Abstract
Patient: Female, 51
Final Diagnosis: Fecal incontinence
Symptoms: Constipation • fecal incontinence
Medication: —
Clinical Procedure: Sacral nerve stimulator
Specialty: Gastroenterology and Hepatology
Objective:
Rare co-existance of disease or pathology
Background:
Fecal incontinence and constipation are common gastrointestinal complaints, but rarely occur concurrently. Management of these seemingly paradoxical processes is challenging, as treatment of one symptom may exacerbate the other.
Case Report:
A 51-year-old female with lifelong neurogenic bladder secondary to spina bifida occulta presented with progressive symptoms of daily urge fecal incontinence as well as hard bowel movements associated with straining and a sensation of incomplete evacuation requiring manual disimpaction. Pelvic floor testing showed poor ability to squeeze the anal sphincter, which indicated sphincter weakness as a major contributor to her fecal incontinence symptoms. Additionally, on defecography she was unable to widen her posterior anorectal angle or relax the anal sphincter during defecation consistent with dyssynergic defecation. A sacral nerve stimulator was placed for management of her fecal incontinence. Interestingly, her constipation also dramatically improved with sacral neuromodulation.
Conclusions:
This unique case highlights the emerging role of sacral nerve stimulation in the treatment of complex pelvic floor dysfunction with improvement in symptoms beyond fecal incontinence in a patient with dyssynergic-type constipation.
MeSH Keywords: Constipation, Fecal Incontinence, Spina Bifida Occulta
Background
Fecal incontinence (FI), the unintentional loss of solid or liquid stool, can be further classified as urge, which occurs despite active efforts to retain stool or passively occurring with little or no forewarning [1]. Additionally, some patients may complain of fecal seepage, which is defined as leakage following a bowel movement. The internal anal sphincter provides much of the involuntary (resting) tone, while the external anal sphincter (EAS) and puborectalis complex provide the majority of the voluntary squeeze tone. Management initially involves use of medications to improve stool consistency and Kegel exercises. Patients who do not respond may further benefit from biofeedback, which is a minimally invasive therapy that provides visual feedback aimed at re-education and strengthening of pelvic floor muscles [2]. Constipation is usually characterized as fewer than three weekly bowel movements, passage of lumpy or hard stools, sensation of incomplete evacuation and/or anorectal blockage, straining excessively, or manual maneuvers to facilitate defecation. Approximately 23% of patients with constipation have dyssynergic defecation (DD), which is defined as inadequate relaxation of the anal sphincters and pelvic floor during defecation [3]. Treatment typically involves use of biofeedback targeted at retraining the pelvic floor to relax and is effective in 72% of patient completing therapy [4–7]. FI and DD patients who are refractory to medications and pelvic floor biofeedback pose a significant dilemma to practitioners, but a promising emerging therapy, sacral neuromodulation (SNS), has shown significant benefit in patients with FI and may offer hope for patients with DD.
Case Report
A 51-year-old G1P1 Caucasian female with lifelong neurogenic bladder secondary to spina bifida occulta was referred for symptoms of constipation and (FI). She averaged one Bristol Type 1–2 stool every 5 days requiring frequent manual disimpaction. Additionally, she reported twice weekly episodes of urgent fecal seepage, which required the use of daily continence pads. Her symptoms did not improve with the addition of psyllium and bisacodyl suppositories. A defecography suggested atrophy of the puborectalis and poor squeeze with EAS muscle atrophy. Anorectal manometry (ARM) showed a normal resting pressure with no augmentation of squeeze pressure, consistent with weak EAS (Figure 1). During bearing down, fixed perineal descent was noted with the inability to widen the posterior anorectal angle and poor evacuation of contrast with straining, consistent with DD. With pushing, ARM similarly demonstrated type IV DD, which is classified as inability to generate adequate propulsive forces along with absent or incomplete relaxation of the anal sphincter [8] (Figure 2). Reflex and sensory testing indicated an intact rectoanal inhibitory reflex and rectal hypersensitivity. The patient failed management with home and conventional biofeedback therapy. Following a successful trial of temporary SNS with improvement in FI symptoms by 75%, the patient had a permanent SNS placed. One year later, the patient reports sustained improvement in constipation and FI symptoms.
Figure 1.

Resting and squeeze: defecography and ARM showing complementary findings of normal sphincter integrity and resting tone, suggestive of intact internal anal sphincter function. Additionally, during voluntary squeeze there was inability to contract the sphincter or augment sphincter pressure suggestive of external anal sphincter weakness.
Figure 2.
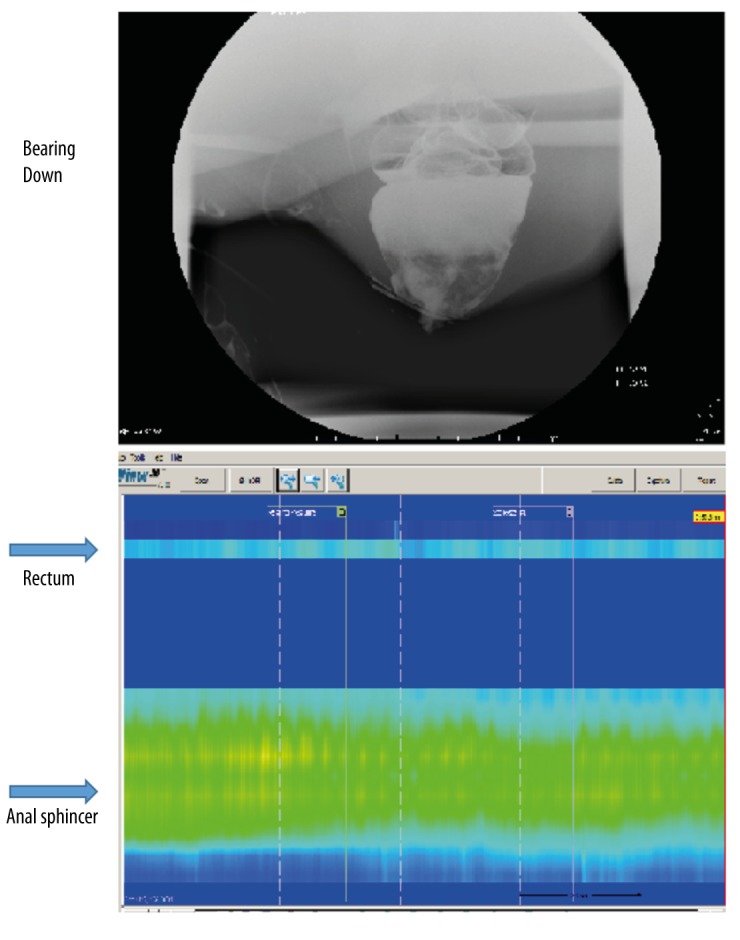
Bearing Down: no significant evacuation of contrast or opening of anorectal angle on defecography. On ARM, there was no increase in rectal pressure, nor was there relaxation of the anal sphincter as compared to resting.
Discussion
Spina bifida is a birth defect leading to incomplete closure of the backbone and membranes surrounding the spinal cord. Spina bifida occulta, a milder subtype affecting up to 20% of healthy individuals, can be associated with a tethered cord. This causes abnormal stretching of the spinal cord over time resulting in neurological symptoms like bowel or bladder dysfunction [9]. Initially, conservative treatment is recommended for FI and/or DD, including dietary advice, medications, and biofeedback. SNS is indicated for FI management when conservative therapy fails. This involves stimulation of the anterior ramus of S3 or S4, which is thought to provoke pelvic afferent and central pathways. This patient presented with overlapping complaints of constipation, fecal urgency, and seepage. Her constipation symptoms were consistent with an evacuation disorder, which was attributable to her inability to relax her puborectalis muscle during defecation. Non-relaxation of the puborectalis with pushing prevents opening of the anorectal angle because the puborectalis is the strap muscle which supports the rectum. The patient’s fecal urgency and seepage may have been related to a combination of findings noted on her defecography and ARM, including rectal hypersensitivity, external anal sphincter weakness, and overflow related to her DD.
Up to 89% of FI patients who undergo permanent SNS following a successful temporary trial will report long-term symptom improvement [10]. There is no apparent correlation of response to SNS with age, symptom duration, BMI, type of FI (passive, urge, seepage), or ARM findings; however, the greater the symptom improvement during the temporary trial, the more likely the patient is to maintain long-term benefits [11]. Prior to the temporary SNS trial, our patient kept a bowel diary, which was used to determine symptom improvement during the temporary trial known as percutaneous nerve evaluation (PNE). PNE involves an office procedure or operating room procedure where flexible leads are placed in the S3 or S4 foramen and connected to an externally controlled device and left in place for one to four weeks. Following the temporary trial, a permanent SNS that can be implanted underneath the skin may be offered to patients who have >50% improvement in FI.
A few case series of patients with FI have shown increased retrograde movement and contractility during defecation on colon scintigraphy and colonic manometry, which suggest a possible role for SNS in the treatment of constipation [12,13]. Additionally, SNS has been shown to increase anterograde and high amplitude pressure sequences on colonic manometry in eight patients [14]. Unfortunately, SNS has not been shown to consistently benefit patients suffering from constipation in clinical trials [15]. Large trials looking at subsets of constipation, including slow-transit and DD, have not yet been done.
This unique case illustrates constipation symptom improvement with SNS in a patient with the inability to adequately relax her pelvis during defecation, who interestingly also suffered from weakness of the sphincter during squeeze, which contributed to her FI.
Conclusions
This case illustrates the uncommon occurrence of overlapping DD and FI with paradoxical inadequate pelvic relaxation and sphincter weakness. While SNS is increasingly being used in patients with refractory FI, its role in treatment of refractory constipation is unclear. This case suggests that SNS may have benefit in the dyssynergic subtype of constipation. Further studies evaluating SNS in this specific subset of constipation sufferers are needed.
Acknowledgments
The authors would like to graciously thank Indiana University School of Medicine Faculty Dr. Dean Maglinte for his radiologic interpretation and Dr. Bruce Robb for his surgical expertise and help with management of this patient.
Footnotes
Statement
The authors declare no relevant conflicts of interests.
References:
- 1.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: Management of benign anorectal disorders. Am J Gastroenterol. 2014;109(8):1141–57. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 2.Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–37. doi: 10.1007/DCR.0b013e3181b55455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130(5):1510–18. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130(3):657–64. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Boo SJ, Jung KW, et al. Long-term efficacy of biofeedback therapy in patients with dyssynergic defecation: Results of a median 44 months follow-up. Neurogastroenterol Motil. 2015;27(6):787–95. doi: 10.1111/nmo.12552. [DOI] [PubMed] [Google Scholar]
- 6.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5(3):331–28. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Rao SS, Valestin J, Brown CK, et al. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized control trial. Am J Gastroenterol. 2010;105(4):890–96. doi: 10.1038/ajg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am. 2008;37(3):569–86. doi: 10.1016/j.gtc.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus CJ, Wood HM. Congenital causes of neurogenic bladder and the transition to adult care. Transl Androl Urol. 2016;5(1):39–50. doi: 10.3978/j.issn.2223-4683.2015.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hull T, Giese C, Wexner SD, et al. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–45. doi: 10.1097/DCR.0b013e318276b24c. [DOI] [PubMed] [Google Scholar]
- 11.Maeda Y, Norton C, Lundby L, et al. Predictors of the outcome of percutaneous nerve evaluation for faecal incontinence. Br J Surg. 2010;97(7):1096–102. doi: 10.1002/bjs.7028. [DOI] [PubMed] [Google Scholar]
- 12.Michelsen HB, Christensen P, Krogh K, et al. Sacral nerve stimulation for faecal incontinence alters colorectal transport. Br J Surg. 2008;95(6):779–84. doi: 10.1002/bjs.6083. [DOI] [PubMed] [Google Scholar]
- 13.Patton V, Wiklendt L, Arkwright JW, et al. The effect of sacral nerve stimulation on distal colonic motility in patients with faecal incontinence. Br J Surg. 2013;100(7):959–68. doi: 10.1002/bjs.9114. [DOI] [PubMed] [Google Scholar]
- 14.Dinning PG, Fuentealba SE, Kennedy ML, et al. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis. 2007;9(2):123–32. doi: 10.1111/j.1463-1318.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 15.Dinning PG, Hunt LM, Arkwright JW, et al. Pancolonic motor response to sub-sensory and suprasensory sacral nerve stimulation in patients with slow-transit constipation. Br J Surg. 2012;99(7):1002–10. doi: 10.1002/bjs.8760. [DOI] [PubMed] [Google Scholar]


