Scholar]
- 23. Saito S, Katoh M, Masumoto M, Matsumoto S, Masuho Y. Involvement of MMP-1 and MMP-3 in collagen degradation induced by IL-1 in rabbit cartilage explant culture. Life Sci. 1998;62(22):359-65. [DOI] [PubMed] [Google Scholar]
- 24. Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271(38):23577-81. [DOI] [PubMed] [Google Scholar]
- 25. Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roach HI, Yamada N, Cheung KS, Tilley S, Clarke NM, Oreffo RO, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52(10):3110-24. [DOI] [PubMed] [Google Scholar]
- 27. Gepstein A, Shapiro S, Arbel G, Lahat N, Livne E. Expression of matrix metalloproteinases in articular cartilage of temporomandibular and knee joints of mice during growth, maturation, and aging. Arthritis Rheum. 2002;46(12):3240-50. [DOI] [PubMed] [Google Scholar]
- 28. Halbwirth F, Niculescu-Morzsa E, Zwickl H, Bauer C, Nehrer S. Mechanostimulation changes the catabolic phenotype of human dedifferentiated osteoarthritic chondrocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):104-11. [DOI] [PubMed] [Google Scholar]
- 29. Fernandes AM, Herlofsen SR, Karlsen TA, Küchler AM, Fløisand Y, Brinchmann JE. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS One. 2013;8(5):e62994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neri S, Mariani E, Cattini L, Facchini A. Long-term in vitro expansion of osteoarthritic human articular chondrocytes do not alter genetic stability: a microsatellite instability analysis. J Cell Physiol. 2011;226(10):2579-85. [DOI] [PubMed] [Google Scholar]
- 31. Tallheden T, Bengtsson C, Brantsing C, Sjögren-Jansson E, Carlsson L, Peterson L, et al. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7(3):R560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]